Background: rRNAs are produced as precursors and require nucleases for maturation.
Results: Recombinant Rcl1 cleaves pre-rRNA at the in vivo A2 site, and mutations abolish cleavage in vivo and in vitro.
Conclusion: Rcl1 is the nuclease for co-transcriptional separation of rRNAs for the large and small subunit.
Significance: Identification of the nucleases for rRNA production will allow accumulation and study of novel intermediates.
Keywords: Enzyme Kinetics, Nucleic Acid Enzymology, Ribosome Assembly, rRNA Processing, Yeast, Endonuclease
Abstract
In all forms of life, rRNAs for the small and large ribosomal subunit are co-transcribed as a single transcript. Although this ensures the equimolar production of rRNAs, it requires the endonucleolytic separation of pre-rRNAs to initiate rRNA production. In yeast, processing of the primary transcript encoding 18 S, 5.8 S, and 25 S rRNAs has been studied extensively. Nevertheless, most nucleases remain to be identified. Here, we show that Rcl1, conserved in all eukaryotes, cleaves pre-rRNA at so-called site A2, a co-transcriptional cleavage step that separates rRNAs destined for the small and large subunit. Recombinant Rcl1 cleaves pre-rRNA mimics at site A2 in a reaction that is sensitive to nearby RNA mutations that inhibit cleavage in vivo. Furthermore, mutations in Rcl1 disrupt rRNA processing at site A2 in vivo and in vitro. Together, these results demonstrate that the role of Rcl1 in eukaryotic pre-rRNA processing is identical to that of RNase III in bacteria: to co-transcriptionally separate the pre-rRNAs destined for the small and large subunit. Furthermore, because Rcl1 has no homology to other known endonucleases, these data also establish a novel class of nucleases.
Introduction
Eukaryotic ribosome biogenesis is a complex process that requires the transient association of ∼200 proteins, including nucleases, RNA helicases, GTPases, and ATPases, to promote the modification, folding, and cleavage of rRNA and the binding of ribosomal proteins to the pre-rRNA (1). Three of the four rRNAs are co-transcribed as a single transcript referred to in Saccharomyces cerevisiae as the 35 S precursor, which is subsequently cleaved in a partially ordered process to release the mature 18 S, 5.8 S, and 25 S rRNAs (Fig. 1A) (2). After initial cleavages to generate the mature 5′-end of 18 S rRNA, co-transcriptional cleavage at so-called site A2 separates rRNA destined for the small and large subunits (3, 4). For 40 S maturation, this is followed by Nob1-dependent 3′-end formation (5, 6). Despite decades of research, only three of eight endonucleases in pre-rRNA processing have been identified. This is surprising considering the conservation of cleavage steps and the central importance of processing for rRNA production. Knowledge of the A2 nuclease would also allow for accumulation of novel pre-40 S intermediates that have so far been only inferred because yeast strains specifically inhibited for A2 cleavage are lacking.
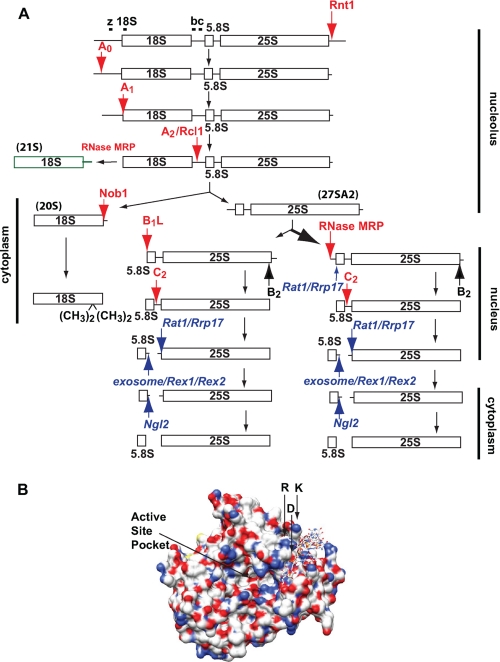
FIGURE 1.
A, schematic of the pre-RNA processing pathway. Endonucleolytic and exonucleolytic cleavage sites are highlighted with red and blue arrows, respectively, with the name of the nuclease involved if known or hypothesized. The 21 S rRNA is shown. It is not converted to 18 S rRNA. The locations of Northern probes are indicated with bars. B, Rcl1 structure (adapted from Ref. 12). For illustration purposes, a single-stranded RNA substrate is modeled into the suggested 3′-substrate binding site. The R327A/D328A/K330A residues and putative active site are indicated.
Rcl1 (RNA 3′-phosphate cyclase-like) is a conserved S. cerevisiae nucleolar protein (7, 8) named for its similarity to RNA cyclases. Cyclases catalyze the ATP-dependent formation of a 2′,3′-cyclic phosphodiester from a 3′-phosphate (9). However, the Rcl1 protein contains an insertion not present in cyclases, and thus, Rcl1 lacks cyclase activity (7). Rcl1 is required for co-transcriptional steps in 18 S rRNA biogenesis (7) and is delivered to pre-ribosomes by Bms1, an essential GTPase that is also required for assembly of the 40 S ribosomal subunit (8, 10, 11). The regulation of this protein by a GTPase suggests that its function may represent an irreversible gatekeeper in ribosome assembly.
Here, we show that recombinant Rcl1 cleaves pre-rRNA transcripts at site A2 in a concentration-dependent manner. This cleavage is abolished by rcl1 mutations that inhibit this co-transcriptional cleavage step in vivo and substantially slowed by RNA mutations around the cleavage site that are known to inhibit A2 cleavage in vivo. These results provide strong evidence that Rcl1 is the long sought nuclease responsible for co-transcriptional cleavage at site A2, the separation point of the large and small subunit rRNAs.
MATERIALS AND METHODS
Cloning
The rcl1 gene-including promoter and terminator was amplified from genomic DNA and cloned into the yeast expression vector pRS315 using primers 1 and 2. Mutations were generated using the QuikChange method. Rcl1, rcl(1–309), and rcl(1–341) were also cloned into pRS416TEF using primers 3–6. Maltose-binding protein (MBP)3-tagged rcl1 was cloned into pSV272 using primers 4 and 7. Plasmids are described in supplemental Table S2.
Transcriptions
rDNA fragments were cloned as described (14, 15). Transcriptions were carried out as described (27), and RNA was purified using an RNA Maxi Kit (Qiagen).
RNA 5′-End Labeling
300 pmol of RNA was treated with Antarctic phosphatase (New England Biolabs) for 1.5 h at 37 °C. After heat inactivation at 65 °C for 5 min, the RNA was 5′-end labeled using T4 polynucleotide kinase (New England Biolabs) and [γ-32P]ATP at 37 °C for 2 h and then gel-purified.
Expression and Purification of Rcl1
His-tagged or MBP-tagged Rcl1 were expressed and purified over nickel-nitrilotriacetic acid agarose as described previously (11). Elution fractions containing Rcl1 were pooled and dialyzed overnight into 50 mm NaCl and 25 mm Tris (pH 7.5) at 4 °C. Dialyzed protein was loaded onto a MonoS column equilibrated in 25 mm KCl, 50 mm Tris (pH 7.5), and 0.5 mm EDTA and eluted in a salt gradient to a final concentration of 1 m KCl over 12 column volumes. Fractions containing Rcl1 were pooled and concentrated for further purification over a Superdex75 column in 200 mm KCl, 50 mm Tris (pH 7.5), 10% glycerol, 1 mm DTT, and 1 mm Tris(2-carboxyethyl)phosphine. Concentrated protein was flash frozen for storage at −80 °C. Protein concentration was determined using an extinction coefficient of 35,410 m−1 cm−1.
RNA Cleavage Assay
RNA was folded as described (11, 14). Rcl1 was pre-incubated at 30 °C for 15–20 min. Aliquots were removed at the indicated time points and immediately phenol/chloroform extracted and precipitated. Samples were boiled for 2 min at 95 °C and held on ice before separation on a 6% acrylamide, 50 mm MES (pH 6.2), 8 m urea gel. Gels were dried and then exposed overnight. Cleavage gels were quantified using phosphoimaging analysis and graphs fit with Equation 1.
 |
Yeast Strain
Yeast strain YKK55, generated by PCR-based recombination, has a galactose-inducible promoter upstream of the Rcl1 open reading frame. The insertion was verified by PCR and Western blotting.
Northern Blotting
Northern blotting was carried out as described previously (28).
RESULTS
Rcl1 Cleaves Pre-rRNA
Rcl1 depletion leads to defects in early co-transcriptional cleavages of pre-rRNA, with effects on cleavage at site A2 being most pronounced (Billy et al., 2000). This observation, as well as the sequence homology of Rcl1 to RNA 3′-cyclases, and the fact that 2′,3′-cyclic phosphates are common endonuclease products, led to the proposal that Rcl1 could be the nuclease for A2 cleavage (7, 11), although that proposal has been recently challenged (12), and alternative proposals have been made (13).
To test whether Rcl1 is responsible for cleavage at site A2, we produced recombinant S. cerevisiae Rcl1 in Escherichia coli. The resulting 40-kDa protein was purified to homogeneity over three columns, and mass spectrometry confirmed that the full-length protein was obtained (supplemental Fig. S1 and data not shown). As substrates, we transcribed pre-rRNA mimics that include the 3′-minor region of 18 S rRNA (starting at helix 44, H44) and ITS1 (internal transcribed spacer I), the spacer region that separates 5.8 S from 18 S rRNAs and harbors cleavage site A2 (see Fig. 2A for rRNA nomenclature). These rRNAs are folded into unique and stable structures relevant to 18 S processing and 40 S subunit assembly in vivo (5, 14, 15).
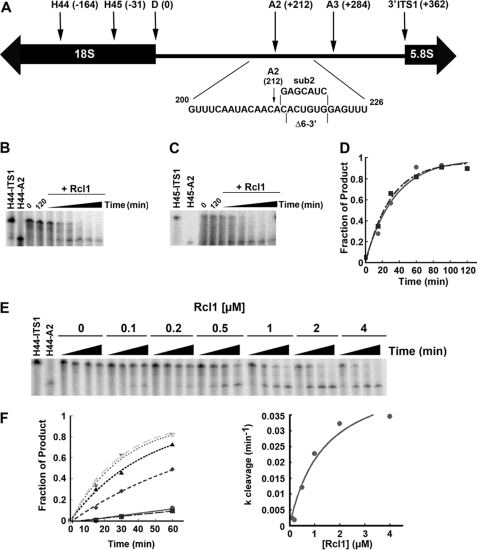
FIGURE 2.
Rcl1 cleaves at site A2. A, schematic view of ITS1. Start (H44 and H45) and end sites (A2 and ITS1) of pre-rRNA constructs, and substitution and deletion mutations around the A2 site are indicated. B, 1 μm Rcl1 was incubated with H44-ITS1 substrate, and samples were collected after 0, 15, 60, 90, and 120 min. Samples incubated without Rcl1 were collected at 0 and 120 min. C, 1 μm Rcl1 was incubated with H45-ITS1 substrate, and samples were collected as described above. D, plots of the reactions with H44-ITS1 (●) and H45-ITS1 (■) were fit to Equation 1. E, increasing concentrations of Rcl1 were incubated with H44-ITS1 substrate, and samples were removed at 0, 15, 30, and 60 min. F, the Rcl1 concentration dependence was fit with the Michaelis-Menten equation to yield kcleav = 0.049 min−1 and K½ = 1.5 μm.
To test whether Rcl1 cleaves pre-rRNA containing the A2 cleavage site, we incubated Rcl1 with prefolded 5′-end labeled H44-ITS1 rRNA and separated the resulting RNAs on denaturing polyacrylamide gels. As shown in Fig. 2B, Rcl1 quantitatively cleaves H44-ITS1 in a time-dependent manner, liberating an RNA fragment corresponding to the in vitro-transcribed H44-A2 product. This reaction is enhanced by increasing the Rcl1 concentration from 0 to 4 μm (Fig. 2, E and F). Furthermore, 3′-end labeling demonstrates that cleavage is endonucleolytic as the 3′-product, A2-ITS1, is also formed (supplemental Fig. S2).
Prior to A2 cleavage, pre-18 S rRNA is in a conformation different from that during cleavage at the 3′-end of 18 S rRNA and in mature 18 S rRNA. H44-ITS1 RNA in this early structure is stabilized by base pairing between the top of H44 and nucleotides immediately downstream of site A2 (5). To test whether the early structure is required for cleavage at site A2, we used an alternative substrate (H45-ITS1), which lacks H44 and thus assumes the post-A2 cleavage structure. Rcl1 cleaves this pre-rRNA with kinetics indistinguishable from those observed for the H44-ITS1 substrate (Fig. 2, C and D). This finding is consistent with the previous observation that the pre-A2 structure is not required for A2 cleavage in vivo (19), and suggests that Rcl1 is indifferent to secondary structure changes near the A2 cleavage site.
To test whether the A2 sequence was sensitive to any nuclease or specifically cleaved by Rcl1, we also incubated H45-ITS1 RNA with Nob1, the endonuclease for D-site cleavage (5, 6). As expected (5) Nob1 cleaves at site D, not at site A2 (supplemental Fig. S3), demonstrating that cleavage at site A2 is specific to Rcl1 and not simply achieved by any nuclease.
Rcl1 Cleaves at Site A2 in Vitro
A2 cleavage in vivo occurs after C(+212) (16). To further confirm that Rcl1 cleaves at the same site in vitro, we mapped the cleavage site by comparing it with RNA sequence ladders generated by RNase T1 digestion and alkaline hydrolysis (supplemental Fig. S4). RNase T1 cleaves after guanosine residues and thereby produces a partial sequencing read. In vitro cleavage of H45-ITS1 by Rcl1 produced the expected product at C(+212) as well as two additional products resulting from cleavages at positions C(+209) and C(+214). These cleavages occur in the same CA sequence context as the main site and are also observed in vivo, albeit to a lesser extent (16).4 Sequence mapping with the larger H44-ITS1 rRNA produced two products, including the expected product at +212 (data not shown). However, the existence of a third product cannot be ruled out, as the larger size of H44-A2 RNA might prevent the resolution of a third band.
Rcl1-dependent Cleavage in Vitro Is Inhibited by rRNA Mutations That Inhibit A2 Cleavage in Vivo
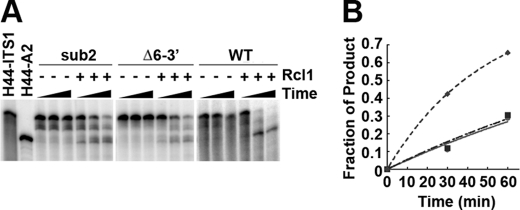
To further demonstrate that Rcl1 is responsible for A2 cleavage and to better characterize the sequence elements recognized by Rcl1, we performed cleavage assays with mutated RNA substrates. These substrates contain a deletion (Δ6–3′) or substitution (sub2) 3′ to the A2 site and inhibit A2 cleavage (at the most abundant site) in vivo (Fig. 2A) (16). Although not abolished, cleavage of either mutant substrate was hindered compared with the wild type RNA substrate (Fig. 3). Residual cleavage most likely results at the aberrant sites three nucleotides upstream or two nucleotides downstream of A2 as reported previously (16). The finding that Rcl1-dependent rRNA cleavage in vitro has the same requirement as A2 cleavage in vivo further supports the hypothesis that Rcl1 is the endonuclease that cleaves at the A2 site.
FIGURE 3.
Rcl1 cleavage of mutant pre-rRNA substrates. A, H44-ITS1 WT, sub2, and Δ6–3′ substrates were incubated for 0, 30, or 60 min in the absence or presence of 3 μm Rcl1. B, data were fit to Equation 1 to yield rate constants of 0.020, 0.0061, and 0.0057 min−1 for WT (♦), sub2 (■), and Δ6–3′ (●), respectively. Fig. 2 shows nucleotide changes.
Mutations in rcl1 Inhibit A2-dependent Cleavage in Vivo and in Vitro
To demonstrate that rRNA cleavage is due to Rcl1 and not a minor E. coli contaminant and to gain insight into the mechanism of Rcl1, we generated an inactive point mutant. We first used truncation analysis to determine a region in rcl1 necessary for activity in vivo. A yeast strain with the endogenous rcl1 gene under the control of a galactose-inducible promoter was created, and plasmids containing no rcl1, wild type, or truncated rcl1 were transformed. Yeast were grown overnight, serially diluted, and plated on media containing either galactose or glucose. As expected, all strains grow indistinguishably on galactose medium, whereas growth on glucose requires functional Rcl1 (Fig. 4A). This analysis indicated that amino acids between residues 309 and 341 are required for Rcl1 function, as Rcl(1–341) rescues growth but Rcl(1–309) does not. This latter truncation corresponds almost exactly to a recently described non-functional truncation of Kluyveromyces lactis Rcl1 (12). Subsequent mutation analysis in this region yielded the triple mutant R327A/D328A/K330A, which grows slowly on glucose-containing medium, indicating that this mutant version of Rcl1 is not fully functional (Fig. 4A). Western analysis of sucrose gradient-fractionated lysates demonstrates that wild type, mutant, and truncated Rcl1 are stable and similarly distributed in pre-ribosomes (supplemental Fig. S5).
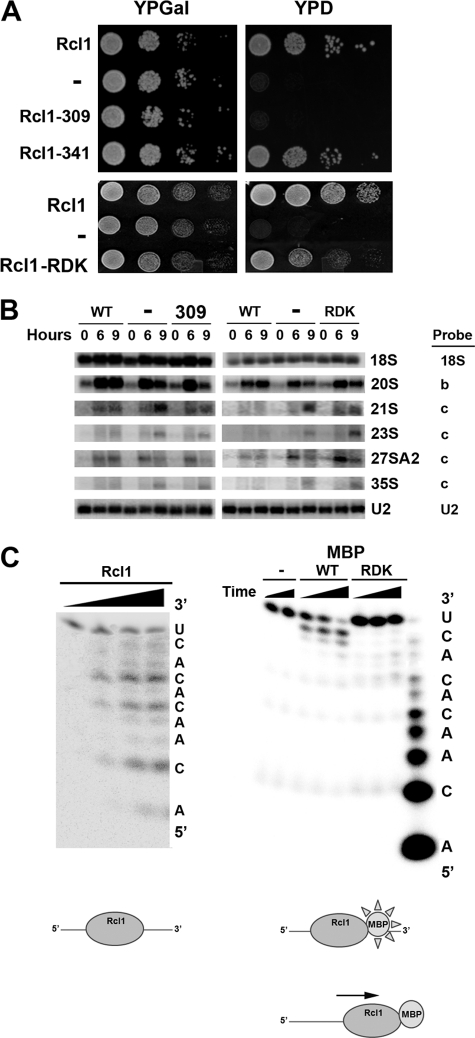
FIGURE 4.
Rcl1 mutants are impaired in A2 site cleavage in vivo and in vitro. A, 10-fold serial dilutions of S. cerevisiae strain YKK55 harboring genomic Rcl1 under the control of a galactose-inducible promoter transformed with plasmids carrying no insert, wild type Rcl1, truncations at amino acids 309 and 341, or point mutations at R327A, D328A, and K330A (RDK) were plated on galactose and glucose solid media (YPGal and YPD, respectively) and incubated for 2 days. B, Northern analysis of pre-rRNAs from the strains in 0, 6, and 9 h (A) after depletion of endogenous Rcl1 by switching to glucose media. U2 RNA was used as a loading control. Probes are indicated next to the panels, and their locations are noted in Fig. 1A. 21 S rRNA was detected with probe c, but not probe z, indicating processing had occurred at site A1. C (left), Rcl1 cleaves an RNA oligonucleotide (ACAACACACU) containing the A2 cleavage site at the three CA sites observed with longer transcripts. Right, cleavage of the same RNA by purified MBP-tagged WT Rcl1 and Rcl1-R327A/D328A/K330A. Bottom, model for the interaction of Rcl1 and MBP-tagged Rcl1 with RNA.
Northern analysis of mutant and truncated Rcl1 shows reduced levels of 20 S and 27 S A2 rRNAs, the products from A2 cleavage, as well as accumulation of 21 S rRNA (Fig. 4B). This intermediate is cleaved at the A0 and A1 sites, but not at site A2 (but instead at the independent, downstream A3 site). Together, these data demonstrate that mutations in rcl1 specifically inhibit cleavage at site A2 in vivo, whereas cleavages at sites A0, A1, and A3 are not affected.
His-tagged mutant or truncated Rcl1 are insoluble (data not shown), perhaps not surprising, as both human and Schizosaccharomyces pombe Rcl1 are also insoluble (12). Addition of a MBP tag solubilized these proteins, and therefore, MBP-tagged wild type and mutant Rcl1 were prepared. However, MBP-tagged wild type Rcl1 was inactive in the RNA cleavage assay using H44-ITS1 (data not shown). We reasoned that this lack of activity might be due to steric inhibition from the MBP tag, which is the same size as Rcl1 itself (see “Discussion” for further details about steric inhibition in the context of the Rcl1 structure). To test whether inhibition could be relieved by use of a smaller substrate that is less prone to steric inhibition, we used a chemically synthesized 10-mer RNA oligonucleotide comprising the cleavage site. Wild type Rcl1 cleaves this oligonucleotide at the same sites as larger substrates, consistent with recognition of a single-stranded region of limited size (Fig. 4C). MBP-tagged Rcl1 also cleaves this oligonucleotide. However, the main site of cleavage is at a downstream site, consistent with steric clashes arising from the MBP-tag that preclude binding at the correct site. In this model, the N-terminal end of the protein, which contains the MBP tag, is oriented toward the RNA 3′-end (Fig. 4C). To avoid steric clashes between the MBP tag and RNA, the protein reorients downstream so that the tag does not contact RNA. Importantly, the Rcl1 R327A/D328A/K330A mutant was incapable of cleaving these oligonucleotides at any site, demonstrating that RNA cleavage arises from the Rcl1 protein.
DISCUSSION
Rcl1 Is the Nuclease That Cleaves at A2
In all forms of life, rRNAs from the small and large subunit are co-transcribed in a single transcript; in eukaryotes, this operon encodes 18 S, 5.8 S, and 25 S rRNAs. Here, we demonstrate that Rcl1 is the nuclease required for the co-transcriptional cleavage that separates 18 S rRNA from 5.8 S and 25 S rRNAs. Recombinant S. cerevisiae Rcl1 purified from E. coli cleaves in vitro-transcribed pre-rRNA mimics containing the A2 cleavage site in a site-specific manner to generate products identical to those found in vivo. Point mutations in rcl1 and pre-rRNA abolish rRNA cleavage in vivo and in vitro.
Our data are consistent with previous in vivo primer extension data demonstrating that Rcl1 depletion has no effect on cleavage at sites A0 and A1 when assayed within 10 h of the shift to glucose (7). Lateron effects on the non-essential cleavage at site A0 are also observed, consistent with our observation that 35 S also accumulates at later time-points.
Rcl1 is delivered to pre-ribosomes by the GTPase Bms1 (11). Our finding that Rcl1 is the nuclease required for cleavage at site A2 puts this observation in a new light, as it explains why Rcl1 delivery is regulated in a GTP-dependent manner: ribosome assembly is facilitated by a large machinery comprising ∼200 cofactors, which transiently interact with assembling ribosomes before dissociating. Because binding and dissociation steps are generally freely reversible, this creates the problem of a lack of flux through the pathway. rRNA cleavage steps (and other steps such as GTP or ATP hydrolysis) can solve this problem as they are irreversible. As observed in metabolic pathways, which also often contain freely reversible as well as irreversible steps, these irreversible steps are often the point of regulation. This appears also to hold true for ribosome biogenesis, where not just the Rcl1 endonuclease is regulated by the GTPase Bms1, but similarly, the Nob1 and RNase MRP endonucleases are regulated by a conformational switch and an RNA-dependent helicase, Dbp3, respectively (11, 5, 18).
Rcl1 has no homology to other known nucleases, and although it has significant structural homology to the RNA cyclase RtcA, most residues around the active site are not conserved (12). Thus, Rcl1 represents a novel endonuclease, with no additional homologs in yeast. It is possible that among the four or more missing endonucleases are additional novel endonucleases, which might have substantially stalled their identification, as they would not be identified as nucleases computationally.
Rcl1 Sequence Specificity
Our data indicate that Rcl1 has specificity for cleavage between C and A. Furthermore, the observation that Rcl1 cleavage is limited to the correct A2 site as well as only two CA sites just up and downstream (despite the presence of 32 CA dinucleotides in H44-ITS1 RNA), suggests that there is an additional Rcl1 recognition element. Substitutions and mutations that affect A2 cleavage are all located 3′ to the A2 site, suggesting that a recognition site may reside there. Reanalysis of previous data (16, 19) and data herein suggests that Rcl1 recognizes a sequence of C↓ACAC, where the arrow indicates the site of cleavage. The C 5′ to the cleavage site can be replaced with an A (supplemental Fig. S4B). Immediately 3′ to this sequence element is a region that forms base pairs with H44 in early pre-40 S ribosomes (5). This region is outside of the Rcl1 recognition site, as the secondary structure of this element does not affect Rcl1 cleavage in vivo or in vitro. Interestingly, the C↓ACAC sequence appears to be conserved from yeast to humans, with the A immediately 3′ to the cleavage site possibly universally conserved (5). We believe that both the highly conserved A 3′ to the cleavage site, as well as a conserved binding site 3′ but not 5′ to the cleavage site are a vestige of the evolution from the bacterial RtcA. RtcA can convert any 3′-monophosphate into cyclic phosphate, suggesting there is no sequence specific recognition to the 5′ sequence. Conversion of the 3′-monophosphate to the cyclic 2′,3′-phosphate is achieved by activation with ATP, which is specifically bound. We believe that the highly conserved A 3′ to the cleavage site binds Rcl1 where ATP binds RtcA.
Although correct Rcl1-dependent cleavage at the A2 site is observed in vitro, additional cleavage products arising from cleavage two or three nucleotides up or downstream, respectively, are also found. The same miscleavage sites are observed in vivo; however, fidelity is much increased (16). The S1 domain-containing protein Rrp5 is required for cleavage at site A2 in vivo (20–22) and binds near cleavage site A2 (15). In vitro, Rrp5 does not promote Rcl1-dependent cleavage nor increase its fidelity, and its binding is insensitive to the rRNA mutations. Moreover, in vitro pulldowns provide no evidence for a direct interaction between Rrp5 and Rcl1.5 Together, these data indicate that Rrp5 is not directly responsible for fidelity of A2 site cleavage in vivo.
Structure of Rcl1 Nuclease
The structures of Rcl1 and the related bacterial RtcA provide important guidance for further functional studies (12, 23, 24). We have identified three residues in the C terminus, Arg-327, Asp-328, and Lys-330, where mutations render Rcl1 non-functional in vivo and in vitro. The observation that individual mutations of the R327A/D328A/K330A residues have no appreciable effect on Rcl1 function in vivo (data not shown) suggests that these residues are not directly involved in the catalytic step. Instead, we predict that they help binding of the RNA substrate: the active site in Rcl1 is likely near the covalently bound AMP in RtcA (24) where a larger pocket, sufficient to accommodate a CA dinucleotide, is found in Rcl1 (Fig. 1B) (12). At one end of this pocket, Rcl1 displays a groove that is lined with basic amino acids, where the conserved R327A/D328A/K330A residues are located. RtcA has a similar, although more narrow, groove. Furthermore, this groove does not display positive charges in RtcA. We propose that this groove forms the binding site for the RNA element 3′ to the cleavage site recognized by Rcl1 as described above. Because a cyclase works on a RNA 3′-end, it would not have such a binding site. This model explains the findings that specificity elements in the RNA are located 3′ to the cleavage site, and that N-terminally tagged Rcl1 cleaves at the very 3′-end of an RNA substrate: the N terminus is located at the end of this channel (12) such that the MBP tag would interfere with RNA binding, requiring a shift in the RNA binding mode.
The recently published Rcl1 structure includes an extensive mutational analysis where 14 individual point mutants around the putative active site pocket were analyzed in a yeast growth assay (12). We have mutated a partially overlapping set of residues around that site and obtained the same result (data not shown). Although this was interpreted as evidence against nuclease activity for Rcl1, we want to point out that it simply demonstrates that these side chains are not required for cell growth. A2 cleavage is not rate-limiting for 40 S ribosome production in vivo, which could obscure small effects on catalysis. Furthermore, backbone amides can be used to bind and stabilize developing negative charge in the transition state, as demonstrated for the oxyanion hole in serine proteases (25). Similarly, backbone carbonyls as well as the RNA substrate itself could provide ligands for essential metal ions, which could function in acid/base catalysis. Side chain mutagenesis would not affect these potential functional groups and can thus not be used to rule out (or in) any functional role for the putative active site or the entire protein. Nevertheless, the surprising lack of any effects from mutagenesis clearly indicates that additional experiments are required to understand how this novel enzyme works.
Rcl1 as Functional Analog of RNase III
rRNA processing in bacteria is initiated by RNase III-dependent cleavage 3′ to the 16 S coding region, which separates pre-16 S rRNA from pre-23 S rRNA and pre-tRNAs encoded between the 16 S and 23 S rRNA genes. Thus, RNase III performs a reaction that is analogous to the one carried out by Rcl1 in eukaryotes. It is not known why eukaryotes, which have a functional RNase III homolog that is involved in 3′-end formation of 25 S rRNA (26), evolved a specialized enzyme to separate rRNAs from the two subunits. Perhaps RtcA, suggested to be involved in tRNA repair, was localized to pre-rRNA by the tRNAs encoded between bacterial 16 S and 23 S rRNAs and co-evolved into Rcl1 with the evolving ITS1. It is tempting to speculate that additional enzymes allow for a finer regulation of pre-rRNA cleavage in higher organisms.
Supplementary Material
Acknowledgments
We thank the members of our laboratory for many helpful discussions and comments on the manuscript.
This work was supported by National Science Foundation Grant MCB0845156.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, Tables S1 and S2, Figs. S1–S5, and additional references.
It is also possible that cleavage at the upstream site is overestimated in our assay, as cleavage at the correct site followed by cleavage at the upstream site would appear only as upstream cleavage. In contrast, the in vivo primer extension assay is biased to cleavage downstream. Sequencing of RNaseT1-digested 3′-ends of 20 S rRNA provided evidence for a single product one nucleotide shorter than the upstream cleavage product, as well as demonstrating that in at least 20% of the material at least one nucleotide was lost in the work up (17). Using existing assays, it is thus not possible to determine the exact distribution of cleavage sites in vivo and in vitro.
C. L. Young, S. L. Mason, and K. Karbstein, unpublished data.
- MBP
- maltose-binding protein.
REFERENCES
- 1. Strunk B. S., Karbstein K. (2009) RNA 15, 2083–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Venema J., Tollervey D. (1999) Annu. Rev. Gen. 33, 261–311 [DOI] [PubMed] [Google Scholar]
- 3. Kos M., Tollervey D. (2010) Mol. Cell 37, 809–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osheim Y. N., French S. L., Keck K. M., Champion E. A., Spasov K., Dragon F., Baserga S. J., Beyer A. L. (2004) Mol. Cell 16, 943–954 [DOI] [PubMed] [Google Scholar]
- 5. Lamanna A. C., Karbstein K. (2011) J. Mol. Biol. 405, 3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pertschy B., Schneider C., Gnädig M., Schäfer T., Tollervey D., Hurt E. (2009) J. Biol. Chem. 284, 35079–35091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Billy E., Wegierski T., Nasr F., Filipowicz W. (2000) EMBO J. 19, 2115–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wegierski T., Billy E., Nasr F., Filipowicz W. (2001) RNA 7, 1254–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Genschik P., Billy E., Swianiewicz M., Filipowicz W. (1997) EMBO J. 16, 2955–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karbstein K., Doudna J. A. (2006) J. Mol. Biol. 356, 432–443 [DOI] [PubMed] [Google Scholar]
- 11. Karbstein K., Jonas S., Doudna J. A. (2005) Mol. Cell 20, 633–643 [DOI] [PubMed] [Google Scholar]
- 12. Tanaka N., Smith P., Shuman S. (2011) RNA 17, 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bleichert F., Granneman S., Osheim Y. N., Beyer A. L., Baserga S. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9464–9469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lamanna A. C., Karbstein K. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14259–14264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Young C. L., Karbstein K. (2011) RNA 17, 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allmang C., Henry Y., Wood H., Morrissey J. P., Petfalski E., Tollervey D. (1996) RNA 2, 51–62 [PMC free article] [PubMed] [Google Scholar]
- 17. De Jonge P., Klootwijk J., Planta R. J. (1977) Eur. J. Biochem. 72, 361–369 [DOI] [PubMed] [Google Scholar]
- 18. Weaver P. L., Sun C., Chang T. H. (1997) Mol. Cell Biol. 17, 1354–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindahl L., Archer R. H., Zengel J. M. (1994) Nucleic Acids Res. 22, 5399–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Torchet C., Jacq C., Hermann-Le Denmat S. (1998) RNA 4, 1636–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Venema J., Tollervey D. (1996) RRP5 is required for formation of both 18 S and 5.85 rRNA in yeast. EMBO J. 15, 5701–5714 [PMC free article] [PubMed] [Google Scholar]
- 22. Vos H. R., Faber A. W., de Gier M. D., Vos J. C., Raue H. A. (2004) Eukaryot. Cell 3, 1504–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palm G. J., Billy E., Filipowicz W., Wlodawer A. (2000) Structure Fold Des. 8, 13–23 [DOI] [PubMed] [Google Scholar]
- 24. Tanaka N., Smith P., Shuman S. (2010) Structure 18, 449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birktoft J. J., Blow D. M. (1972) J. Mol. Biol. 68, 187–240 [DOI] [PubMed] [Google Scholar]
- 26. Elela S. A., Igel H., Ares M., Jr. (1996) Cell 85, 115–124 [DOI] [PubMed] [Google Scholar]
- 27. Karbstein K., Carroll K. S., Herschlag D. (2002) Biochemistry 41, 11171–11183 [DOI] [PubMed] [Google Scholar]
- 28. Woolls H. A., Lamanna A. C., Karbstein K. (2011) J. Biol. Chem. 286, 2578–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.