Abstract
Phosphorylation of heat shock protein 20 (Hsp20) by protein kinase A (PKA) is now recognized as an important regulatory mechanism modulating contractile activity in the human myometrium. Thus agonists that stimulate cyclic AMP production may cause relaxation with resultant beneficial effects on pathologies that affect this tissue such as the onset of premature contractions prior to term. Here we describe for the first time that acetylation of Hsp20 is also a potent post-translational modification that can affect human myometrial activity. We show that histone deacetylase 8 (HDAC8) is a non-nuclear lysine deacetylase (KDAC) that can interact with Hsp20 to affect its acetylation. Importantly, use of a selective linkerless hydroxamic acid HDAC8 inhibitor increases Hsp20 acetylation with no elevation of nuclear-resident histone acetylation nor marked global gene expression changes. These effects are associated with significant inhibition of spontaneous and oxytocin-augmented contractions of ex vivo human myometrial tissue strips. A potential molecular mechanism by which Hsp20 acetylation can affect myometrial activity by liberating cofilin is described and further high-lights the use of specific effectors of KDACs as therapeutic agents in regulating contractility in this smooth muscle.
Keywords: Cytoskeleton, Heat Shock Protein, Histone Deacetylase, Histone Deacetylase Inhibitors, Smooth Muscle, Hsp20, Myometrium, Acetylation
Introduction
In developed countries premature birth prior to 37 weeks gestation accounts for nearly 75% of newborn deaths and is related to a high risk for survivors of long-term physical or mental disability (1). There are still no effective and safe therapeutic treatments for decreasing the incidence of premature deliveries. Accordingly, an increased understanding of the molecular mechanisms underlying myometrial activity is required to aid in the development of new strategies for the treatment of premature labor. To this end, recent evidence indicates a novel role for post-translational modification by acetylation in regulating myometrial activity. This was first highlighted by the class I/II histone deacetylase inhibitors (HDACIs)3 TSA, VPA, and SBHA effecting relaxations of ∼60% to spontaneously or oxytocin-mediated contracting human myometrial tissues ex vivo (2). Potential epigenetic events resulting from increased histone acetylation seem to be excluded due to the comparatively short time period observed for contractile inhibition of 20–60 min. This pointed to the above HDACIs having non-epigenetic effects involving increased acetylation of lysine residues of protein components of the myometrial contractile machinery. This possibility is supported by recent proteomic findings of Kim et al. (3) and Choudhary et al. (4). They, respectively, showed that administration of the HDACIs TSA/Sirtinol to HeLa or SAHA/MS-275 to leukemia MV4–11 cell cultures resulted in acetylation of a range of non-nuclear proteins. Importantly, proteins involved in regulating the cytoskeletal/filamentous architecture of cells were observed to be acetylated. These included actin, cofilin, 14-3-3 as well as the heat shock family protein member Hsp27. With respect to the latter, heat shock proteins such as Hsp27 and Hsp20 are well known actin-binding proteins in smooth muscle cells (5, 6). Both of the above proteomic studies (3, 4) also indicated that acetylation occurred in protein components of large macromolecular complexes such as chromatin, microtubules and, of relevance to the study presented here, the actin-cytoskeleton. If large macromolecule protein structures such as the actin-cytoskeleton may have the potential to be modified by acetylation then this would thus necessitate a role for both non-nuclear histone acetyltransferases (HATs) and histone deacetylases (HDACs). Indeed we now know that HDAC function is not restricted to the nucleus. HDAC6 can regulate cell motility by destabilization of microtubules via reversible deacetylation of non-nuclear tubulin in the cytoplasm of the cell (7) and also the extent of microtubular-directed mitochondrial transport (8). Similarly, HDAC4 can affect cardiomyocyte contractility by binding to the z-disc protein MLP (9). Most importantly, HDAC8 a class I HDAC family member able to deacetylate all core histones in vitro (10, 11) and inhibited by TSA (10), has recently been found to be a non-nuclear marker of smooth muscle differentiation (12) and associates with smooth muscle α-actin (α-SMA) (13). Therefore, HDAC8 may exhibit specific intracellular localization within smooth muscle cells and have a major regulatory function in controlling their contractile capacities. Interestingly, HDAC8 itself is a target of post-translational modification by PKA resulting in a decrease of its activity (14). Because myometrial cyclic AMP-PKA signaling components are known to be up-regulated during pregnancy (15–17) this may potentially lead to increased HDAC8 phosphorylation and its subsequent inhibition resulting in increased acetylation of target proteins and maintenance of uterine quiescence.
Consequently the purpose of the present study was to shed light on the non-epigenetic mechanism(s) by which administration of HDACIs, and resultant changes in protein acetylation, inhibited human myometrial contractility (2). We report that HDAC8 is a non-nuclear protein that interacts with the actin-binding proteins Hsp20 and cofilin. Administration of a selective HDAC8 inhibitor alters Hsp20 acetylation and reduces myometrial contractility. Finally, we deduce that a novel, reciprocal regulatory arrangement exists between acetylation and PKA-directed phosphorylation that alters HDAC8/Hsp20/cofilin function and, thereby, myometrial contractility.
EXPERIMENTAL PROCEDURES
Tissue collection, treatment of myometrial strips and cell cultures, subcellular protein extraction of myometrial tissues and cells, protein lysate preparation and SDS-PAGE, fluorescent immunocytochemistry, Western-blotting, and immunoprecipitation used in the study are detailed in the supplemental Materials and Methods (18–20).
In Vitro HDAC8 Activity Assay
In vitro activity of TSA and Compound 2 in inhibiting HDAC8 was measured using the HDAC8 Fluorometric Drug Discovery kit (Enzo Life Sciences) according to the accompanying protocols detailed in the supplemental Materials and Methods.
Myometrial Contractility Studies
Myometrial contractility experiments employing the HDAC8 inhibitor Compound 2 are detailed in the supplemental Materials and Methods (21).
Gene Chip Microarray Analysis
Gene microarray was performed by ServiceXS (Netherlands) on the 12 sample HumanHT-12 format of Ilumina BeadStation Platform. Changes in gene expression profiles with TSA and Compound 2 compared with untreated controls were analyzed using GeneSpring GX 11 (Agilent). RNA isolation, Gene chip analysis and statistical evaluation are detailed in the supplemental Materials and Methods.
Mass Spectrometry Identification of Proteins from Acetylated ϵ-Lysine Antibody Immunoprecipitations
Immunoprecipitates were separated by SDS-PAGE, stained with colloidal Coomassie Brilliant Blue and bands to be identified were cut out manually. Gel pieces were washed, destained, and proteins were reduced with DTT and alkylated with iodoacetamide, followed by an in gel digest using elastase. The resulting peptides were eluted using increasing concentrations of acetonitrile (MeCN), reduced in volume and identified by reversed phase LCMSMS on a Dionex Ultimate 3000 nano-HPLC system coupled to a Thermo LTQ XL Orbitrap mass spectrometer. Peaklists were generated from raw data files Proteome Explorer 1.1 (Thermo) and the human subset of swissprot (2010.4) was searched using X1Tandem through the gpm interface.
RESULTS
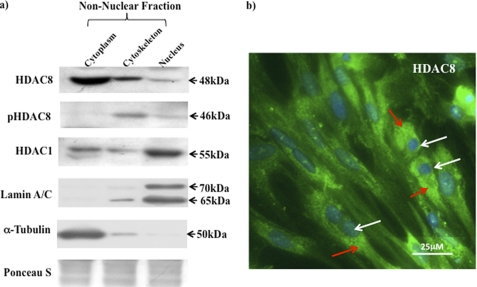
To determine whether HDAC8 had a similar role in the human myometrium as detailed for the vasculature (12, 13), myometrial cell cultures were separated into nuclear, cytoplasmic, and cytoskeletal protein fractions (non-nuclear), employing the procedure as detailed by Waltregny et al. (13), and subjected to Western blotting with HDAC8, phosphorylated HDAC8 (pHDAC8), HDAC1 (also a class I HDAC), lamin A/C (a nuclear marker protein), and α-tubulin (a cytoplasmic/cytoskeletal marker protein) antibodies. HDAC8 and pHDAC8 were primarily localized in the cytoplasmic/cytoskeletal subcellular region whereas HDAC1 was mainly present in the nucleus (Fig. 1a). Such in situ localization of HDAC8 was confirmed by immunofluorescent microscopy (Fig. 1b).
FIGURE 1.
Intracellular localization of HDAC8 in human myometrial cells. a, localization of the HDAC1, HDAC8 and pHDAC8 in cytoplasmic/cytoskeletal (non-nuclear) and nuclear extracts from NP myometrial cells. Purity of the individual fractions was monitored with antibodies to Lamin A/C (nuclear fractions) and to α-tubulin (cytoplasmic/cytoskeletal) fractions. Ponceau S was used to stain gels for equal loading. b, immunofluorescent microscopy staining with the HDAC8 antibody (green and indicated by red arrows) and DAPI (nuclei, blue and indicated by white arrows). Results were repeated in a further two individual patient samples.
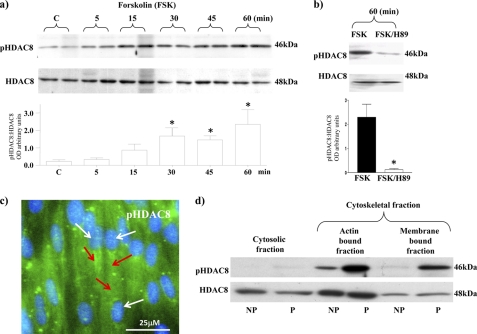
Because Lee et al. (14) indicated that HDAC8 was a substrate for phosphorylation by PKA in HeLa cells, we next determined whether this was the case in human myometrium. Cell cultures treated with forskolin, to activate adenylyl cyclase, for 5–60 min resulted in increased phospho-HDAC8 (pHDAC8) levels (Fig. 2a). Co-incubation of cells with forskolin and the PKA inhibitor H89 for 60 min resulted in a substantial decrease in pHDAC8 levels whereas HDAC8 levels remained unchanged (Fig. 2b). Immunofluorescent microscopy indicated that pHDAC8 is also mainly localized in the cytoplasmic/cytoskeletal region of myometrial cells (Fig. 2c). Similar localizations were found in myometrial cells of fresh ex vivo tissue biopsies. Note that for pHDAC8 there were substantially higher levels observed in tissues of pregnant women (P) compared with tissues from non-pregnant women (NP) (see Fig. 2d). This would seem to reflect the increased cAMP and PKA activity reported from P tissues (15–17). It may imply HDAC8 activity is decreased in comparison to NP tissues. Note that no change in expression levels of total HDAC8 was observed in the cytosolic/cytoskeletal fractions between NP and P tissues.
FIGURE 2.
PKA phosphorylation of HDAC8 and cytoskeletal expression of pHDAC8 in myometrial cells. a, forskolin (FSK) (10 μm) treatment increased phosphorylation of HDAC8 (pHDAC8) from 5–60 min. HDAC8 staining was used as a control for total levels of expression. Data are expressed as mean ± S.E. *, p < 0.05, Students t test compared with control untreated cells, n = 4. b, co-treatment with FSK (10 μm) and the PKA inhibitor H89 (10 μm) for 60 min decreased pHDAC8 levels with no change in total HDAC8 levels. Data are expressed as mean ± S.E. *, n = 3. c, immunofluorescent microscopy staining of myometrial cells with the pHDAC8 antibody (green and indicated by red arrows) and DAPI (nuclei, blue and indicated by white arrows). d, subcellular localization of pHDAC8 and HDAC8 in NP and P tissues. Results were repeated in a further two individual patient samples.
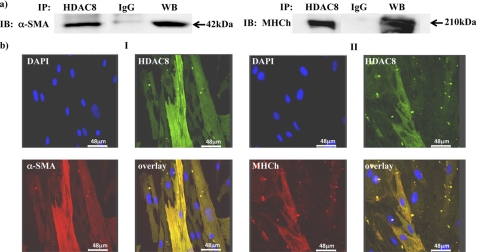
HDAC8 expression in the cytoplasmic/cytoskeletal regions of myometrial cells suggests it may interact with and modulate components of the contractile machinery. To determine if this occurred; immunoprecipitation assays were initially carried out to observe whether HDAC8 formed complexes with the two main contractile proteins associated with smooth muscle cells α-SMA and myosin heavy chain (MHCh). Fig. 3a indicates this to be the case and co-localization with these proteins was further confirmed by immunofluorescent microscopy with HDAC8, α-SMA, and MHCh antibodies (Fig. 3b).
FIGURE 3.
Immunoprecipitation and co-localization of HDAC8 with α-SMA and MHCh. a, NP myometrial cell lysates were immunoprecipitated with the HDAC8 antibody and control IgG sera and precipitated proteins subjected to Western blotting with α-SMA and MHCh antibodies. WB, positive control. b, immunofluorescent microscopy staining showing co-localization of HDAC8 and cytoskeletal proteins. Panel I: HDAC8 antibody (green), α-SMA (red) DAPI (nuclei, blue), and the overlay (yellow); Panel II: HDAC8 antibody (green), ΜΗCh (red) DAPI (blue), and the overlay (yellow). n = 4 individual patient samples.
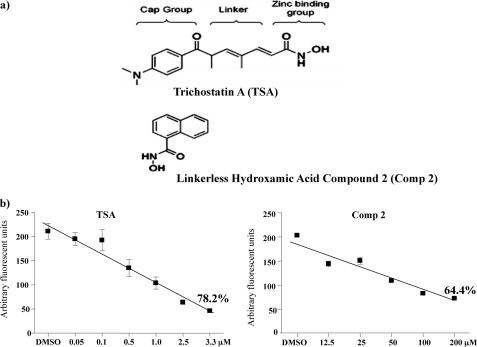
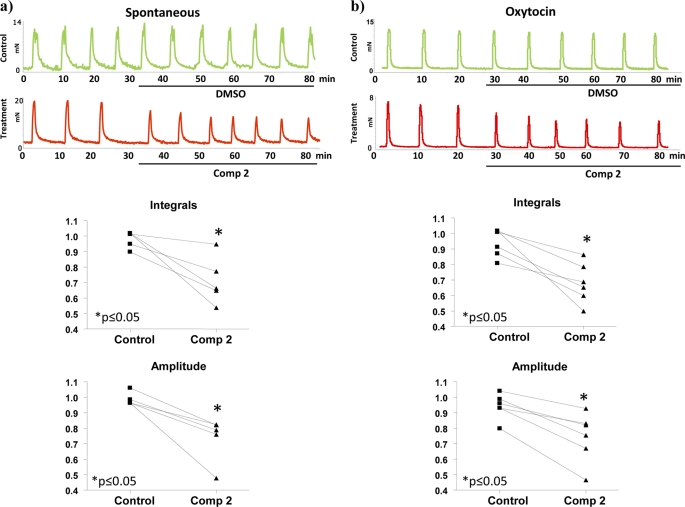
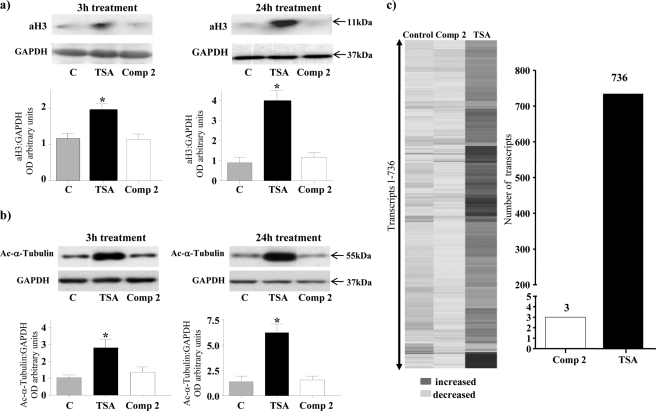
The functional consequence of the interaction of HDAC8 with α-SMA and MHCh was further explored using treatment of myometrial tissue strips with either TSA (a general class I/II HDAC inhibitor) or a highly selective linkerless hydroxamic acid HDAC8 inhibitor termed Compound 2 (22) (Fig. 4a). We already indicated above that TSA can significantly inhibit both spontaneous and oxytocin-induced myometrial contractions (2). TSA actions arise by binding to two internal structural cavities common to all class I/II HDACs (23, 24). In contrast, Compound 2 (22) specifically inhibits HDAC8 activity by binding to an internal groove within its structure which is unique to this HDAC isoform (24). Initially an in vitro assay was employed to determine the degree of inhibition by Compound 2 in comparison to TSA. Dose-response data from these assays indicated that Compound 2 inhibited HDAC8 activity by ∼64% at 100–200 μm concentrations; whereas TSA gave rise to a 78.2% inhibition at 3.3 μm (Fig. 4b). The higher concentrations of Compound 2 (≥ 100 μm) required for inhibition in comparison to TSA (1–5 μm) are likely as a result of having to access this internal groove of HDAC8 (22, 24). Myography studies were subsequently initiated to define the effect of Compound 2 on contracting myometrium obtained from pregnant (P) non-laboring women. Treatment with Compound 2 reduced both spontaneous and oxytocin-induced contractions for up to 1 h (Fig. 5, a and b). It was important to determine whether Compound 2 affected histone H3 acetylation (as a marker of possible epigenetic actions) and α-tubulin acetylation (as compound 2 has a weak efficacy toward the tubulin deacetylase HDAC6 (22)) in these tissues. No increase in aH3 or acetylated α-tubulin levels were observed even after 24 h with Compound 2 whereas TSA caused significant increases in both proteins as expected of a pan-class I/II HDAC inhibitor (Fig. 6, a and b). Thus the relaxatory actions induced by Compound 2 were unlikely to be due to either epigenetic affects on myometrial gene/protein expression or by analogous actions on HDAC6. Microarray analysis confirmed the assertion that little change in gene expression occurred upon incubations with Compound 2 (expression of only 3 genes changed: tumor necrosis factor receptor 6b, chemokine ligand 20 (CCL20) and the Duffy blood group chemokine receptor (DARC)) in comparison to TSA (Fig. 6c). The list of genes (736) whose expression changed via TSA administration is provided as supplemental data S1.
FIGURE 4.
TSA and Compound 2 inhibit HDAC8 activity in vitro. a, structure of TSA in comparison to the linkerless hydroxamic acid derivative Compound 2 (Comp 2). b, concentration response curve of HDAC8 activity as measured by an in vitro assay. Results are derived from three assays done in duplicate. TSA and Comp 2 inhibited HDAC8 activity by 78.2 and 64.4% at 3.3 μm and at 100–200 μm, respectively.
FIGURE 5.
Compound 2 inhibits spontaneous and oxytocin-induced myometrial contractility. Treatment with Compound 2 (100 μm) reduced both (a) spontaneous and (b) oxytocin-augmented (2 nm) contractions for up to 1 h compared with vehicle-treated controls. Integrals for contractions were calculated from three consecutive peaks (25–35 min). Data points on the graphs represent ratios of the integrals before and after treatment with vehicle or Compound 2. Significant decreases in spontaneous and oxytocin induced contractions (Integrals) were observed upon treatment with Compound 2. (p ≤ 0.05 Wilcoxon matched pair test) due to significant decreases in peak amplitudes. The duration of contractions and half peak durations were not significantly changed. n = 5–6 individual patient samples.
FIGURE 6.
The effect TSA and Compound 2 on acetylation of histone H3/α-tubulin and myometrial gene expression. Treatment of P myometrial strips with TSA (3.3 μm) increased levels of (a) acetylated histone H3 (aH3) and (b) acetylated α-tubulin after 3 h and 24 h treatment. Compound 2 (Comp 2) (100 μm) did not affect acetylation of either of these proteins. GAPDH staining was used as a loading control. Data are expressed as mean ± S.E. *, p < 0.05, Student's t test TSA compared with control and Comp 2. n = 5 individual patient samples. c, P myometrial strips were incubated in the presence or absence of Compound 2 (Comp 2) (100 μm) or TSA (3.3 μm) for 24 h and mRNA extracted and subjected to gene microarray analysis. A heat-map of genes that change with Compound 2 (Comp 2) and TSA treatment compared with control is shown as well as the number of genes that change with Compound 2 (Comp 2) and TSA treatment normalized to the control. (n = 4 individual patient samples).
The above findings indicated that one would expect inhibition of HDAC8 by Compound 2, or TSA, to give rise to an increase in acetylated target proteins within non-nuclear regions of myometrial cells. To maximize the opportunity to identify proteins that were targets for acetylation, myometrial strips from NP women were initially treated for 24 h with TSA and protein lysates immunoprecipitated with anti-acetylated ϵ-lysine antibodies. Note that, as remarked upon above, myometrial tissues from P women have elevated cAMP/PKA activities and these are associated with increases in pHDAC8 and, one presumes, reduced activity of HDAC8. The likelihood of envisaging changes in acetylated substrates upon TSA treatment therefore seemed greater for tissues from NP women that had less inhibitory influences of cAMP/PKA activity.
First, to ensure that TSA treatment resulted in acetylation of protein lysates, the immunoprecipitates were probed with an anti aH3 antibody. Fig. 7a indicates that TSA treatment resulted, as anticipated, in the substantial accumulation of aH3 in the immunoprecipitates. Second, since HDAC8 immunoprecipitates and co-localizes with α-SMA and MHCh (Fig. 3), they represented potential targets for increased acetylation upon inhibition of HDACs by TSA. Lysates immunoprecipitated with a pool of three anti- acetylated ϵ-lysine antibodies were thus probed with α-SMA and MHCh. However, surprisingly neither protein was precipitated indicating that they were unlikely to be targets for HDAC8 activity (see supplemental Fig. S1a). Thus the HDAC8 co-immunoprecipitation/co-localization with α-SMA and MHCh (Fig. 3) likely occurred indirectly via complexing with other protein components of the contractile machinery. Consequently, acetylated lysine immunoprecipitates were probed further. Initially this was against calmodulin and Hsp27 antibodies since both proteins have been implicated as important regulators of smooth muscle contractility and, moreover, been found to be targets for acetylation in the non-muscle studies of Kim et al. (3). Neither calmodulin nor Hsp27 appeared to be acetylated via TSA treatment of myometrial strips (see supplemental Fig. S1b).
FIGURE 7.
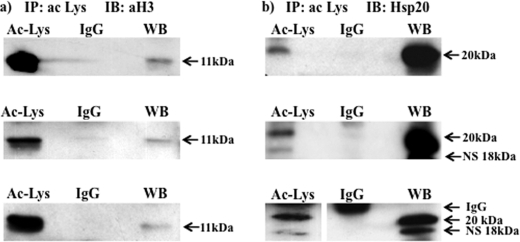
Acetylated lysine antibodies immunoprecipitate Hsp20 in TSA-treated tissues. NP myometrial strips were treated with TSA (3.3 μm) for 24 h, and protein lysates subjected to immunoprecipitation with a pool of 3 acetylated ϵ-lysine antibodies and then Western blotting with (a) an acetylated H3 (aH3) antibody and (b) an Hsp20 antibody. Note for the third replicates in (b) lanes were run on the same gel but were non-contiguous (line). WB, positive control. Note that the Millipore Hsp20 antibody also recognizes a nonspecific (NS) band of 18 kDa.
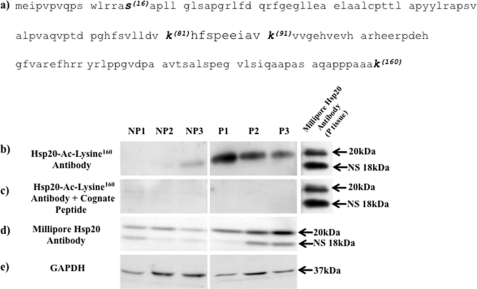
The closely related heat shock protein Hsp20 has also been shown to be an important contractile regulator in the myometrium whereby it co-localizes with α-SMA and its phosphorylation by PKA promotes relaxation (25). Importantly, there is evidence in airway smooth muscle that Hsp20, when phosphorylated by PKA, enacts a sequence of events resulting in the dephosphorylation of cofilin (26, 27) and its increased binding to F-actin (28, 29) and promotion of depolymerization at membrane-associated dense bodies (30, 31). Phosphorylated cofilin has been suggested to bind to the intracellular scaffolding protein 14-3-3 (28, 29) and stabilize F-actin filaments. PKA-phosphorylated Hsp20 (26, 27) has a binding domain for 14-3-3 (32) that displaces phophorylated cofilin which enables it to then be targeted by intracellular phosphatases. Such a scheme has implicated roles for cofilin, 14-3-3 and Hsp20 in regulating smooth muscle cell contractility. Cofilin and 14-3-3 were also identified as substrates for acetylation in the non-muscle cell studies of Kim et al. (3) and Choudhary et al. (4). As such, acetylated lysine immunoprecipitates of human myometrial tissues were also probed with antibodies specific to these proteins. Neither cofilin nor 14-3-3 appeared to be acetylated by TSA treatment of myometrial strips for 24 h (see supplemental Fig. S1b). However, acetylated Hsp20 was observed in the immunoprecipitate by Western blotting (Fig. 7b) and this observation was confirmed using electrospray mass spectrometry (see supplemental data S2 and supplemental Fig. S2). Note that the Millipore anti-Hsp20 antibody employed here and in the assays below predominantly recognizes a band of mass 20 kDa. A smaller band ∼18 kDa is also recognized by this antibody, which remains to be identified but may represent another acetylated protein present in human myometrial tissues that is immunoprecipitated by the acetylated ϵ-lysine antibodies.
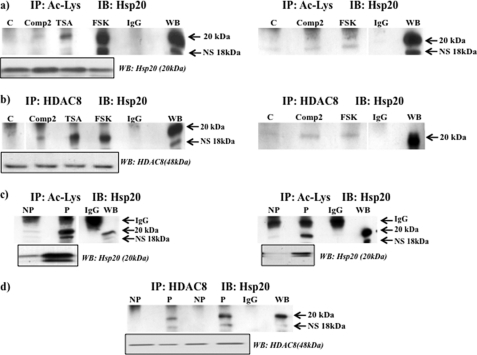
Immunoprecipitates on tissue lysates were carried out to determine the efficacy of Compound 2 and forskolin in acetylating Hsp20 in comparison to TSA. Either Compound 2 or forskolin 24h treatment of myometrial strips resulted in immunoprecipitation of acetylated Hsp20 being observed. (Fig. 8a, see also supplemental Fig. S3a for replicate treatment with Compound 2 and forskolin). Treatments did not change total levels of Hsp20 (Fig. 8a, boxed insert). To define that inhibition of HDAC8 activity was involved in the increased acetylation of Hsp20, the same tissue lysates were immunoprecipitated with the HDAC8 antibody and Western blots probed with the Hsp20 antibody. In each instance HDAC8 was observed to precipitate Hsp20 (Fig. 8b, see also supplemental Fig. S3b for replicate treatment with Compound 2 and forskolin). Treatments did not change total levels of HDAC8 (Fig. 8b, boxed insert).
FIGURE 8.
Acetylation of Hsp20 involves inhibition of HDAC8 in myometrial tissues. a, NP tissues were treated in the absence or presence of Compound 2 (Comp 2) (100 μm), TSA (3. 3 μm), and forskolin (FSK) (10 μm) for 24 h and lysates immunoprecipitated with a pool of 3 acetylated ϵ-lysine antibodies with the appropriate IgG sera control and then Western blotting with the Hsp20 antibody. Boxed insert below indicates total Hsp20 protein levels in the tissues. b, treated NP tissue lysates as in (a) were immunoprecipitated with the HDAC8 antibody and then subjected to Western blotting with the Hsp20 antibody. Lanes were run on the same gel but were non-contiguous (line). Boxed insert below indicates total HDAC8 protein levels in the tissues. Replicates of treatments with Compound 2 and forskolin in (a) and (b) are also shown. Here, lanes were run on the same gels but were non-contiguous (lines). Further data is provided in supplemental Fig. S3, a and b. c, NP and P tissue lysates were immunoprecipitated with a pool of 3 acetylated ϵ-lysine antibodies and subjected to Western blotting with the Hsp20 antibody. Boxed insert below indicates total Hsp20 protein levels in the tissues. Lanes were run on the same gel but were non-contiguous (line). Replicates of (c) are also shown. Further data is provided in supplemental Fig. S3c. d, NP and P tissue lysates were immunoprecipitated with the HDAC8 antibody and subjected to Western blotting with the Hsp20 antibody. Boxed insert below indicates total HDAC8 protein levels in the tissues. Further data are provided in supplemental Fig. S3d. Note that the Millipore Hsp20 antibody also recognizes a nonspecific (NS) band of 18 kDa.
As previously stated, NP tissues have lower cAMP/PKA activity levels in comparison to P tissues (15–17) which could affect HDAC8 phosphorylation (see Fig. 2d) and thus its activity. This would be reflected by the amount of acetylated Hsp20 protein that could be precipitated. Consequently more acetylated Hsp20 should be observed in P compared with NP tissues. To determine this was the case a comparison was made between NP and P acetylated lysine immunoprecipitates. Here substantially more acetylated Hsp20 was precipitated in P compared with NP tissues (Fig. 8c, see also supplemental Fig. S3c). Immunoprecipitation of NP and P tissue lysates with the HDAC8 antibody and probing the precipitates with the Hsp20 antibody showed that increased Hsp20 acetylation in P tissues was associated with binding of HDAC8 (Fig. 8d, see also supplemental Fig. S3d). However, control Westerns with the Hsp20 and HDAC8 antibodies indicated that substantially more Hsp20 was expressed in P compared with NP tissue lysates whereas HDAC8 levels remained unaltered between the two tissue types (Fig. 8, c and d, boxed inserts). This indicated that precipitation of acetylated Hsp20 and binding to HDAC8 in P compared with NP tissues is also dependent on the level of expression of Hsp20 but not on the degree of expression of HDAC8. To further confirm that Hsp20 is susceptible to acetylation in the myometrium we generated a rabbit polyclonal antibody against a peptide sequence derived from Hsp20 that encompassed acetylated lysine160 (see Fig. 9a). Employing this Hsp20-Ac-Lysine160 antibody in Western blotting of NP and P tissues confirmed that Hsp20 is indeed acetylated and that acetylated Hsp20 is highly expressed in P compared with NP myometrial tissues (Fig. 9b). Thus correlating with the increased expression of total levels of Hsp20 in these tissues (Fig. 8, c and d). Pre-incubation of the antibody with the cognate peptide indicated specificity for acetylated Hsp20 (Fig. 9c).
FIGURE 9.
Hsp20 is acetylated on the lysine 160 residue and is highly expressed in P compared with NP tissues. a, amino acid sequence of Hsp20 indicating the phosphorylation (S-Serine16) and potential acetylation (K-Lysine81,91,160) sites within the protein. The Hsp20-Ac-Lysine160 antibody was raised against a peptide sequence containing amino acids 155–160 of human Hsp20 that encompassed acetylated lysine160 and used at 1:3,000 dilution. b, Western blotting with the Hsp20-Ac-Lysine160 antibody indicates that the lysine160 residue is acetylated in the myometrium and that acetylated Hsp20 is highly expressed in P compared with NP tissues. c, no bands were observed when the Hsp20-Ac-Lysine160 antibody was pre-incubated for 1 h with the cognate peptide (40 μg/ml) to which it was raised prior to Western-blotting of NP and P tissues; thus indicating specificity of the 20 kDa band seen in b. d, control Western blotting with the Millipore Hsp20 antibody (see Table 1) confirmed increased total Hsp20 expression levels in P compared with NP tissues. e, Western blots were re-probed with the GAPDH antibody as a loading control. For b–e, samples were run on the same gels but were non-contiguous (lines). For b and c, P tissues were probed with the Millipore Hsp20 antibody as a positive control. Note that the Millipore antibody also recognizes a nonspecific (NS) band of 18 kDa. Data were obtained from three individual NP and P patient samples.
TABLE 1.
Antibodies used in the study
| Antibody | Source | WB dilution | ICC dilution | co-IP (μg/reaction) |
|---|---|---|---|---|
| 14-3-3γ | Abcam | 1:2,000 | – | 2 |
| Rabbit Ac-Lys | ImmuneChem | 1:2,000 | – | 2 |
| Rabbit Ac-Lys (9441) | Cell Signaling | – | – | 1 |
| Mouse Ac-Lys (9681) | Cell Signaling | – | – | 1 |
| Ac-H3 (06–599) | Upstate | 1:10,000 | – | – |
| α-SMA (A2547) | Sigma | 1:105 | 1:20,000 | 1 |
| α-tubulin | Sigma | 1:10,000 | – | – |
| Ac-α-tubulin (T7451) | Sigma | 1:10,000 | – | – |
| Ac-H3 (06–599) | Millipore | 1:10,000 | – | – |
| Calmodulin (05–173) | Millipore | 1:7,500 | – | – |
| Cofilin | S. Cruz | 1:3,000 | – | – |
| Phospho-Cofilin | Abcam | 1:3,000 | – | – |
| GAPDH (sc-25778) | S. Cruz | 1:2000 | – | – |
| HADC1 (ab7028) | Abcam | 1:2,000 | – | – |
| HDAC8 (ab39664) | Abcam | 1:2,500 | 1:100 | 3 |
| Phospho-HDAC8 | Abcam | 1:500 | 1:100 | – |
| Hsp20 (07–490) | Millipore | 1:10,000 | – | 2 |
| Hsp27 (06–478) | Millipore | 1:20,000 | – | 2 |
| IgG rabbit (ab46540) | Abcam | – | – | 2 |
| IgG mouse (12–371) | Millipore | – | – | 2 |
| Lamin A/C | Cell Signaling | 1:5,000 | – | – |
| MHCh | Sigma | 1:5,000 | 1:100 | – |
| acLys160 | CRB | 1:3,000 | – | – |
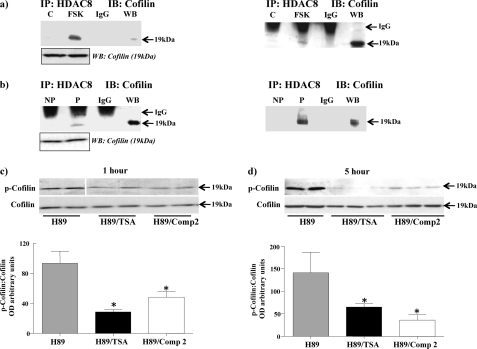
Because HDAC8 forms a complex with Hsp20 and the latter potentially can interact with 14-3-3 to displace cofilin, it is possible that HDAC8 may also form complexes with either 14-3-3 and/or cofilin in the myometrium without affecting their acetylation. To determine this, NP tissue strips treated with forskolin were immunoprecipitated with the HDAC8 antibody and precipitates probed with the 14-3-3 and cofilin antibodies. No immunoprecipitates were observed with 14-3-3 but HDAC8 was observed to immunoprecipitate cofilin (Fig. 10a) thus implicating formation of a HDAC8/Hsp20/cofilin complex. No change in total cofilin levels was observed between the tissues (Fig. 10a, boxed insert). To determine if similar complexes were formed in P tissues where cAMP/PKA activity is high, lysates from both NP and P tissues were immunoprecipitated with the HDAC8 antibody and Westerns carried out with the cofilin antibody. Here cofilin was observed to immunoprecipitate with HDAC8 in P compared with NP tissues (Fig. 10b). No change in total cofilin levels was observed between the tissues (Fig. 10b, boxed insert).
FIGURE 10.
Inhibition of HDAC8 results in immunoprecipitation with cofilin and a decrease in p-cofilin levels. a, immunoprecipitation of lysates from NP myometrial strips treated with forskolin (FSK) (10 μm) for 24 h with the HDAC8 antibody and subjected to Western blotting with a cofilin antibody. Boxed insert below indicates total cofilin protein levels in the tissues. b, immunoprecipitation of lysates from NP and P myometrial strips with the HDAC8 antibody and subjected to Western blotting with a cofilin antibody. Boxed insert below indicates total cofilin protein levels in the tissues. Replicates of a and b are also shown. IgG, negative control sera. WB, positive control. c and d, H89 (10 μm) treated NP myometrial strips were incubated with or without TSA (3.3 μm) and Compound 2 (Comp 2) (100 μm) for 1 h and 5 h, respectively and protein lysates subjected to Western blotting with a p-cofilin antibody and a cofilin antibody to monitor total cofilin levels. Lanes in c were run on the same gel but were non-contiguous (line). Results were derived from three individual patient samples and are expressed as mean ± S.E. *, p < 0.05, Student's t test H89/TSA and H89/Comp 2 compared with H89 controls.
In airway smooth muscle Komalavilas et al. (26) indicated that phosphorylation of Hsp20 by PKA resulted in displacement of phosphorylated cofilin from 14-3-3 followed by dephosphorylation by specific phosphatases. This was monitored as a reduction in phosphorylated cofilin employing a specific phospho-cofilin antibody. Because HDAC8/Hsp20/cofilin complexes are formed in the myometrium and inhibition of HDAC8 results in increased acetylated Hsp20 levels; the possibility existed that acetylated Hsp20 behaves in a similar manner to phosphorylated Hsp20 in decreasing phosphorylated cofilin levels. To test this premise NP myometrial tissue strips were incubated with H89 to inhibit PKA so as to limit the possibility of phosphorylated Hsp20 reducing levels of phosphorylated cofilin. These strips were then incubated in the presence or absence of either TSA or Compound 2 and the level of phosphorylated cofilin monitored. Both TSA and Compound 2 were observed to decrease phospho-cofilin levels after 1 h (Fig. 10c) and 5 h (Fig. 10d) treatments, with no changes in total cofilin levels being detected. These data would seem to indicate that acetylation of Hsp20, independent of its phosphorylation state, can increase levels of dephosphorylated cofilin.
DISCUSSION
It is now recognized that phosphorylation of Hsp20 by PKA plays an important role in regulating myometrial smooth muscle contractility (25). We now show for the first time that acetylation of Hsp20 is another post-translation modification (PTM) with the potential to affect the contractile machinery within the human myometrium. Our data indicate that phosphorylation and acetylation of Hsp20 appear to be independent processes regulating Hsp20 activity. However both PTMs may be affected by a common signaling pathway in that the increased PKA activity observed during pregnancy (15–17) not only stimulates Hsp20 phosphorylation but also its acetylation as a result of inhibition of HDAC8, a component of the acetylation mechanism.
With respect to HDAC8, this lysine deacetylase (KDAC) is primarily situated within the cytoplasmic/cytoskeletal region of myometrial cells. This is in contrast to HDAC1, traditionally assigned with HDAC8 to be a class I HDAC, which is localized mainly in the nucleus. Importantly we show that HDAC8 is phosphorylated by PKA and that pHDAC8 is also principally expressed in the cytoplasmic/cytoskeletal region of myometrial cells. pHDAC8 levels are elevated in P myometrial tissues indicative of the high PKA activity in comparison to non-pregnant tissues. Our use of tissues from both NP and P women has tested the effectiveness of the pan-HDAC inhibitor TSA and a selective HDAC8 inhibitor Compound 2 in affecting acetylation of Hsp20 as well as the functional consequence of this PTM on myometrial contractility. In vitro, both TSA and compound 2 substantially inhibit HDAC8 activity although apparently via different structural mechanisms. Of interest, acetylation of Hsp20 and binding of HDAC8 appears to be minimal in control, non-treated NP tissues compared with P tissues. This may indicate that inhibition of HDAC8 by PKA and TSA/Compound 2 results in greater interaction with Hsp20 and the observed increase in its acetylation when NP tissues are treated. In this context we also provide evidence indicating that the lysine160 residue within Hsp20 is acetylated and that acetylated Hsp20 is more highly expressed in P compared with NP tissues; which correlates with the observed increase in total levels of expression of Hsp20 in these tissues. At present it remains to be elucidated whether lysine81 and lysine91 within Hsp20 are also susceptible to acetylation.
Employing P myometrium in myography studies, we show that Compound 2 can inhibit both spontaneous and oxytocin induced contractions even though these tissues have presumably high PKA activity and Hsp20 levels which would inherently dampen contractility. The inhibitory effect of this selective HDAC8 inhibitor was not associated with an increase in aH3 or major changes in gene expression. Similarly no change in α-tubulin acetylation was observed indicating that the effects of Compound 2 did not involve changes in activity of HDAC6. The specificity of Compound 2 and similar linkerless hydroxamic derivatives (33) in inhibiting HDAC8 may thus have potential as novel therapeutics in preventing the onset of premature contractions; although a caveat to be overcome is the high concentrations required.
Because acetylation tends to occur in large macromolecular complexes (3, 4) it is important to note that Hsp20 and Hsp27 also exist in smooth muscle cells in macromolecular complexes ranging from dimers to multimers of 500–700 kDa (34). These complexes are dynamically regulated by protein kinases such as PKA, which phosphorylates serine 16 within Hsp20 (26–28). It would thus seem likely that macromolecular complexes of Hsp20 within the myometrium are also regulated by acetylation of lysine residues within Hsp20.
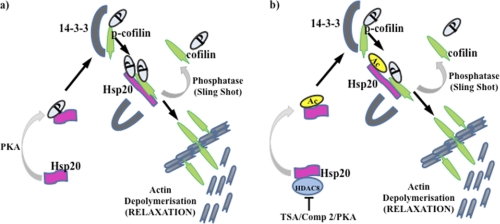
Our data indicated that HDAC8 interacts with both α-SMA and MHCh however we were unable to immunoprecipitate either protein with acetylyated lysine antibodies following treatment of tissues with HDAC8 inhibitors. Moreover since α-SMA forms a complex with Hsp20 in the human myometrium as detailed by Tyson et al. (25) it seems most probable that the localization of HDAC8 at myosin-actin filaments results from the formation of complexes of HDAC8/Hsp20/α-SMA/MHCh at these sites. This may thus implicate a function for acetylated Hsp20 in modulating tension within these structures (20, 35, 36) An alternative mode of action of Hsp20 involves the displacement of phospho-cofilin from the scaffolding protein 14-3-3 upon phosphorylation of Hsp20 by PKA (26–28, 36) with the subsequent release of cofilin affecting actin depolymerization. Here we indicate that acetylation of Hsp20 may act in a similar manner (see Fig. 11 for model). First, we show that HDAC8/Hsp20/cofilin complexes are formed when NP tissues are treated with forskolin. This treatment not only stimulates acetylation of Hsp20 by inhibiting HDAC8 but also its phosphorylation via increased PKA activity. Importantly we show that these complexes are also formed in P tissues. Second, we show that acetylation of Hsp20 alone can decrease levels of phospho-cofilin when NP tissues are treated with TSA or Compound 2 in the presence of the PKA inhibitor H89. In conclusion we have identified Hsp20 as a target for acetylation and provide evidence for a role for acetylation of Hsp20 in regulating the release of cofilin with its subsequent effects on actin filament dynamics and thus myometrial activity.
FIGURE 11.
Model of Hsp20 phosphorylation and acetylation regulating myometrial actin dynamics. a, phosphorylation of Hsp20 by protein kinase A (PKA) results in displacement of p-cofilin from 14-3-3 with subsequent dephosphorylation by phosphatases such as “sling shot” resulting in liberation of cofilin, which influences the depolymerization of actin at membrane-associated dense bodies. b, acetylation of Hsp20 may act independently or in conjunction with phosphorylation to further modulate actin filamental dynamics. P, phosphorylation, Ac, acetylation.
Supplementary Material
Acknowledgments
We thank Dr. Daniel Swan in the Bio-informatics unit, Faculty of Medicine at Newcastle University, for processing the microarray data, Karen Lowdon and Sarah Smith (NEPAF) for excellent technical assistance with the proteomics, and the research midwives at the Royal Victoria Infirmary Newcastle upon Tyne for identification and consenting of patients for myometrial sample collection.
This work was supported by Grant G00800202 from the Medical Research Council, UK.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Materials and Methods, Data, and Figs. S1–S3.
- HDACI
- histone deacetylase inhibitors
- Hsp
- heat shock protein
- HDAC
- histone deacetylase
- TSA
- trichostatin A.
REFERENCES
- 1. Goldenberg R. L., Culhane J. F., Iams J. D., Romero R. (2008) Lancet 371, 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moynihan A. T., Hehir M. P., Sharkey A. M., Robson S. C., Europe-Finner G. N., Morrison J. J. (2008) Am. J. Obstet Gynecol 199, 167.e1–7 [DOI] [PubMed] [Google Scholar]
- 3. Kim S. C., Sprung R., Chen Y., Xu Y., Ball H., Pei J., Cheng T., Kho Y., Xiao H., Xiao L., Grishin N. V., White M., Yang X. J., Zhao Y. (2006) Mol. Cell 23, 607–618 [DOI] [PubMed] [Google Scholar]
- 4. Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009) Science 325, 834–840 [DOI] [PubMed] [Google Scholar]
- 5. MacIntyre D. A., Tyson E. K., Read M., Smith R., Yeo G., Kwek K., Chan E. C. (2008) Endocrinology 149, 245–252 [DOI] [PubMed] [Google Scholar]
- 6. Salinthone S., Tyagi M., Gerthoffer W. T. (2008) Pharmacol. Ther. 119, 44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X. F., Yao T. P. (2002) Nature 417, 455–458 [DOI] [PubMed] [Google Scholar]
- 8. Chen S., Owens G. C., Makarenkova H., Edelman D. B. (2010) PLoS One 5, e10848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta M. P., Samant S., Smith S. H., Shroff S. G. (2008) J. Biol. Chem. 283, 10135–10146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu E., Chen Z., Fredrickson T., Zhu Y., Kirkpatrick R., Zhang G. F., Johanson K., Sung C. M., Liu R., Winkler J. (2000) J. Biol. Chem. 275, 15254–15264 [DOI] [PubMed] [Google Scholar]
- 11. Buggy J. J., Sideris M. L., Mak P., Lorimer D. D., McIntosh B., Clark J. M. (2000) Biochem. J. 350, 199–205 [PMC free article] [PubMed] [Google Scholar]
- 12. Waltregny D., De Leval L., Glénisson W., Ly Tran S., North B. J., Bellahcène A., Weidle U., Verdin E., Castronovo V. (2004) Am. J. Pathol 165, 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waltregny D., Glénisson W., Tran S. L., North B. J., Verdin E., Colige A., Castronovo V. (2005) Faseb J. 19, 966–968 [DOI] [PubMed] [Google Scholar]
- 14. Lee H., Rezai-Zadeh N., Seto E. (2004) Mol. Cell. Biol. 24, 765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Europe-Finner G. N., Phaneuf S., Watson S. P., López Bernal A. (1993) Endocrinology 132, 2484–2490 [DOI] [PubMed] [Google Scholar]
- 16. Europe-Finner G. N., Phaneuf S., Tolkovsky A. M., Watson S. P., López Bernal A. (1994) J. Clin. Endocrinol Metab 79, 1835–1839 [DOI] [PubMed] [Google Scholar]
- 17. MacDougall M. W., Europe-Finner G. N., Robson S. C. (2003) J. Clin. Endocrinol. Metab. 88, 2194–2205 [DOI] [PubMed] [Google Scholar]
- 18. Karolczak-Bayatti M., Loughney A. D., Robson S. C., Europe-Finner G. N. (2009) J. Cell Mol. Med. 15, 94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chapman N. R., Europe-Finner G. N., Robson S. C. (2004) J. Clin. Endocrinol. Metab. 89, 5683–5693 [DOI] [PubMed] [Google Scholar]
- 20. Phaneuf S., Europe-Finner G. N., Varney M., MacKenzie I. Z., Watson S. P., López Bernal A. (1993) J. Endocrinol. 136, 497–509 [DOI] [PubMed] [Google Scholar]
- 21. Bugg G. J., Riley M. J., Johnston T. A., Baker P. N., Taggart M. J. (2006) Eur. J. Clin. Invest. 36, 133–140 [DOI] [PubMed] [Google Scholar]
- 22. Krennhrubec K., Marshall B. L., Hedglin M., Verdin E., Ulrich S. M. (2007) Bioorg Med. Chem. Lett. 17, 2874–2878 [DOI] [PubMed] [Google Scholar]
- 23. Thangapandian S., John S., Sakkiah S., Lee K. W. (2010) Eur. J. Med. Chem. 45, 4409–4417 [DOI] [PubMed] [Google Scholar]
- 24. Estiu G., West N., Mazitschek R., Greenberg E., Bradner J. E., Wiest O. (2010) Bioorg Med. Chem. 18, 4103–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tyson E. K., Macintyre D. A., Smith R., Chan E. C., Read M. (2008) Endocrinology 149, 6157–6165 [DOI] [PubMed] [Google Scholar]
- 26. Komalavilas P., Penn R. B., Flynn C. R., Thresher J., Lopes L. B., Furnish E. J., Guo M., Pallero M. A., Murphy-Ullrich J. E., Brophy C. M. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 294, L69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dreiza C. M., Brophy C. M., Komalavilas P., Furnish E. J., Joshi L., Pallero M. A., Murphy-Ullrich J. E., von Rechenberg M., Ho Y. S., Richardson B., Xu N., Zhen Y., Peltier J. M., Panitch A. (2005) Faseb J. 19, 261–263 [DOI] [PubMed] [Google Scholar]
- 28. Birkenfeld J., Betz H., Roth D. (2003) Biochem. J. 369, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gohla A., Bokoch G. M. (2002) Curr. Biol. 12, 1704–1710 [DOI] [PubMed] [Google Scholar]
- 30. Gohla A., Birkenfeld J., Bokoch G. M. (2005) Nat. Cell Biol. 7, 21–29 [DOI] [PubMed] [Google Scholar]
- 31. Niwa R., Nagata-Ohashi K., Takeichi M., Mizuno K., Uemura T. (2002) Cell 108, 233–246 [DOI] [PubMed] [Google Scholar]
- 32. Chernik I. S., Seit-Nebi A. S., Marston S. B., Gusev N. B. (2007) Mol. Cell Biochem. 295, 9–17 [DOI] [PubMed] [Google Scholar]
- 33. Balasubramanian S., Ramos J., Luo W., Sirisawad M., Verner E., Buggy J. J. (2008) Leukemia 22, 1026–1034 [DOI] [PubMed] [Google Scholar]
- 34. Brophy C. M., Dickinson M., Woodrum D. (1999) J. Biol. Chem. 274, 6324–6329 [DOI] [PubMed] [Google Scholar]
- 35. Yoshino Y., Sakurai W., Morimoto S., Watanabe M. (2005) Jpn J. Physiol. 55, 373–378 [DOI] [PubMed] [Google Scholar]
- 36. San Martin A., Lee M. Y., Williams H. C., Mizuno K., Lassègue B., Griendling K. K. (2008) Circ. Res. 102, 432–438 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.