Abstract
Solar UV radiation is a major environmental factor that causes DNA damage, inflammation, and even skin cancer. T-LAK cell-originated protein kinase (TOPK) is expressed widely in both normal and cancer cells and functions to inhibit apoptosis and promote carcinogenesis. However, its function in inflammation is not known. The p38 MAPK signaling pathway plays an important role in solar UV light-induced inflammation. In this study, we found that TOPK negatively regulated the activity of p38α by phosphorylating the p38α-specific phosphatase MKP1 and enhancing the stability of MKP1. Notably, the absence of TOPK in mice resulted in a striking increase in skin inflammation. Therefore, we conclude that TOPK has a protective function in solar UV light-induced inflammation.
Keywords: Cancer Tumor Promoter, Cyclooxygenase (COX) Pathway, MAP Kinases (MAPKs), p38, Tumor Necrosis Factor (TNF), Solar Ultraviolet (UV) Radiation, TOPK
Introduction
UV light is a well established carcinogen of squamous-type tumors in mouse skin (1). UV light acts as both an initiator, presumably by causing DNA damage leading to gene mutations, and as a tumor promoter (2, 3). Because UV irradiation cannot penetrate farther than the skin in humans, this organ is the primary target for UV light-induced damage and carcinogenesis. Solar UV light can be very harmful to human health, causing DNA damage, inflammation, erythema, sunburn, immunosuppression, photoaging, gene mutations, and skin cancer (4). The inflammation produced by exposure to UV light has been well documented clinically and histologically (5). The MAPKs, especially p38, have been reported to be involved in UV light-induced inflammation and related signal transduction (4).
The p38 MAPK pathway is a key regulator of proinflammatory cytokine biosynthesis, including TNF-α, IL-1β, and cyclooxygenase-2, at the transcriptional and translational levels (6). In addition, p38 also acts downstream of cytokines, such as TNF-α, mediating some of their effects. Thus, p38 has been the subject of extensive efforts in both basic research and drug discovery (7). The p38 protein can be phosphorylated by MKK3 and MKK6 within a few minutes after exposure to diverse stimuli (8). The MAPK phosphatase MKP1, an archetypal member of the MKP family, plays a pivotal role in the deactivation of p38 through a dephosphorylation reaction. Studies using MKP1 knock-out mice have defined the critical importance of MKP1 in the regulation of proinflammatory cytokine synthesis in vivo during the host response to Toll-like receptor ligands (9). Our kinase array assay indicated that the p38 protein is activated strongly by solar UV radiation3 and therefore might play a pivotal role in solar UV light-induced inflammation.
T-LAK cell-originated protein kinase (TOPK),4 a newly identified member of the MEK3/6-related MAPKK family, is expressed in a wide range of proliferating cells and tissues, including cancer cells and testis. TOPK (Thr-9) is phosphorylated by the Cdk1-cyclin B complex and associates with mitotic spindles during mitosis (10). TOPK phosphorylation of histone H2AX prevents arsenite-induced apoptosis in RPMI7951 melanoma cells (11). A positive feedback loop occurs between TOPK and ERK2 through their phosphorylation of each other, resulting in increased tumorigenesis properties of HCT116 colorectal cancer cells, which makes TOPK a new potential therapeutic target (12). The phosphorylation level of p38 was reported to be up-regulated after transfection of the TOPK gene into COS-7 cells (13). However, we found that the phosphorylation level of p38 was increased in TOPK−/− mouse embryonic fibroblasts (MEFs) compared with TOPK+/+ MEFs after stimulation with solar UV light. Our observations reported here unveil a novel role for TOPK. TOPK negatively regulates p38 activity through enhancement of the stability of MKP1, which appears to result in decreased UV light-induced inflammation.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfections
TOPK+/+ and TOPK−/− MEFs and HEK293T and RPMI7951 mock siRNA (siMock) and TOPK siRNA (siTOPK) human malignant melanoma epithelium-like cell lines were maintained in HyClone DMEM with 10% FBS and in Eagle's minimal essential medium with 10% FBS, respectively. Cells were starved for 24 h in serum-free medium before treatment with solar UV light. Transfection of the various expression vectors was conducted using jetPEITM cationic polymer transfection reagent (Polyplus-transfection Inc., New York, NY) according to the manufacturer's suggested protocol.
Construction of Expression Vectors
For purification of the His-MKP1 fusion protein, the pBluescriptR-MKP1 plasmid (Thermo Scientific, Inc., Huntsville, AL) was amplified by PCR using 5′-GACGACGACAAGATGGTCATGGAAGTGGGCACCCTG-3′ as the sense primer and 5′-GAGGAGAAGCCCGGTTCAGCAGCTGGGAGAGGTCGTAATG-3′ as the antisense primer. The PCR products were digested and cloned into the pET-46Ek/LIC vector (Novagen). For expression of the full-length V5-MKP1 fusion protein in HEK293T cells, the pBluescriptR-MKP1 plasmid was amplified by PCR using 5′-CGGGATCCCGATGGTCATGGAAGTGGGCACCCTG-3′ as the sense primer and 5′-CGTCTAGACGCCCAGCAGCTGGGAGAGGTCGTAAT-3′ as the antisense primer. For N-terminal expression of the MKP1 rhodanese (Rho) fragment, 5′-CGTCTAGACGCCAGAAAGGGCAGGATTTCCACCGGGCCACC-3′ was used as the antisense primer. For C-terminal expression of the MKP1 dual specificity phosphatase catalytic (DSPc) fragment, 5′-CGGGATCCCGATGTACCTGGGCAGTGCGTATCAC-3′ was used as the sense primer. The PCR products were digested and cloned into the pcDNA3.1/V5-HisA vector (Invitrogen).
Bacterial Expression and Purification of the His-MKP1 Fusion Protein
The His-MKP1 fusion protein was expressed in Rosetta 2(DE3)pLysS bacteria (Novagen). Bacteria were grown at 37 °C to an absorbance of 0.8–0.9 at 660 nm, induced with 0.5 mm isopropyl β-d-thiogalactopyranoside overnight at 25 °C, and harvested by centrifugation. Cell pellets were suspended in 50 mm NaH2PO4 lysis buffer (pH 8.0) containing 300 mm NaCl and 10 mm imidazole. After sonication and centrifugation, the supernatant fraction was incubated with nickel-nitrilotriacetic acid-agarose beads (Qiagen, Valencia, CA) overnight at 4 °C. Beads were washed with lysis buffer and PBS and then eluted with 250 mm imidazole. After protein quantitation, samples were separated by 10% SDS-PAGE, and visualized by Coomassie Brilliant Blue staining or Western blotting with anti-MKP1 antibody.
In Vitro Kinase Assay
To detect γ-32P incorporation, 2 μg of V5-MKP1 was mixed with active PDZ-binding kinase/TOPK kinase (0.2 μg/50-μl reaction; Cell Signaling) in 5× kinase buffer containing 10 μm unlabeled ATP and 10 μCi of [γ-32P]ATP (New England Biolabs) and incubated at 30 °C for 30 min, and the reaction was stopped by the addition of 6× SDS loading buffer. Samples were separated by 10% SDS-PAGE and visualized by autoradiography or Western blotting with anti-MKP1 antibody.
Western Blotting and Immunoprecipitation
Cells (2 × 106) were grown to 70–80% confluence and starved for 24 h in serum-free medium before being stimulated by solar UV light and then harvested in 300 μl of radioimmune precipitation assay buffer, followed by disruption by sonication and centrifugation at 12,000 rpm for 10 min. The quantity of protein was determined by the Bradford method (14). Protein samples were subjected to SDS-PAGE and then Western blotting. Antibody-bound proteins were detected by chemiluminescence (ECFTM, Amersham Biosciences) and analyzed using a Storm 840 scanner (Molecular Dynamics, Sunnyvale, CA). Untreated cells were used as negative controls. For immunoprecipitation, HEK293T cells were treated with solar UV light (60 kJ/m2) at 48 h after transfection and then disrupted in 200 μl of 1% CHAPS buffer. Equal amounts of protein (0.5–1 mg) were subjected to immunoprecipitation, followed by Western blot analysis.
Animal Study
Adult TOPK+/+ and TOPK−/− mice (6–8 weeks old, five per group for a total of three groups) were shaved 3 days before being irradiated with 142 kJ/m2 solar UV light. Dorsal trunk skin samples were harvested at 0, 12, and 24 h after irradiation. One-half of the samples were immediately fixed in 10% neutral buffered Zamboni's reagent and processed for H&E staining and immunohistochemistry. The other samples were frozen and used for Western blot analysis.
Statistical Analysis
Significant differences were determined by one-way analysis of variance.
RESULTS
TOPK Inhibits Phosphorylation of p38α after Stimulation with Solar UV Light
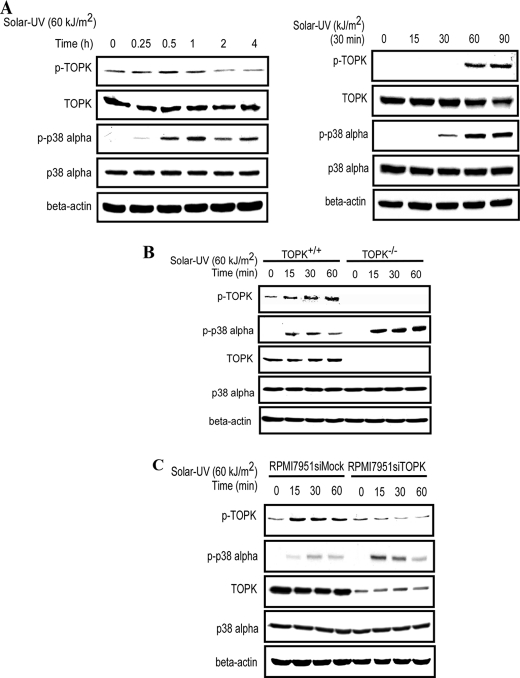
TOPK is a serine/threonine kinase that is overexpressed and activated in many kinds of tumors, including melanoma (15), colon cancer (12), breast cancer (16), and hematological malignancies (17). Multiple biological functions of TOPK have been reported and include cell proliferation and transformation, cell cycle regulation, tumorigenesis, and anti-apoptotic actions. To elucidate the role of TOPK in solar UV light-induced inflammation, which is characterized by high activities of the p38 MAPK signaling pathway, we used MEFs to study the activities of TOPK and p38α following solar UV treatment. We found that the phosphorylation levels of both TOPK and p38α increased in a time- and dose-dependent manner, with the peak phosphorylation level of p38α occurring later than that of TOPK (Fig. 1A). The phosphorylation levels of ERK1/2 and JNK1/2 induced by solar UV light were also determined because of their strong relationship to the UVB signaling pathway (1) and their reported cross-talk with the p38 signaling pathway (18, 19). However, only a very slight increase could be detected in the phosphorylation of ERK1/2 and JNK1/2 induced by solar UV light (supplemental Fig. S1A). We further studied the effects of TNF-α, IL-1β, and LPS on the phosphorylation level of p38 in TOPK+/+ and TOPK−/− MEFs exposed or not exposed to solar UV light. We found that p38 could be negatively regulated by TOPK only upon solar UV stimulation. TNF-α, IL-1β, or LPS had no effect (supplemental Fig. S1B). To further confirm these results, we measured LPS-induced IL-6 production in TOPK+/+ and TOPK−/− MEFs. We found that the concentration of IL-6 decreased dramatically in TOPK+/+ cells stimulated with LPS (supplemental Fig. S1C), which is consistent with the results of Western blotting. Thus, the p38 signaling pathway was identified as the primary MAPK signaling pathway activated by solar UV light. TOPK was first cloned and reported to be a novel MEK3/6-related MAPKK, and p38α was identified as one of the substrates of TOPK (13). To study the role of TOPK in the phosphorylation level of p38α, we treated TOPK+/+ and TOPK−/− MEFs with solar UV light (60 kJ/m2) and harvested the cells at different time points. Unexpectedly, we found that the phosphorylation level of p38α was dramatically increased in TOPK knock-out (TOPK−/−) MEFs compared with wild-type TOPK (TOPK+/+) MEFs (Fig. 1B). The same pattern of changes was also observed in RPMI7951 siMock and RPMI7951 siTOPK melanoma cell lines (Fig. 1C), which express high levels of TOPK and knocked down TOPK, respectively. These data demonstrate that solar UV light induces the phosphorylation of TOPK and p38α and that TOPK negatively regulates the activity of p38α.
FIGURE 1.
TOPK inhibits the phosphorylation of p38α. A, the phosphorylation levels of TOPK and p38α were increased in a time- and dose-dependent manner. MEFs (2 × 106/10-cm dish) were stimulated with solar UV light (60 kJ/m2) after 24 h of serum-free starvation, whole cell lysates were collected at different time points as indicated (upper panels) or stimulated with different doses of solar UV light as indicated (lower panels), and whole cell lysates were collected at 30 min. The phosphorylation levels of TOPK (Thr-9) and p38α (Thr-180/Tyr-182) were measured by Western blotting using specific antibodies. β-Actin was used as a loading control to verify equal protein loading. B, the phosphorylation level of p38α was enhanced in TOPK−/− MEFs. TOPK+/+ and TOPK−/− MEFs were stimulated with solar UV light (60 kJ/m2) after 24 h of serum-free starvation, and whole cell lysates were collected at different time points as indicated. The phosphorylation levels of TOPK (Thr-9) and p38α (Thr-180/Tyr-182) were detected by Western blotting. C, the phosphorylation level of p38α was enhanced in RPMI7951 siTOPK melanoma cells. RPMI7951 siMock and RPMI7951 siTOPK melanoma cells were used for Western blotting as described for B.
TOPK Interacts with MKP1
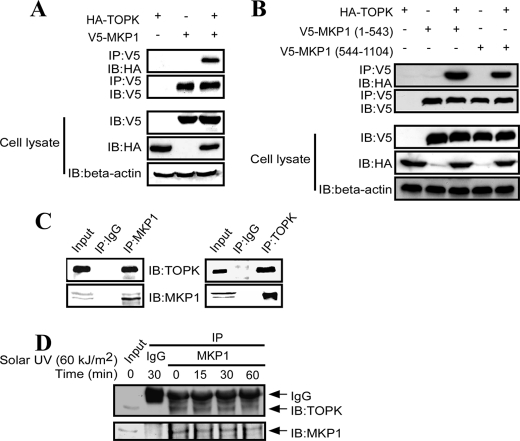
To study the mechanism of the negative regulation of p38α by TOPK, we first determined the effect of TOPK on the activities of the p38α upstream kinase MKK3/6. We found that the phosphorylation level of MKK3/6 was not changed in TOPK−/− MEFs compared with TOPK+/+ MEFs after treatment with solar UV light (supplemental Fig. S2). Because MKP1 is a specific phosphatase for p38α and plays an important role in the regulation of inflammation, we reasoned that TOPK might affect the function of MKP1. To test this hypothesis, we first determined whether an interaction occurs between TOPK and MKP1 in HEK293 cells overexpressing HA-TOPK and V5-MKP1. The results indicated that HA-TOPK was detected in the immunoprecipitates obtained with anti-V5 antibody (Fig. 2A). MKP1 comprises two domains with different functions: an N-terminal regulatory Rho domain, which is believed to determine substrate specificity by binding the substrate, and a C-terminal DSPc domain. On the basis of these domains, we constructed the Rho (1–543 bp) and DSPc (544–1104 bp) domains in the pcDNA3.1A-V5 plasmid, respectively, and cotransfected each with HA-TOPK into HEK293T cells. HEK293T cell extracts were immunoprecipitated with anti-V5 antibody, and HA-TOPK was detected in the immunoprecipitates obtained with anti-V5 antibody in both the Rho and DSPc constructs (Fig. 2B). Next, we assessed the interaction between endogenous TOPK and MKP1. RPMI7951 melanoma cell extracts were immunoprecipitated with anti-MKP1 antibody or control IgG. TOPK was detected in the immunoprecipitates obtained with anti-MKP1 antibody but not in those obtained with control IgG (Fig. 2C, left panels). Conversely, endogenous MKP1 was readily immunoprecipitated with a TOPK-specific monoclonal antibody but not with the control antibody (Fig. 2C, right panels). We also studied the kinetics of the interaction of TOPK with MKP1 when stimulated by solar UV light. We found that, after treatment with solar UV light, the interaction of TOPK with MKP1 decreased in a time-dependent manner (Fig. 2D). These data suggest that TOPK interacts with MKP1.
FIGURE 2.
TOPK interacts with MKP1. A, TOPK bound to MKP1 in HEK293 cells after transient transfection. The pcDNA3-HA-TOPK and pcDNA3-V5-MKP1 plasmids were cotransfected into HEK293 cells. After 48 h, whole cell lysates were immunoprecipitated (IP) with anti-V5 antibody and then probed with anti-HA antibody. The transfection efficiency and equal protein loading were verified by Western blotting using whole cell lysates. IB, immunoblot. B, the Rho (1–543 bp) and DSPc (544–1104 bp) domains were constructed in the pcDNA3.1A-V5 plasmid, respectively, and each was cotransfected with HA-TOPK into HEK293T cells. Cell extracts were immunoprecipitated with anti-V5 antibody and detected with anti-HA antibody. C, TOPK bound to MKP1 in RPMI7951 melanoma cells. Endogenous MKP1 was immunoprecipitated from RPMI7951 melanoma cells using anti-MKP1 antibody or control IgG and then probed with anti-TOPK antibody (left panels), or endogenous TOPK was immunoprecipitated from RPMI7951 melanoma cells using anti-TOPK antibody or control IgG and then probed with anti-MKP1 antibody (right panels). D, RPMI7951 melanoma cells were stimulated with solar UV light (60 kJ/m2) after 24 h of serum-free starvation, and whole cell lysates were collected at different time points as indicated. Endogenous MKP1 was immunoprecipitated from RPMI7951 cell lysates using anti-MKP1 antibody or control IgG and then probed with anti-TOPK antibody.
TOPK Phosphorylates MKP1 at Ser-359 in Vitro and ex Vivo
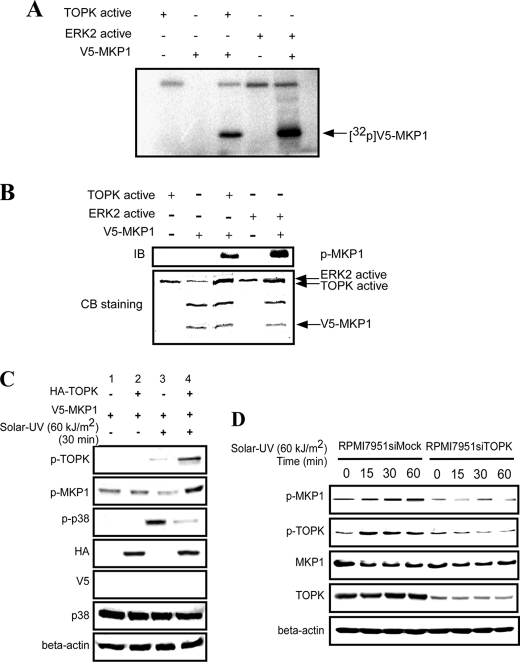
When a kinase binds to another protein, a phosphorylation reaction might occur with high probability. TOPK is a serine/threonine kinase, and therefore, we first examined whether TOPK can phosphorylate MKP1 in vitro. His-MKP1 was expressed and purified from Escherichia coli (supplemental Fig. S3) and then used as a substrate together with active TOPK in the presence of [γ-32p]ATP in an in vitro kinase assay. Phosphorylation of inactive MKP1 by active ERK2 was used as a positive control. The results indicated that active TOPK could phosphorylate MKP1 (Fig. 3A). We further confirmed the results of the in vitro kinase assay by Western blotting with a specific antibody to detect phosphorylation of MKP1 at Ser-359. Phosphorylation of MKP1 by active ERK2 was used as a positive control, and Coomassie Blue staining was used to confirm equal protein loading. The results indicated that TOPK could phosphorylate MKP1 at Ser-359 (Fig. 3B). To further confirm that TOPK can phosphorylate MKP1 and to investigate its effect on the activity of p38α ex vivo, TOPK was cotransfected with MKP1 into HEK293 cells and then stimulated with solar UV light (60 kJ/m2, 30 min), followed by Western blotting to determine the phosphorylation levels of TOPK, MKP1, and p38α. The results indicated that solar UV light could dramatically induce phosphorylation of TOPK and p38α (Fig. 3C, lanes 3 and 4) compared with the controls (lanes 1 and 2). Solar UV light-induced phosphorylation of TOPK was increased considerably in TOPK-overexpressing HEK293 cells (Fig. 3C, lane 3 versus lane 4), which corresponded with the high phosphorylation level of MKP1. Also, corresponding with the high phosphorylation level of MKP1, the endogenous phosphorylation of p38α decreased markedly (Fig. 3C, lane 3 versus lane 4). The results indicated that, after stimulation with solar UV light, overexpression of TOPK resulted in a high phosphorylation level of MKP1, which was associated with inhibition of p38α phosphorylation. To confirm the relationship of TOPK and MKP1 in RPMI7951 melanoma cells highly expressing TOPK (Fig. 1C), we compared RPMI7951 siTOPK and RPMI7951 siMock cells. The results indicated that the phosphorylation level of MKP1 decreased substantially after TOPK was blocked by siTOPK (Fig. 3D). Overall, these data indicate that, after stimulation with solar UV light, TOPK phosphorylates MKP1 at Ser-359 in vitro and ex vivo, which corresponds with the down-regulation of p38α phosphorylation.
FIGURE 3.
TOPK phosphorylates MKP1 at Ser-359 in vitro and ex vivo. A, active TOPK phosphorylated MKP1 in vitro in the presence of [γ-32P]ATP and was visualized by autoradiography. ERK2 phosphorylation of MKP1 was used as a positive control. B, TOPK phosphorylated MKP1 in vitro at Ser-359. The kinase reaction mixtures were used for Western blotting with anti-phospho-MKP1 (Ser-359) antibody. ERK2 phosphorylation of MKP1 was used as a positive control. Equal protein loading was visualized by Coomassie Blue (CB) staining. IB, immunoblot. C, TOPK promoted phosphorylation of MKP1 in HEK293 cells. HEK293 cells were transfected with pcDNA3-HA-TOPK and pcDNA3-V5-MKP1 as indicated, and at 48 h after transfection, cells were stimulated with solar UV light (60 kJ/m2) and harvested 30 min later. Whole cell lysates were then analyzed by Western blotting. D, the phosphorylation level of MKP1 was attenuated in RPMI7951 siTOPK melanoma cells. RPMI7951 siMock and RPMI7951 siTOPK melanoma cells were stimulated with solar UV light (60 kJ/m2) after 24 h of serum-free starvation, and whole cell lysates were collected at different time points as indicated. The phosphorylation levels of TOPK (Thr-9) and MKP1 (Ser-359) were detected by Western blotting.
TOPK Increases the Stability of MKP1
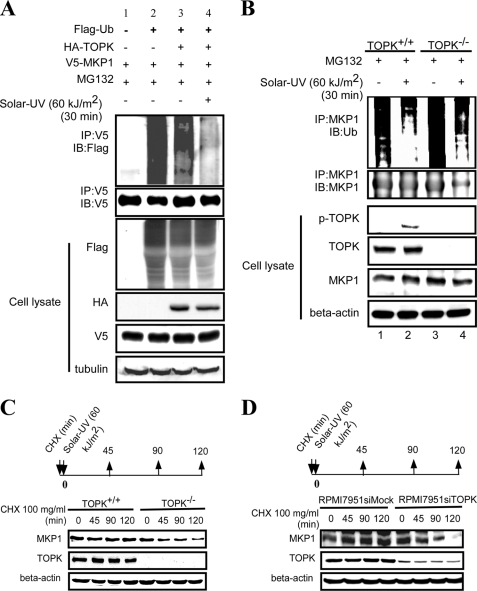
The phosphorylation of MKP1 might result in two functional changes: one to modify its activity and the other to affect its stability. According to Brondello et al. (20), the phosphorylation of MKP1 at Ser-359 has no effect on the enzyme activity of MKP1. Therefore, we determined whether this phosphorylation has an effect on the stability of the MKP1 protein. V5-MKP1 was expressed in HEK293 cells together with FLAG-ubiquitin and HA-TOPK. After treatment with MG132 and solar UV light, cell extracts from each group were immunoprecipitated with anti-V5 antibody, and FLAG-ubiquitin was detected in the immunoprecipitates by Western blotting. The results indicated that overexpression of TOPK decreased the ubiquitination level of MKP1 compared with the control (Fig. 4A, lane 3 versus lane 4). After treatment with solar UV light, the ubiquitination level of MKP1 decreased much more compared with the untreated groups (Fig. 4A, lane 2 versus lane 3). Transfection efficiency and equal protein loading were confirmed by Western blotting using whole cell lysates. To determine the effect of TOPK on the endogenous ubiquitination of MKP1, TOPK+/+ and TOPK−/− MEFs were used. After treatment with MG132 and solar UV light, cell extracts from each group were immunoprecipitated with anti-MKP1 antibody, and ubiquitin was detected in the immunoprecipitates with anti-ubiquitin antibody. Compared with TOPK−/− MEFs, the ubiquitination level of MKP1 decreased in TOPK+/+ MEFs following treatment with MG132 (Fig. 4B). After stimulation with solar UV light, the ubiquitination level of MKP1 decreased dramatically in both cell types, but the ubiquitination of MKP1 in TOPK+/+ MEFs was much less than that observed in TOPK−/− MEFs. Western blot analysis of whole cell lysates confirmed equal protein loading. To further confirm these results, the half-life of endogenous MKP1 in TOPK+/+ and TOPK−/− MEFs was examined after simultaneous treatment with solar UV light and cycloheximide. Compared with TOPK+/+ MEFs, the half-life of MKP1 in TOPK−/− MEFs decreased to 90 min (Fig. 4C). RPMI7951 siMock and RPMI7951 siTOPK cells were used to validate the effect of TOPK on the half-life of MKP1. Compared with RPMI7951 siMock cells, the half-life of MKP1 in RPMI7951 siTOPK cells also decreased to 90 min (Fig. 4D). Collectively, these data demonstrate that, after stimulation with solar UV light, TOPK increases the stability of MKP1 to extend its half-life.
FIGURE 4.
TOPK enhances the stability of MKP1. A, TOPK decreased the ubiquitination of MKP1 in HEK293 cells after treatment with solar UV light (60 kJ/m2). HEK293 cells were transfected with pCMV-FLAG-ubiquitin (Flag-Ub), pcDNA3-HA-TOPK, and pcDNA3-V5-MKP1 as indicated. At 48 h after transfection, cells were stimulated with MG132 (10 μm) for 4 h, followed by exposure to solar UV light (60 kJ/m2) and harvesting 30 min later. The samples were immunoprecipitated (IP) with anti-V5 antibody and detected with anti-FLAG antibody by Western blotting. The transfection efficiency and equal protein loading were verified by Western blotting using the whole cell lysate. IB, immunoblot. B, endogenous ubiquitination of MKP1 in TOPK+/+ MEFs was decreased compared with TOPK−/− MEFs after treatment with solar UV light (60 kJ/m2). TOPK+/+ and TOPK−/− MEFs were pretreated with MG132 (10 μm) for 4 h, followed by stimulation with solar UV light (60 kJ/m2) and harvesting 30 min later. The samples were immunoprecipitated with anti-MKP1 antibody, and ubiquitin was detected with anti-ubiquitin antibody by Western blotting. The phosphorylation of TOPK (Thr-9) and equal protein loading were confirmed by Western blotting using the whole cell lysate. C, the half-life of MKP1 was shorter in TOPK−/− MEFs compared with wild-type cells. TOPK+/+ and TOPK−/− MEFs were stimulated with cycloheximide (CHX; 100 μg/ml) and solar UV light (60 kJ/m2) simultaneously. The protein level of MKP1 was detected by Western blotting. D, the half-life of MKP1 was shorter in RPMI7951 siTOPK melanoma cells compared with RPMI7951 siMock cells. The half-life of MKP1 was assessed in RPMI7951 siMock and RPMI7951 siTOPK melanoma cells as described for C.
TOPK Attenuates Inflammation by Inhibiting p38 Signaling
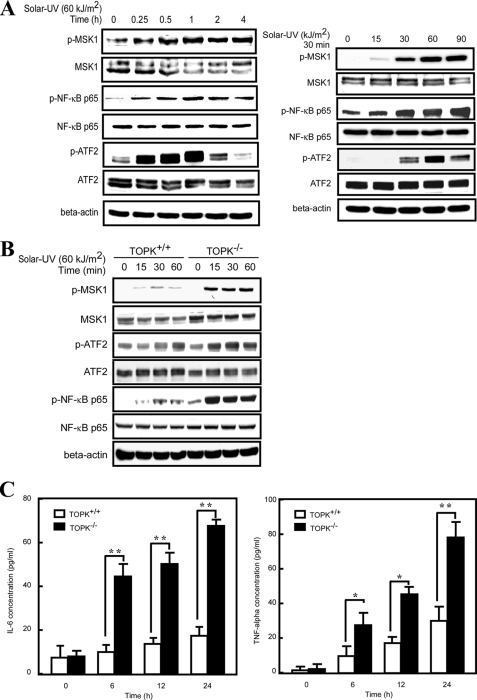
p38 MAPK plays a central role in regulating the function of inflammatory cells and in cytokine signaling. This protein kinase has been studied intensively in several chronic cytokine-dependent inflammatory diseases, including rheumatoid arthritis, Crohn disease, psoriasis, and asthma (21). In this study, we found that TOPK could negatively regulate the activity of p38α by increasing the stability of its specific phosphatase, MKP1. Therefore, we hypothesized that TOPK might play a role in the regulation of inflammation induced by solar UV light. We first examined the effect of solar UV light on inflammation-related signaling downstream of the p38α signaling pathway. MSK1 (mitogen- and stress-activated protein kinase 1) can be activated through phosphorylation by p38α (6), which in turn phosphorylates NF-κB p65 at Ser-276 and promotes its DNA binding and transcriptional activity (22). ATF2 (activating transcription factor 2) is activated by inflammatory signals and is directly phosphorylated by p38 MAPK (23). ATF2 is essential for maximal immediate induction of adhesion molecules and cytokine genes, including the adhesion molecules E-selectin, P-selectin, and VCAM-1, as well as the cytokines TNF-α, IL-1β, and IL-6 (24). After stimulation with solar UV light, the phosphorylation levels of both MSK1 and ATF2 increased in a time- and dose-dependent manner, and the MSK1 downstream NF-κB p65 also displayed a similar pattern of change (Fig. 5A). To explore the function of TOPK in inflammation, TOPK+/+ and TOPK−/− MEFs were used to determine the signaling pathway changes downstream of p38 after stimulation with solar UV light and harvesting at different time points. The phosphorylation levels of MSK1, NF-κB p65, and ATF2 were much higher in TOPK−/− MEFs compared with TOPK+/+ MEFs (Fig. 5B). To determine whether the signaling pathway changes could affect the secretion of inflammatory cytokines, we determined the concentrations of IL-6 and TNF-α in the culture media of TOPK+/+ and TOPK−/− MEFs after treatment with solar UV light and harvesting at different time points. Solar UV light-induced secretion of IL-6 and TNF-α was much higher in TOPK−/− MEFs compared with TOPK+/+ MEFs (Fig. 5C). In particular, solar UV light (60 kJ/m2) had little effect on the secretion of IL-6 in TOPK+/+ MEFs. Overall, TOPK inhibits the inflammation-related downstream signaling pathway of p38α and the secretion of cytokines induced by solar UV light.
FIGURE 5.
TOPK inhibits the downstream p38 signaling pathway and the secretion of cytokines induced by solar UV light. A, signaling downstream of p38α increased in a time- and dose-dependent manner following solar UV light treatment. MEFs (2 × 106/10-cm dish) were stimulated with solar UV light (60 kJ/m2) after 24 h of serum-free starvation, and whole cell lysates were collected at different time points as indicated (left panels), or MEFs were stimulated with different doses of solar UV light as indicated, and whole cell lysates were collected 30 min later (right panels). The phosphorylation levels of MSK1 (Ser-376), NF-κB p65 (Ser-276), and ATF2 (Thr-69/Thr-71) were determined by Western blotting using specific antibodies. β-Actin was used as a control to verify equal protein loading. B, signaling downstream of p38α was increased in TOPK−/− MEFs compared with wild-type cells after treatment with solar UV light. TOPK+/+ and TOPK−/− MEFs were stimulated with solar UV light (60 kJ/m2) after 24 h of serum-free starvation, and whole cell lysates were collected at different time points as indicated. The phosphorylation levels of MSK1 (Ser-376), NF-κB p65 (Ser-276), and ATF2 (Thr-69/Thr-71) were determined by Western blotting. C, TOPK inhibited the secretion of IL-6 and TNF-α induced by solar UV light. TOPK+/+ and TOPK−/− MEFs were seeded at a density of 7 × 105/well in 6-well plates. Cells were stimulated with solar UV light (60 kJ/m2) after 24 h of serum-free starvation, and the culture media were collected at different time points as indicated. The concentrations of IL-6 and TNF-α were determined using ELISA kits. Significant differences were determined by one-way analysis of variance. *, p < 0.05; **, p < 0.01.
TOPK Inhibits Inflammation Induced by Solar UV Light in Mouse Skin
To explore the role of TOPK in solar UV light-induced inflammation in mouse skin, adult TOPK+/+ and TOPK−/− mice were irradiated with one dose of solar UV light (142 kJ/m2), and dorsal trunk skin samples for H&E staining were harvested at 0, 12, and 24 h after irradiation. After treatment with solar UV light, most of the horny layers of the epidermis were lost in TOPK−/− mice compared with TOPK+/+ mice (Fig. 6A). Epidermal thickness, caused by edema and epithelial cell proliferation, represents typical skin histological inflammatory alterations (25). UV light-induced skin inflammation is usually quantitated by measurement of the thickness of the epidermis (26). The protective effect of compounds in UV light-induced skin inflammation is evaluated by the decrease in epidermal thickness (26–28). In this study, we found that the epidermis thickness increased in a time-dependent manner in TOPK−/− mice but was not obviously changed in TOPK+/+ mice (Fig. 6B). Inflammation is always accompanied by increasing deposition of the extracellular matrix (29), and moderate fibrosis was observed in TOPK−/− mice compared with TOPK+/+ mice. To determine the molecular mechanism, we prepared protein samples from mouse skin tissue for Western blot analysis. We found that, after treatment with solar UV light, the expression level of MKP1 was decreased in TOPK−/− mice compared with TOPK+/+ mice (Fig. 6C). Furthermore, the phosphorylation levels of p38α and NF-κB p65 increased dramatically in TOPK−/− mice compared with TOPK+/+ mice (Fig. 6C). Collectively, these data demonstrate that TOPK protects mouse skin from solar UV light-induced inflammation by inhibiting the activity of the p38α signaling pathway.
FIGURE 6.
TOPK inhibits inflammation in mouse skin exposed to solar UV light. A, TOPK inhibited inflammation induced by solar UV light in mouse skin tissue. Adult TOPK+/+ and TOPK−/− mice were irradiated with one dose of solar UV light (142 kJ/m2), and dorsal trunk skin samples were harvested at 0, 12, and 24 h after irradiation and stained with H&E. Representative staining from 12 samples is shown. B, the thickness of the epidermis from each of the two groups at three time points was quantified using the EC-Z1 FreeViewer software program (Version 3.20). Data are presented as means ± S.D. of values from 12 samples. Significant difference are indicated (*, p < 0.05; **, p < 0.01). Proteins from skin samples were extracted by skin tissue-specific lysis buffer (20 mm Tris-HCl (pH 8.0), 150 mm NaCl, and protease inhibitors). C, representative protein samples from the two groups at three time points were subjected to Western blot analysis to visualize the expression level of MKP1 and the phosphorylation levels of p38 (Thr-180/Tyr-182) and NF-κB p65 (Ser-276). β-Actin was used to verify equal loading of protein.
Next, we tested the effect of a specific p38 inhibitor, SB202190, on our inflammatory model. We found that SB202190 had no effect on the phosphorylation level of p38, but it markedly inhibited the phosphorylation level of NF-κB p65 (supplemental Fig. S4A). We used an ELISA assay kit (eBioscience) to compare the effect of SB202190 on the production of IL-6 in TOPK+/+ and TOPK−/− MEFs exposed or not exposed to solar UV light. We found that SB202190 suppressed the secretion of IL-6 in both TOPK+/+ and TOPK−/− MEFs (supplemental Fig. S4B). Finally, we examined the effect of SB202190 on a mouse model of solar UV light-induced inflammation. We found that SB202190 inhibited solar UV light-induced skin inflammation in both TOPK+/+ and TOPK−/− mice (supplemental Fig. S4C). These data suggest that TOPK is an upstream kinase of p38 and that inhibiting p38 activity can block the function of TOPK under solar UV conditions.
DISCUSSION
The p38α protein kinase is known to be essential in modulating the expression of inflammatory cytokines, such as TNF-α, IL-6, and IL-12, in response to proinflammatory signals. It plays a pivotal role in inflammation and has been the subject of extensive efforts in both basic research and drug discovery (30). The p38α (but not ERK or JNK) signaling pathway is the primary pathway activated by solar UV light. The specific p38α kinase inhibitor SB202190 not only suppresses the production of IL-6 in MEFs after treatment with solar UV light but also protects the epidermis from solar UV light-induced inflammatory responses in a mouse model (31). Thus, the p38α signaling pathway plays a pivotal role in solar UV light-induced inflammation.
The function of TOPK in solar UV light-induced skin inflammation is not yet known. However, our data suggest that TOPK negatively regulates p38α activity by enhancing the stability of the p38α-specific phosphatase MKP1 after treatment with solar UV light. We found that TOPK inhibits p38α downstream signaling, which is closely associated with inflammation. TOPK also suppresses the secretion of TNF-α and IL-6 in MEFs after stimulation with solar UV light.
The results from the animal study further confirmed that TOPK negatively regulates the p38α signaling pathway, resulting in inhibition of mouse skin inflammation as evidenced by a dramatic decrease in the thickness of the epidermis and decreases in the infiltration of immunocytes after treatment with solar UV light. Therefore, we reasoned that TOPK plays a protective function in solar UV light-induced inflammation by negatively regulating p38α activity.
Although chronic inflammation might promote tumor formation, acute inflammation might very well inhibit tumorigenesis and is indeed used therapeutically to prevent tumor formation (32). Therefore, the up-regulated acute inflammation observed in TOPK−/− mice might have a protective function in solar UV light-induced skin carcinogenesis. Our laboratory reported that TOPK can enhance cell transformation and promote tumorigenesis by the positive regulation of ERK and JNK signal pathways. Thus, the function of TOPK in solar UV light-induced skin carcinogenesis might be decided primarily by the role of its downstream p38α in tumor development. Several studies have described decreased activity of the p38 pathway in different human cancers (33–35). Genetic modification of different members of this pathway revealed that p38α can function as a tumor suppressor. Mice with liver-specific deletion of p38α develop more liver tumors in the diethylnitrosamine/phenobarbital-induced liver cancer model (34). K-RasG12V-induced lung tumorigenesis is greatly enhanced in p38α conditional deletion mice (36). These studies strongly suggest that p38α can suppress cancer development in mice. In our study, TOPK inhibited the activity of p38α upon solar UV exposure. We thus reasoned that TOPK might promote solar UV light-induced skin tumorigenesis. A long-term mouse model study should be carefully designed to address this hypothesis in future research.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants CA120388, R37 CA081064, and ES016548. This work was also supported by The Hormel Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
S. Li, F. Zhu, T. Zykova, M. O. Kim, Y. Y. Cho, A. M. Bode, C. Peng, W. Ma, A. Carper, A. Langfald, and Z. Dong, unpublished data.
- TOPK
- T-LAK cell-originated protein kinase
- MEF
- mouse embryonic fibroblast
- siMock
- mock siRNA
- siTOPK
- TOPK siRNA
- Rho
- rhodanese
- DSPc
- dual specificity phosphatase catalytic.
REFERENCES
- 1. Bode A. M., Dong Z. (2003) Sci. STKE 2003, RE2. [DOI] [PubMed] [Google Scholar]
- 2. (1992) IARC Monogr. Eval. Carcinog. Risks Hum. 55, 1–316 [PMC free article] [PubMed] [Google Scholar]
- 3. Ley R. D. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jinlian L., Yingbin Z., Chunbo W. (2007) J. Biomed. Sci. 14, 303–312 [DOI] [PubMed] [Google Scholar]
- 5. Hruza L. L., Pentland A. P. (1993) J. Invest. Dermatol. 100, 35S–41S [DOI] [PubMed] [Google Scholar]
- 6. Vermeulen L., Vanden Berghe W., Beck I. M., De Bosscher K., Haegeman G. (2009) Trends Biochem. Sci. 34, 311–318 [DOI] [PubMed] [Google Scholar]
- 7. Schieven G. L. (2005) Curr. Top. Med. Chem. 5, 921–928 [DOI] [PubMed] [Google Scholar]
- 8. Remy G., Risco A. M., Iñesta-Vaquera F. A., González-Terán B., Sabio G., Davis R. J., Cuenda A. (2010) Cell. Signal. 22, 660–667 [DOI] [PubMed] [Google Scholar]
- 9. Li L., Chen S. F., Liu Y. (2009) Int. J. Clin. Exp. Med. 2, 48–67 [PMC free article] [PubMed] [Google Scholar]
- 10. Matsumoto S., Abe Y., Fujibuchi T., Takeuchi T., Kito K., Ueda N., Shigemoto K., Gyo K. (2004) Biochem. Biophys. Res. Commun. 325, 997–1004 [DOI] [PubMed] [Google Scholar]
- 11. Zykova T. A., Zhu F., Lu C., Higgins L., Tatsumi Y., Abe Y., Bode A. M., Dong Z. (2006) Clin. Cancer Res. 12, 6884–6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu F., Zykova T. A., Kang B. S., Wang Z., Ebeling M. C., Abe Y., Ma W. Y., Bode A. M., Dong Z. (2007) Gastroenterology 133, 219–231 [DOI] [PubMed] [Google Scholar]
- 13. Abe Y., Matsumoto S., Kito K., Ueda N. (2000) J. Biol. Chem. 275, 21525–21531 [DOI] [PubMed] [Google Scholar]
- 14. Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 15. Zykova T. A., Zhu F., Vakorina T. I., Zhang J., Higgins L. A., Urusova D. V., Bode A. M., Dong Z. (2010) J. Biol. Chem. 285, 29138–29146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park J. H., Lin M. L., Nishidate T., Nakamura Y., Katagiri T. (2006) Cancer Res. 66, 9186–9195 [DOI] [PubMed] [Google Scholar]
- 17. Li G., Hundemer M., Wolfrum S., Ho A. D., Goldschmidt H., Witzens-Harig M. (2006) Ann. Hematol. 85, 583–590 [DOI] [PubMed] [Google Scholar]
- 18. Junttila M. R., Li S. P., Westermarck J. (2008) FASEB J. 22, 954–965 [DOI] [PubMed] [Google Scholar]
- 19. Wang Z., Yang H., Tachado S. D., Capó-Aponte J. E., Bildin V. N., Koziel H., Reinach P. S. (2006) Invest. Ophthalmol. Vis. Sci. 47, 5267–5275 [DOI] [PubMed] [Google Scholar]
- 20. Brondello J. M., Pouysségur J., McKenzie F. R. (1999) Science 286, 2514–2517 [DOI] [PubMed] [Google Scholar]
- 21. Coulthard L. R., White D. E., Jones D. L., McDermott M. F., Burchill S. A. (2009) Trends Mol. Med. 15, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reber L., Vermeulen L., Haegeman G., Frossard N. (2009) PloS ONE 4, e4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waas W. F., Lo H. H., Dalby K. N. (2001) J. Biol. Chem. 276, 5676–5684 [DOI] [PubMed] [Google Scholar]
- 24. Reimold A. M., Kim J., Finberg R., Glimcher L. H. (2001) Int. Immunol. 13, 241–248 [DOI] [PubMed] [Google Scholar]
- 25. Gambichler T., Boms S., Stücker M., Moussa G., Kreuter A., Sand M., Sand D., Altmeyer P., Hoffmann K. (2005) Arch. Dermatol. Res. 297, 218–225 [DOI] [PubMed] [Google Scholar]
- 26. Cole N., Sou P. W., Ngo A., Tsang K. H., Severino J. A., Arun S. J., Duke C. C., Reeve V. E. (2010) Int. Arch. Allergy Immunol. 152, 87–97 [DOI] [PubMed] [Google Scholar]
- 27. Vicentini F. T., Fonseca Y. M., Pitol D. L., Iyomasa M. M., Bentley M. V., Fonseca M. J. (2010) J. Pharm. Pharm. Sci. 13, 274–285 [DOI] [PubMed] [Google Scholar]
- 28. Jin X. J., Kim E. J., Oh I. K., Kim Y. K., Park C. H., Chung J. H. (2010) J. Korean Med. Sci. 25, 930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee E. J., Jeon M. S., Kim B. D., Kim J. H., Kwon Y. G., Lee H., Lee Y. S., Yang J. H., Kim T. Y. (2010) Free Radic. Biol. Med. 48, 1133–1143 [DOI] [PubMed] [Google Scholar]
- 30. Schieven G. L. (2009) Curr. Top. Med. Chem. 9, 1038–1048 [DOI] [PubMed] [Google Scholar]
- 31. Hildesheim J., Awwad R. T., Fornace A. J., Jr. (2004) J. Invest. Dermatol. 122, 497–502 [DOI] [PubMed] [Google Scholar]
- 32. Sgambato A., Cittadini A. (2010) Eur. Rev. Med. Pharmacol. Sci. 14, 263–268 [PubMed] [Google Scholar]
- 33. Mendoza R. A., Moody E. E., Enriquez M. I., Mejia S. M., Thordarson G. (2011) J. Endocrinol. 208, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hui L., Bakiri L., Mairhorfer A., Schweifer N., Haslinger C., Kenner L., Komnenovic V., Scheuch H., Beug H., Wagner E. F. (2007) Nat. Genet. 39, 741–749 [DOI] [PubMed] [Google Scholar]
- 35. Comes F., Matrone A., Lastella P., Nico B., Susca F. C., Bagnulo R., Ingravallo G., Modica S., Lo Sasso G., Moschetta A., Guanti G., Simone C. (2007) Cell Death Differ. 14, 693–702 [DOI] [PubMed] [Google Scholar]
- 36. Ventura J. J., Tenbaum S., Perdiguero E., Huth M., Guerra C., Barbacid M., Pasparakis M., Nebreda A. R. (2007) Nat. Genet. 39, 750–758 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.