Abstract
Background
Selenium may prevent colorectal cancer. However, several previous studies are small and few investigated the associations between selenium and colorectal cancer among women, whose selenium metabolism may differ from men.
Methods
This nested case-control study investigated whether serum selenium concentration and genetic variants in five selenoenzymes (glutathione peroxidase 1-4 and selenoprotein P) were associated with colorectal cancer risk in 804 colorectal cancer cases and 805 matched controls from the Women’s Health Initiative (WHI) Observational Study. A meta-analysis was conducted to compare the WHI result with previous studies including 12 observational studies and two clinical trials on selenium.
Results
Within the WHI, selenium concentrations were relatively high (mean = 135.6 μg/L) and were not associated with colorectal cancer risk (p for trend = 0.10); the adjusted odds ratio (OR) comparing the fifth with first quintile was 1.26 [95% confidence intervals (CI) = 0.91-1.73]. Moreover, genetic variants in selenoenzymes were not significantly associated with colorectal cancer risk. Consistent with the finding in WHI, our meta-analysis showed no association between selenium and colorectal tumor risk in women (OR = 0.97, 95% CI = 0.79-1.18 comparing the highest quantile with the lowest); however, in men, there was a significant inverse association (OR = 0.68, 95% CI = 0.57-0.82) (p = 0.01).
Conclusion
Consistent with previous studies, we observed no protective effect of selenium on colorectal cancer among women.
Impact
Our analyses suggest a population with relatively high selenium concentrations, especially women, would not benefit from increasing selenium intake.
Introduction
The current evidence from in vitro and animal studies strongly supports a protective effect of selenium on colorectal cancer, while the evidence from clinical trials and observational studies is inconclusive (1-15). Secondary analyses of two clinical trials provide conflicting findings (16, 17). An early trial for the prevention of the recurrence of non-melanoma skin cancer [Nutritional Prevention of Cancer Trial (NPCT)] found a statistically significant 61% lower risk of colorectal cancer [95% confidence intervals (CI) = 10 to 83%, p = 0.03] in the selenium supplementation group (17, 18). In contrast, a recent large clinical trial for prostate cancer prevention [Selenium and Vitamin E Cancer Prevention Trial (SELECT)] found no effect of selenium on colorectal cancer, a pre-specified secondary outcome [hazard ratio (HR) = 1.05, 99% CI = 0.66-1.67] (16). Many observational studies (1-9), but not all (10-15), support a protective effect of selenium on risk of colorectal tumors. Most studies or trials were smaller or restricted to men and, hence, did not provide stratified results by gender. Within the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, we reported that serum selenium was inversely associated with colorectal adenoma risk only in men, but not in women (1). Effect modification by gender may be plausible given gender differences in selenium metabolism (19-22).
Selenium is hypothesized to reduce cancer risk due to its antioxidant activity, which is mediated through selenoenzymes (23). Selenoenzymes are encoded by a small number of genes (25 in humans) that often contain selenium as selenocysteine at their active center. Genetic variants in selenoenzymes may affect their activity and thus directly or indirectly affect the risk of cancer.
This study investigated whether serum selenium concentrations and single nucleotide polymorphisms (SNPs) in five selenoenzymes expressed in the colorectal tissue (24-27) [glutathione peroxidase 1-4 (GPX1-4) and selenoprotein P (SEPP1)] were associated with colorectal cancer risk. Data were from a large case-control study nested within the Women’s Health Initiative (WHI) Observational Study, which recruited postmenopausal women from 40 clinical centers throughout the United States. We also performed a meta-analysis to summarize the current evidence on selenium and the risk of colorectal tumors.
Methods
WHI Observational Study
Study population
The WHI Observational Study includes 93,676 postmenopausal women 50 to 79 years of age at the time of recruitment (28). These women were recruited mostly through age-targeted mass mailing and media announcements in areas surrounding the 40 WHI clinical centers across the United States and described in more detail elsewhere (29). Colorectal cancer cases were identified through annual questionnaires or interviews and were adjudicated through local and central reviews of the medical records and pathology reports. When cases and controls were selected for this study (Fall 2005), only 4.7% of the initial WHI participants were lost to or had stopped follow-up. As of August 2005, the agreement rate between local and WHI Clinical Coordinating Center adjudications for colorectal cancer was 96%. In the nested case-control study, cases were diagnosed with colorectal cancer between October 1993 to September 2005 (n=805). Central adjudication determined later that one case was not a colorectal cancer case, resulting in 804 cases for analysis. Controls were selected from all eligible women in the WHI Observational Study who were alive and had not been diagnosed with colorectal cancer at the time of the case’s diagnosis. They were individually matched to cases by age (± 3 years), enrollment date to the study (± 168 days), race/ethnicity (white, black, Hispanic, or other), and hysterectomy (yes or no) (n=805).
Data collection
Participants in the WHI Observational Study had clinic visits at baseline and year 3. Self-administered questionnaires were completed at baseline and annually thereafter. The baseline questionnaire acquired detailed information on demographic and lifestyle characteristics, medical history, reproductive history and hormone regimen (29). Dietary intake was assessed at baseline using a 122-item food frequency questionnaire (30). During the clinic visits at baseline and year 3, interviewers collected data on use of dietary supplements and non-steroidal anti-inflammatory drugs (NSAIDs). Overnight fasting blood samples were collected at baseline and year 3 and maintained at 4°C until serum was separated from cells and stored at -70°C within 2 hours after blood draw (29).
Laboratory methods
Serum selenium concentration was measured using atomic absorption spectrometry (Perkin Elmer, Fremont CA) according to the standard protocol (31-33). Each sample was run in duplicate and considered acceptable if the coefficient of variation (CV) was less than 10%. Internal quality control (QC) samples were run before and after each batch to ensure the quality of the assay and the mean CV of these samples was 7.3%. In addition, blinded duplicates from 96 women who were recruited, but not enrolled in WHI, were included in batches with study samples. Three batches had CVs >21%. All three batches were originally run on two dates. When we re-analyzed all the batches that were originally run on these two dates (three batches with CVs >21% and another with CV <10%), all yielded acceptable CVs. After these reruns, the mean CV for the blinded QCs was 5.8%. The Spearman correlation between blood samples collected at baseline and year 3 visits from 100 control women was 0.68. Of the selected 1609, a total of 1600 (799 cases and 801 controls) had sufficient serum samples available for selenium assay.
Five selenoenzymes (GPX1, GPX2, GPX3, GPX4 and SEPP1) have been reported to be both purportedly associated with oxidative stress and expressed in the colorectal tissue (24-27). Each set of putative functionalnon-synonymous SNPs and tagging SNPs (tagSNP) were genotyped in order to efficiently capture the overall variation of the gene. To select tagSNPs, we first sequenced the genes in European American subjects in HapMap population (34). Using this sequencing data, we selected all SNPs in the selenocysteine insertion sequence, which facilitates selenoenzyme synthesis by a unique stem-loop structure (23), and all nonsynonymous SNPs in exons (35). We then selected additional tagSNPs from our sequencing data (35) using criteria of linkage disequilibrium (LD) of r2 ≥0.8, and minor allele frequency of ≥5% (36). A total of 42 SNPs were genotyped using Matrix-assisted Laser Desorption/ Ionization Time-of-Flight on the Sequenom MassARRAY 7K platform (Sequenom, Inc., San Diego CA). Each plate included 5% blinded duplicates from the study samples as QC. We excluded two SNPs (rs75404373 and rs3763011) due to <90% call rate, three SNPs (rs6888691, rs376301 and rs757229) due to a p-value of <0.01 for Hardy-Weinberg equilibrium and two SNPs (rs2074452 and rs7579) due to low concordance of blinded duplicates (<90%). For the remaining SNPs, the concordance rate of the blinded duplicates was >95% (average 99.9%). Thus, the final analysis included 34 SNPs.
Statistical analyses
We used unconditional logistic regression to estimate odds ratios (ORs) and 95% CI for colorectal cancer risk associated with increasing serum selenium concentrations given that results were similar when we used conditional logistic regression (data not shown) and unconditional analysis may be beneficial with larger sample size (37). A linear trend of the association was tested by assigning each observation the quintile median serum selenium based on the distribution in controls and treating this as a continuous variable. All analyses were adjusted for the following four matching factors: age at screening, enrollment date to the study (days), race/ethnicity, and hysterectomy. In addition, the following known and potential risk factors for colorectal cancer were evaluated as confounders: physical activity (MET-hours/week), body mass index (BMI, kg/m2), smoking (pack-years; never, former or current), alcohol consumption (six categories ranging from non-drinker to seven or more drinks/week), NSAID use [longest duration (days) of any NSAID use; yes or no], postmenopausal hormone use (years of use; never, former or current), family history of colorectal cancer (yes or no), history of colorectal cancer screening (yes, no or missing), history of adenomas and polyps removals (yes or no), education (four categories ranged from less than high school to college degree or higher), total energy intake (kcal/day), red meat intake (serving/day), vegetable and fruit intake (serving/day), dietary and supplemental folate intake (μg/day), dietary fiber intake (g/day), and dietary and supplemental calcium intake (mg/day). For all variables with the numbers of missing values <5% and family history of colorectal cancer, which had 8.8% missing values, the mean value or the most common category were substituted for missing values. The final multivariate model included education and postmenopausal hormone use, since each changed the β-coefficients of the risk estimate for serum selenium by ≥10%.
We also used unconditional logistic regression to examine the association between genetic variations in five selenoenzymes and colorectal cancer. Analyses were adjusted for the four matching factors. Genetic variants were coded based on log-additive model by assigning the number of copies of the minor allele (i.e., zero for homozygote common allele, one for heterozygotes, and two for homozygote rare allele). The OR of the regression model describes the change in the risk of colorectal cancer per minor allele and the p value describes the significance of the linear trend. To account for multiple comparisons, a global gene test was used to test whether the overall variation within each gene was associated with the risk of colorectal cancer. This test was conducted by comparing the log likelihood ratio statistics of the model with and without all SNPs in one gene (each SNP coded as 0/1/2) (38).
Interactions of serum selenium concentrations (continuous) with age at screening (continuous), smoking (never, former or current), postmenopausal hormone use (never, former or current), and genetic variants in selenoenzymes (log-additive model) were also evaluated. Interaction effects were tested by including cross-product terms in the regression models and comparing the log likelihood ratio statistics of the main effect model with the joint effects model.
Meta-Analysis
A meta-analysis was conducted to compare our results from the WHI with previous studies on selenium and risk of colorectal cancer (the number of studies conducted on genetic variants in selenoenzymes were too small to conduct a meta-analysis). Studies were identified through searches of PubMed using the search term, “selenium” and either “colorectal cancer” or “colon cancer” or “colorectal adenoma.” The last search was conducted on September 27, 2010. All articles were screened by two investigators (YT and UP) to assess whether they provided sufficient information. We restricted analysis to observational studies that assessed selenium as blood or toenail concentrations and randomized clinical trials that used selenium supplements. Because questionnaire data do not provide reliable estimates on selenium intake due to large variation in the selenium content of the same food (39-41) and the fact that selenium supplements only present a relative small fraction of the overall selenium intake (27), we did not include studies using questionnaires to assess selenium intake. As colorectal cancer and adenoma tend to have a similar risk profile (42), both outcomes were included in the meta-analysis. In order to include as many studies as possible for overall and gender-specific analyses, if the risk estimate and/or the corresponding 95% CI were missing in a given article, attempts were made to contact the author(s) to obtain this information. Three studies were excluded due to insufficient information (4, 8, 12). No other additional screening or eligibility criteria were used, leaving 15 studies (including the current study) in the meta-analysis. For each article, the following information was extracted: first author name, year of publication, mean or median blood or toenail concentration, sample size, OR/HR and 95% CI. For observational studies, the meta-analysis included the risk estimate comparing the highest quantile (quintile, quartile or tertile) of selenium concentrations with the lowest, except for two studies which reported only the risk estimate based on a given cut-off [median (6) or 75th percentile (7)]. For clinical trials (16, 17), the risk estimate comparing the selenium supplemented with the placebo groups was used. Risk estimates from individual studies were combined and the corresponding summary 95% CI and p-values were obtained under a random-effects meta-analysis model (43, 44). Forest plots were used to display the results from individual studies, as well as the summary results. In order to investigate heterogeneity among studies, we calculated I2 statistics, which is a measure of the percentage of total variation across studies due to heterogeneity beyond chance, and obtained the heterogeneity p-values based on Cochran’s Q statistic (45). Funnel plot was used to assess potential selection or publication bias. Subgroup analyses by gender, biospecimen type (blood vs. toenail selenium), colorectal outcome (colorectal cancer vs. adenoma), and time of selenium assessment (before or after diagnosis of colorectal tumors; clinical trials were included in the before diagnosis group because the effect of selenium supplementation was assessed prospectively) were performed.
Results
WHI Observational Study
Cases and controls did not differ by age and race/ethnicity because they were matched on these criteria (Table 1). Cases were less educated, had slightly higher BMI, were less physically active, smoked more, and used postmenopausal hormone less often than controls. Use of NSAIDs and selenium supplements was similar between cases and controls. Intakes of calcium and dietary fiber were lower in cases than controls. Most cases were diagnosed with cancer in the colon (81.1%). Given that our participants were recruited from 40 different WHI clinical centers across the United States, the range of serum selenium concentrations was wide (81.0-398.7 μg/L); however, the mean concentrations were relatively high (135.6 ± 21.1 μg/L in controls).
Table 1.
Characteristics of the WHI Observational Study, Stratified by Case-Control Status
| Cases* | Controls* | P for difference | |
|---|---|---|---|
| Number | 804 | 805 | - |
|
| |||
| Age (years old) | 66.6 ± 6.9 | 66.7 ± 6.9 | 0.96 |
|
| |||
| Race/ethnicity | |||
| White | 675 (84.0%) | 676 (84.0%) | |
| Black | 82 (10.2%) | 82 (10.2%) | |
| Hispanic | 20 (2.5%) | 20 (2.5%) | |
| Other/ Unknown | 27 (3.3%) | 27 (3.3%) | 0.99 |
|
| |||
| Education | |||
| Less than high school | 40 (5.0%) | 47 (5.9%) | |
| High school diploma/ GED | 122 (15.3%) | 137 (17.2%) | |
| Some school after high school | 355 (44.4%) | 291 (36.5%) | |
| College degree or higher | 282 (35.3%) | 322 (40.4%) | 0.01 |
|
| |||
| BMI (kg/m2) | 28.3 ± 6.1 | 27.5 ± 5.7 | 0.004 |
| Underweight/Normal (BMI < 25 kg/m2) | 267 (33.7%) | 291 (36.5%) | |
| Overweight (25 ≤BMI < 30 kg/m2) | 278 (35.0%) | 302 (37.9%) | |
| Obese (BMI ≥30 kg/m2) | 248 (31.3%) | 204 (25.6%) | 0.05 |
|
| |||
| Physical activity (MET hours) | 11.6 ± 12.3 | 13.2 ± 14.4 | 0.02 |
|
| |||
| Smoking (pack-years) | 13.8 ± 22.4 | 11.1 ± 19.8 | 0.01 |
| Never | 366 (46.2%) | 404 (50.8%) | |
| Former | 366 (46.2%) | 347 (43.7%) | |
| Current | 61 (7.6%) | 44 (5.5%) | 0.08 |
|
| |||
| NSAID use | |||
| None | 564 (70.1%) | 570 (70.8%) | |
| Any at baseline | 240 (29.9%) | 235 (29.2%) | 0.84 |
|
| |||
| Postmenopausal hormone use | |||
| Never | 407 (50.7%) | 352 (43.8%) | |
| Former | 135 (16.8%) | 138 (17.2%) | |
| Current | 261 (32.5%) | 314 (39.0%) | 0.01 |
|
| |||
| Family history of colorectal cancer | 163 (22.0%) | 133 (18.3%) | 0.06 |
|
| |||
| Selenium supplement use | 289 (36.0%) | 299 (37.1%) | 0.63 |
|
| |||
| Folate intake (μg/day)** | 663.1 ± 316.7 | 678.8 ± 336.0 | 0.29 |
|
| |||
| Calcium intake (mg/day)** | 1144.6 ± 679.8 | 1213.0 ± 760.4 | 0.05 |
|
| |||
| Dietary fiber intake (g/day) | 16.0 ± 6.9 | 16.7 ± 7.0 | 0.05 |
|
| |||
| Red meat intake (serving/ day) | 0.62 ± 0.55 | 0.60 ± 0.49 | 0.38 |
|
| |||
| Alcohol consumption (servings/ week) | 2.41 ± 4.81 | 2.52 ± 4.76 | 0.69 |
|
| |||
| Serum selenium concentration (μg/L) | 137.8 ± 24.3 | 135.6 ± 21.1 | 0.05 |
The mean ± standard deviation or number (percentage) is provided. The number of missing values among 1600 participants with serum selenium values was 13 for education, 19 for BMI, 17 for physical activity, 21 for smoking, 2 for postmenopausal hormone use, 141 for family history of colorectal cancer, 71 for nutrient and red meat intakes and 13 for alcohol consumption.
From diet and supplement
Serum selenium concentration was not associated with the risk of colorectal cancer (p for trend = 0.10); the adjusted ORs (95% CIs) for colorectal cancer risk comparing the 2nd, 3rd, 4th, and 5th quintiles of serum selenium concentrations with the 1st quintile were 1.13 (0.82-1.56), 1.16 (0.84-1.59), 1.34 (0.98-1.84) and 1.26 (0.91-1.73), respectively (Table 2). While results of site-specific analyses did not differ between colon and rectum (p = 0.18), the positive association between serum selenium and risk of colon cancer was marginally significant (p = 0.05). There were no statistically significant interactions of serum selenium with age (p for interaction = 0.41), smoking (p for interaction = 0.12), and postmenopausal hormone use (p for interaction = 0.29).
Table 2.
Risk of Colorectal Cancer by Serum Selenium Concentrations in the WHI Observational Study
| Cases/controls | OR (95% CI)* by Quintiles of Selenium Concentrations (μg/L) | P for trend | |||||
|---|---|---|---|---|---|---|---|
| Range | ≤117.5 | 117.6-128.5 | 128.5-138.7 | 138.7-152.6 | >152.6 | ||
| Median | 110.5 | 123.2 | 133.5 | 144.8 | 162.2 | ||
| Adjusted for matching factors only* | 799/801 | reference | 1.09 (0.79-1.49) | 1.11 (0.81-1.52) | 1.27 (0.93-1.73) | 1.17 (0.85-1.60) | 0.22 |
| Fully-Adjusted** | 799/801 | reference | 1.13 (0.82-1.56) | 1.16 (0.84-1.59) | 1.34 (0.98-1.84) | 1.26 (0.91-1.73) | 0.10 |
| Site-specific analysis*** | |||||||
| Colon | 648/801 | reference | 1.01 (0.72-1.42) | 1.14 (0.81-1.60) | 1.34 (0.96-1.87) | 1.28 (0.91-1.79) | 0.05 |
| Rectum | 149/801 | reference | 1.75 (0.99-3.07) | 1.21 (0.66-2.22) | 1.48 (0.83-2.65) | 1.25 (0.68-2.31) | 0.76 |
Analysis was adjusted for matching factors (age, enrollment date, race/ethnicity and hysterectomy)
Analysis was adjusted for matching factors (age, enrollment date, race/ethnicity, hysterectomy) and education and postmenopausal hormone use.
Two cases who had cancer in both colon and rectum were excluded; p for difference obtained by polytomous logistic regression was 0.18.Rectal cancer includes cancer in rectosigmoid junction and rectum
Only one SNP in GPX4 gene (rs8178974) was statistically significantly associated with colorectal cancer risk (p for trend = 0.02); however, the overall variation in GPX4 gene was not associated with the risk of colorectal cancer (global p = 0.20) (Supplementary Table and Supplementary Figure 1). All other SNPs, either tested individually or combined within a gene, were not associated with colorectal cancer risk. When stratified by White and African-Americans, results generally did not differ from the overall analyses. Genetic variants in selenoenzymes did not modify the association between serum selenium and colorectal cancer (global p for interaction = 0.09 to 0.87, Supplementary Figure 2).
Meta-Analysis
The meta-analysis showed a statistically significant inverse association between selenium and colorectal tumor risk (OR = 0.81, 95% CI = 0.71-0.92) (Figure 1); however, results were heterogeneous (I2 = 59.5%; p = 0.001). Similar results were obtained after excluding the two clinical trials (OR = 0.80, 95% CI = 0.70-0.92, I2 = 60.4%, p = 0.001). When stratified by gender, selenium was significantly and inversely associated with colorectal tumor risk in men (OR = 0.68, 95% CI = 0.57-0.82) with no evidence of heterogeneity (I2 = 16.7%; p = 0.29) (Figure 2). Among women, there was no association (OR = 0.97, 95% CI = 0.79-1.18), but results remained heterogeneous (I2 = 49.5%; p = 0.045). The protective association was stronger for studies that measured selenium after colorectal tumor diagnosis compared with those that measured selenium before diagnosis (OR = 0.61, 95% CI = 0.47-0.80, vs. OR = 0.88, 95% CI = 0.76-1.02); however, this did not explain the heterogeneity between studies (Supplementary Figure 3A). The inverse association between selenium and colorectal tumors was limited to studies measuring selenium in serum/plasma (OR = 0.78, 95% CI = 0.68-0.90) and there was no association in studies measuring selenium in toenails, although only three studies were included (OR = 1.00, 95% CI = 0.70-1.44) (Supplementary Figure 3B). The inverse association was stronger for colorectal adenoma (OR = 0.69, 95% CI = 0.57-0.85) than cancer (OR = 0.90, 95% CI = 0.76-1.06; Supplementary Figure 3C). Based on visual inspection of funnel plots, there was potential evidence for publication bias, with a slightly higher number of small studies reporting inverse associations (Supplementary Figures 4A-C), which is in the expected direction.
Figure 1. Meta-analysis of Selenium and the Risk of Colorectal Tumors*.
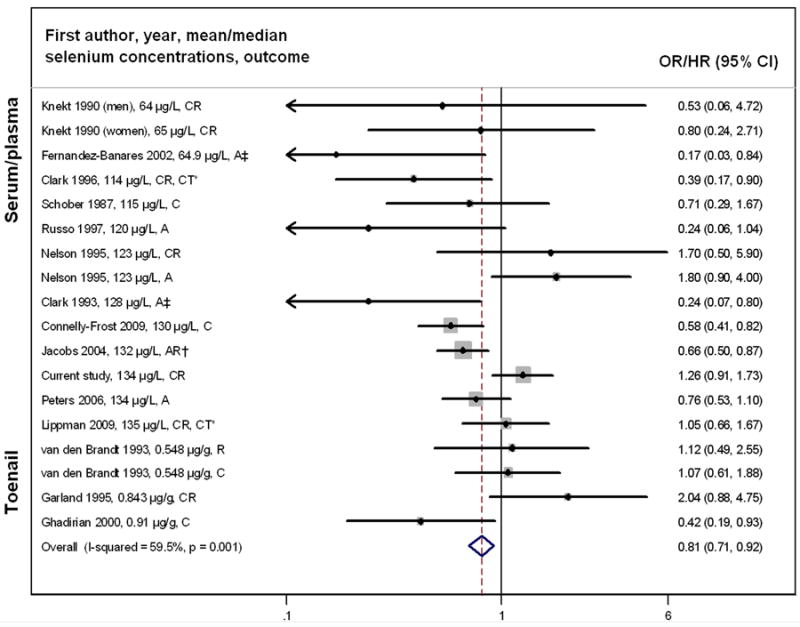
*Ordered by biospecimen type (serum/plasma and toenail samples) and by selenium concentrations within each biospecimen type; C=colon cancer, CR=colorectal cancer, R=rectal cancer, A=adenoma, AR=adenoma recurrence, CT = Clinical trial; The dot in each study indicates the OR/HR, bars represent the 95% CI and the size of gray square box reflects the study’s weight in the meta-analysis. The dashed line indicates the summary OR across all studies. The open diamond on the bottom indicates the 95% CI of the summary OR.
‡Risk estimate is based on a given cut-off (the median for Clark 1995 study and the 75th percentile for Fernandez-Banares 2002 study).
Figure 2. Meta-analysis of Selenium and the Risk of Colorectal Tumors, Stratified by Gender*.
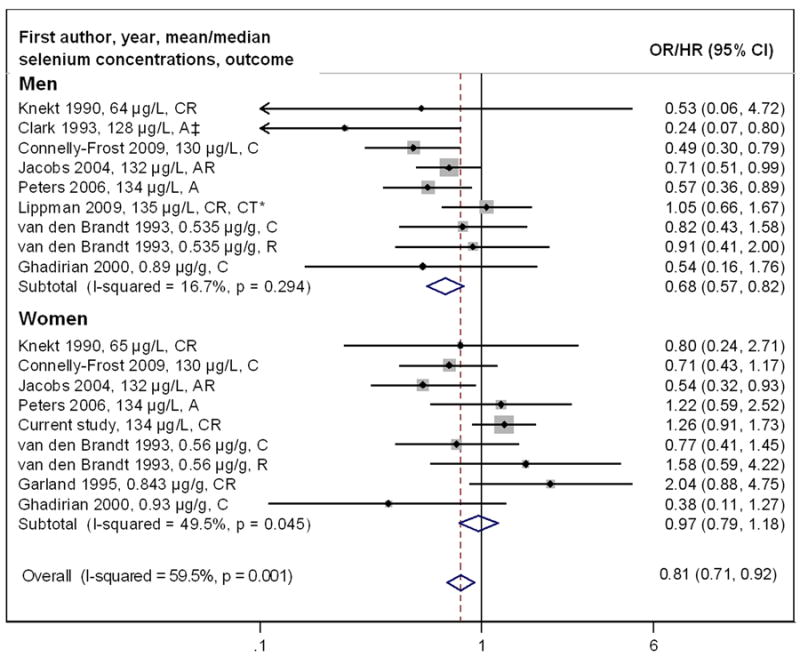
*Ordered by biospecimen type (serum/plasma and toenail samples) and by selenium concentrations within each biospecimen type; C=colon cancer, CR=colorectal cancer, R=rectal cancer, A=adenoma, AR=adenoma recurrence, CT = Clinical trial; The dot in each study indicates the OR/HR, bars represent the 95% CI and the size of gray square box reflects the study’s weight in the meta-analysis. The dashed line indicates the summary OR across all studies. The open diamond on the bottom indicates the 95% CI of the summary OR.
‡Risk estimate is based on a given cut-off (the median for Clark 1995 study).
Discussion
In this large study of postmenopausal women, serum selenium concentrations were not associated with colorectal cancer risk. This finding is consistent with findings from our meta-analysis, showing no association between selenium and colorectal tumors among women and an inverse association among men. Furthermore, there was no evidence for association between genetic variants in selenoenzymes and risk of colorectal cancer and results for serum selenium did not differ by genetic variants in selenoenzymes.
In comparison with selenium supplementation trials, our finding is consistent with secondary analysis from SELECT (16), but inconsistent with those from the NPCT (17). The discrepant findings between these clinical trials could potentially be due to the use of different selenium supplements, selenized yeast (NPCT), which contains a mixture of different selenium forms and purified selenomethionine (SELECT), a major active component of the selenized yeast. Furthermore, the discrepant findings could be explained by a proposed threshold effect of selenium on the GPX1 activity, which plateaus at selenium concentration of approximately 80 to 100 μg/L (46, 47). NPCT specifically recruited participants from areas in the United States with relatively low soil selenium content, resulting in a baseline mean plasma selenium concentration of 114 μg/L (17). In contrast, SELECT recruited participants from areas throughout North America including the United States, Canada and Puerto Rico; a baseline median serum selenium concentration in SELECT was 135 μg/L (16), which was similar to the current WHI study (mean = 136 μg/L). Accordingly, baseline selenium concentrations in SELECT and the WHI study may be in a range where further selenium supplementation has no additional cancer preventive effect. Nonetheless, previous observational studies provide conflicting results regarding a threshold effect of selenium. Three small studies with low average selenium concentrations that were similar to those in the NPCT found no association between selenium and colorectal cancer risk (8, 10, 14), while two studies with selenium concentrations higher than reported in NPCT observed an inverse association (3, 5).
Motivated by previous findings of gender-based differences (1, 13), we conducted a gender-stratified meta-analysis and found statistically significant evidence for association between selenium and colorectal tumors among men only. Note, however, that the largest clinical trial, SELECT, which was only conducted in men, did not find an effect of selenium on colorectal cancer. Results from clinical trials should generally be given more emphasis, because they are not subject to some of the biases in observational studies. The potential gender-difference in the association of selenium with colorectal cancer risk may be due to differences in selenium metabolism and selenoenzyme activity (19-22, 48-51). In particular, men had higher serum selenium concentrations than women in populations in the United States (n = 14,619) (19) and France (n = 13,017) (20). In terms of urinary excretion, women had a higher selenium concentration in urine than men, after adjusting for body weight (21). Hence, these previous findings suggest that men may retain selenium more efficiently than women. GPX1 activity may also be higher in women than in men (50, 51). These findings are intriguing; however, given the limited evidence, the mechanisms behind a potential gender-difference on the effect of selenium need to be further elucidated.
We observed a stronger association between selenium concentrations and colorectal tumor risk among studies that measured selenium after diagnosis of colorectal tumors than those measured before the diagnosis. Although this difference did not explain the observed heterogeneity, this finding is potentially relevant given that it may point to reverse causality in studies where selenium was measured after diagnosis.
Given the reported differences in molecular characteristics of tumors and dietary risk factors between colon and rectum tumors (52, 53), we investigated the effect of selenium for both cancer sites, separately. Our site-specific analysis showed a marginally significant positive association between selenium concentrations and risk of colon cancer. However, given that our study was not powered for site-specific analysis this finding may be due to chance.
Our overall null finding for genetic variants in selenoenzymes and colorectal tumors is consistent with most previous studies (27, 54-58), although not all (56, 59). While our study observed marginal evidence for association between one GPX4 SNP (rs8178974) and colorectal cancer risk, the overall genetic variation in GPX4 was not statistically significantly associated with colorectal cancer risk, suggesting that this finding may be due to chance. Previous studies have found that two other GPX4 SNPs (rs713041 and rs2075710) were associated with the risk of colorectal tumors (56, 57, 59) and with selenoenzyme activity and concentrations (49). However, these two GPX4 SNPs were not in LD with the GPX4 variant (rs8178974) that was associated in our study (r2 = 0.03 and 0.04, respectively). Given that we likely need to expect similar effect sizes as demonstrated in genome-wide association studies for colorectal cancer (OR <1.15) (60), the power of the current and previous studies is limited. This also suggests that an effect, should one exist, is relatively weak.
A strength of our study includes the large number of cases, making it the largest observational study reported to date on serum selenium and colorectal cancer in women. Blood specimens and exposure information were collected in a prospective cohort setting before colorectal cancer was diagnosed. This reduced the possibility of biases and reverse causality, which may be a potential issue for selenium as discussed above. Our quality control measures for blinded QCs demonstrated high quality of the measurements. Although our study population had a wide range of serum selenium concentrations (81.0-398.7 μg/L), this range did not include individuals with selenium-deplete concentrations (<80 μg/L). Our participants who were recruited from areas with similarly low soil selenium content (61) than the NPCT had average selenium concentrations much higher (138 μg/L) than those in the NPCT (114 μg/L). Nonetheless, our average concentrations were similar to those in other recent studies conducted in the United States (1, 2, 5, 16). Hence, the difference in selenium concentrations between the NPCT and our study might be explained by factors other than the soil selenium content, such as global food distribution, which has increased over time. Accordingly, in order to test if selenium has a protective effect in selenium-deplete population, future studies may need to be conducted outside the United States in areas with low selenium concentration and a more local food distribution, such as areas in China.
This study also has some limitations. As previous studies, our study used biospecimens collected at one time point to assess long-term selenium intake. Nonetheless, the correlation between baseline and the year 3 samples measured in 100 control women was relatively high (r = 0.68), suggesting that a single measurement can be used to reflect, although not perfectly, long-term intake, as previously reported (62). Furthermore, selenium concentration in serum may not be reflective of selenium concentration in the target tissue, colorectal tissue in our study, as we previously observed for the prostate (63). Because we selected genetic variants from Caucasians, the genetic coverage of African-Americans and other ancestries in our participants might not be sufficient.
In conclusion, our study is among the largest prospective studies in women to comprehensively investigate the association of serum selenium and genetic variants in selenoenzymes with colorectal cancer risk. This study with relatively high selenium concentrations observed no association between serum selenium and colorectal cancer risk; a result consistent with previous studies in women. In contrast, our meta-analysis found an inverse association in men. Furthermore, we observed no associations between genetic variations in some selenoenzyme genes and colorectal cancer risk, nor did we find evidence of an interaction between genetic variation and serum selenium.
Supplementary Material
Acknowledgments
We would like to thank the study participants of the Women’s Health Initiative Observational Study and the study staff. This study was funded by the National Cancer Institute, National Heart, Lung, and Blood Institute, National Institutes of Health, United States Department of Health and Human Services (R01 CA120582, R01 CA127943, N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221).
References
- 1.Peters U, Chatterjee N, Church TR, et al. High serum selenium and reduced risk of advanced colorectal adenoma in a colorectal cancer early detection program. Cancer Epidemiol Biomarkers Prev. 2006;15(2):315–20. doi: 10.1158/1055-9965.EPI-05-0471. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs ET, Jiang R, Alberts DS, et al. Selenium and colorectal adenoma: results of a pooled analysis. J Natl Cancer Inst. 2004;96(22):1669–75. doi: 10.1093/jnci/djh310. [DOI] [PubMed] [Google Scholar]
- 3.Ghadirian P, Maisonneuve P, Perret C, et al. A case-control study of toenail selenium and cancer of the breast, colon, and prostate. Cancer Detect Prev. 2000;24(4):305–13. [PubMed] [Google Scholar]
- 4.Zhao N. A case-control study of risk factors of colorectal cancer in Shanxi Province. Zhonghua Liu Xing Bing Xue Za Zhi. 1990;11(5):295–8. abstract. [PubMed] [Google Scholar]
- 5.Connelly-Frost A, Poole C, Satia JA, et al. Selenium, folate, and colon cancer. Nutr Cancer. 2009;61(2):165–78. doi: 10.1080/01635580802404188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark LC, Hixson LJ, Combs GF, Jr, et al. Plasma selenium concentration predicts the prevalence of colorectal adenomatous polyps. Cancer Epidemiol Biomarkers Prev. 1993;2(1):41–6. [PubMed] [Google Scholar]
- 7.Fernandez-Banares F, Cabre E, Esteve M, et al. Serum selenium and risk of large size colorectal adenomas in a geographical area with a low selenium status. Am J Gastroenterol. 2002;97(8):2103–8. doi: 10.1111/j.1572-0241.2002.05930.x. [DOI] [PubMed] [Google Scholar]
- 8.Scieszka M, Danch A, Machalski M, Drozdz M. Plasma selenium concentration in patients with stomach and colon cancer in the Upper Silesia. Neoplasma. 1997;44(6):395–7. [PubMed] [Google Scholar]
- 9.Russo MW, Murray SC, Wurzelmann JI, Woosley JT, Sandler RS. Plasma selenium levels and the risk of colorectal adenomas. Nutr Cancer. 1997;28(2):125–9. doi: 10.1080/01635589709514563. [DOI] [PubMed] [Google Scholar]
- 10.Knekt P, Aromaa A, Maatela J, et al. Serum selenium and subsequent risk of cancer among Finnish men and women. J Natl Cancer Inst. 1990;82(10):864–8. doi: 10.1093/jnci/82.10.864. [DOI] [PubMed] [Google Scholar]
- 11.Schober SE, Comstock GW, Helsing KJ, et al. Serologic precursors of cancer. I Prediagnostic serum nutrients and colon cancer risk. Am J Epidemiol. 1987;126:1033–41. doi: 10.1093/oxfordjournals.aje.a114742. [DOI] [PubMed] [Google Scholar]
- 12.Nomura A, Heilbrun LK, Morris JS, Stemmermann GN. Serum selenium and the risk of cancer, by specific sites: case-control analysis of prospective data. J Natl Cancer Inst. 1987;79(1):103–8. [PubMed] [Google Scholar]
- 13.Garland M, Morris JS, Stampfer MJ, et al. Prospective study of toenail selenium levels and cancer among women. J Natl Cancer Inst. 1995;87(7):497–505. doi: 10.1093/jnci/87.7.497. [DOI] [PubMed] [Google Scholar]
- 14.van den Brandt PA, Goldbohm RA, van ’V P, et al. A prospective cohort study on toenail selenium levels and risk of gastrointestinal cancer. J Natl Cancer Inst. 1993;85(3):224–9. doi: 10.1093/jnci/85.3.224. [DOI] [PubMed] [Google Scholar]
- 15.Nelson RL, Davis FG, Sutter E, et al. Serum selenium and colonic neoplastic risk. Dis Colon Rectum. 1995;38(12):1306–10. doi: 10.1007/BF02049157. [DOI] [PubMed] [Google Scholar]
- 16.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial Nutritional Prevention of Cancer Study Group. JAMA. 1996;276(24):1957–63. [PubMed] [Google Scholar]
- 18.Duffield-Lillico AJ, Reid ME, Turnbull BW, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11(7):630–9. [PubMed] [Google Scholar]
- 19.Kafai MR, Ganji V. Sex, age, geographical location, smoking, and alcohol consumption influence serum selenium concentrations in the USA: third National Health and Nutrition Examination Survey, 1988-1994. J Trace Elem Med Biol. 2003;17(1):13–8. doi: 10.1016/S0946-672X(03)80040-8. [DOI] [PubMed] [Google Scholar]
- 20.Arnaud J, Bertrais S, Roussel AM, et al. Serum selenium determinants in French adults: the SU.VI.M.AX study. Br J Nutr. 2006;95(2):313–20. doi: 10.1079/bjn20051528. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez Rodriguez EM, Sanz Alaejos MT, Diaz RC. Urinary selenium status of healthy people. Eur J Clin Chem Clin Biochem. 1995;33(3):127–33. doi: 10.1515/cclm.1995.33.3.127. [DOI] [PubMed] [Google Scholar]
- 22.Waters DJ, Chiang EC, Cooley DM, Morris JS. Making sense of sex and supplements: differences in the anticarcinogenic effects of selenium in men and women. Mutat Res. 2004;551(1-2):91–107. doi: 10.1016/j.mrfmmm.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Moghadaszadeh B, Beggs AH. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology (Bethesda) 2006;21:307–15. doi: 10.1152/physiol.00021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tham DM, Whitin JC, Kim KK, Zhu SX, Cohen HJ. Expression of extracellular glutathione peroxidase in human and mouse gastrointestinal tract. Am J Physiol. 1998;275(6 Pt 1):G1463–G1471. doi: 10.1152/ajpgi.1998.275.6.G1463. [DOI] [PubMed] [Google Scholar]
- 25.Mork H, Lex B, Scheurlen M, et al. Expression pattern of gastrointestinal selenoproteins--targets for selenium supplementation. Nutr Cancer. 1998;32(2):64–70. doi: 10.1080/01635589809514720. [DOI] [PubMed] [Google Scholar]
- 26.Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27(9-10):951–65. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 27.Peters U, Littman AJ, Kristal AR, et al. Vitamin E and selenium supplementation and risk of prostate cancer in the Vitamins and lifestyle (VITAL) study cohort. Cancer Causes Control. 2008;19(1):75–87. doi: 10.1007/s10552-007-9072-y. [DOI] [PubMed] [Google Scholar]
- 28.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 29.Langer RD, White E, Lewis CE, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 30.Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph RE, Vaughan TL, Kristal AR, et al. Serum selenium levels in relation to markers of neoplastic progression among persons with Barrett’s esophagus. J Natl Cancer Inst. 2003;95(10):750–7. doi: 10.1093/jnci/95.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman GE, Schaffer S, Bankson DD, Hughes MP, Omenn GS. Predictors of serum selenium in cigarette smokers and the lack of association with lung and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1069–76. [PubMed] [Google Scholar]
- 33.Ericson SP, McHalsky ML, Rabinow BE, et al. Sampling and analysis techniques for monitoring serum for trace elements. Clin Chem. 1986;32(7):1350–6. [PubMed] [Google Scholar]
- 34.Frazer KA, Ballinger DG, Cox DR, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449(7164):851–61. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster CB, Aswath K, Chanock SJ, McKay HF, Peters U. Polymorphism analysis of six selenoprotein genes: support for a selective sweep at the glutathione peroxidase 1 locus (3p21) in Asian populations. BMC Genet. 2006;7:56. doi: 10.1186/1471-2156-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlson CS, Eberle MA, Rieder MJ, et al. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74(1):106–20. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynn HS, McCulloch CE. When does it pay to break the matches for analysis of a matched-pairs design? Biometrics. 1992;48(2):397–409. [PubMed] [Google Scholar]
- 38.Chapman JM, Cooper JD, Todd JA, Clayton DG. Detecting disease associations due to linkage disequilibrium using haplotype tags: a class of tests and the determinants of statistical power. Hum Hered. 2003;56(1-3):18–31. doi: 10.1159/000073729. [DOI] [PubMed] [Google Scholar]
- 39.Levander OA. Considerations on the assessment of selenium status. Fed Proc. 1985;44(9):2579–83. [PubMed] [Google Scholar]
- 40.Finley J, Matthys L, Shuler T, Kotynta E. Selenium content of foods purchased in North Dakota. Nutr Res. 1996;16:865–71. [Google Scholar]
- 41.Holden J, Gebhardt R, Davis C, Lurie D. A nationwide study of the selenium content and variability in white bread. Journal Food Composition Analysis. 1991;4:183–95. [Google Scholar]
- 42.Morimoto LM, Newcomb PA, Ulrich CM, et al. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1012–8. [PubMed] [Google Scholar]
- 43.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–74. [PubMed] [Google Scholar]
- 44.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 45.Ioannidis JP, Patsopoulos NA, Evangelou E. Heterogeneity in meta-analyses of genome-wide association investigations. PLoS One. 2007;2(9):e841. doi: 10.1371/journal.pone.0000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfthan G, Xu GL, Tan WH, et al. Selenium supplementation of children in a selenium-deficient area in China: blood selenium levels and glutathione peroxidase activities. Biol Trace Elem Res. 2000;73(2):113–25. doi: 10.1385/BTER:73:2:113. [DOI] [PubMed] [Google Scholar]
- 47.Duffield AJ, Thomson CD, Hill KE, Williams S. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr. 1999;70(5):896–903. doi: 10.1093/ajcn/70.5.896. [DOI] [PubMed] [Google Scholar]
- 48.Meplan C, Crosley LK, Nicol F, et al. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study) FASEB J. 2007;21(12):3063–74. doi: 10.1096/fj.07-8166com. [DOI] [PubMed] [Google Scholar]
- 49.Meplan C, Crosley LK, Nicol F, et al. Functional effects of a common single-nucleotide polymorphism (GPX4c718t) in the glutathione peroxidase 4 gene: interaction with sex. Am J Clin Nutr. 2008;87(4):1019–27. doi: 10.1093/ajcn/87.4.1019. [DOI] [PubMed] [Google Scholar]
- 50.Malling TH, Sigsgaard T, Andersen HR, et al. Sex determines the influence of smoking and gene polymorphism on glutathione peroxidase activity in erythrocytes. Scand J Clin Lab Invest. 2009;69(2):295–302. doi: 10.1080/00365510802632155. [DOI] [PubMed] [Google Scholar]
- 51.Massafra C, Gioia D, De FC, et al. Gender-related differences in erythrocyte glutathione peroxidase activity in healthy subjects. Clin Endocrinol (Oxf) 2002;57(5):663–7. doi: 10.1046/j.1365-2265.2002.01657.x. [DOI] [PubMed] [Google Scholar]
- 52.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403–8. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 53.Li FY, Lai MD. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10(3):219–29. doi: 10.1631/jzus.B0820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen R, Saebo M, Skjelbred CF, et al. GPX Pro198Leu and OGG1 Ser326Cys polymorphisms and risk of development of colorectal adenomas and colorectal cancer. Cancer Lett. 2005;229(1):85–91. doi: 10.1016/j.canlet.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Hansen RD, Krath BN, Frederiksen K, et al. GPX1 Pro(198)Leu polymorphism, erythrocyte GPX activity, interaction with alcohol consumption and smoking, and risk of colorectal cancer. Mutat Res. 2009;664(1-2):13–9. doi: 10.1016/j.mrfmmm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Peters U, Chatterjee N, Hayes RB, et al. Variation in the selenoenzyme genes and risk of advanced distal colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1144–54. doi: 10.1158/1055-9965.EPI-07-2947. [DOI] [PubMed] [Google Scholar]
- 57.Meplan C, Hughes DJ, Pardini B, et al. Genetic variants in selenoprotein genes increase risk of colorectal cancer. Carcinogenesis. 2010;31(6):1074–9. doi: 10.1093/carcin/bgq076. [DOI] [PubMed] [Google Scholar]
- 58.Al Taie OH, Uceyler N, Eubner U, et al. Expression profiling and genetic alterations of the selenoproteins GI-GPx and SePP in colorectal carcinogenesis. Nutr Cancer. 2004;48(1):6–14. doi: 10.1207/s15327914nc4801_2. [DOI] [PubMed] [Google Scholar]
- 59.Bermano G, Pagmantidis V, Holloway N, et al. Evidence that a polymorphism within the 3’UTR of glutathione peroxidase 4 is functional and is associated with susceptibility to colorectal cancer. Genes Nutr. 2007;2(2):225–32. doi: 10.1007/s12263-007-0052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hindorff LA, Sethupathy P, Junkins HA, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106(23):9362–7. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.U.S.Department of the Interior. U.S. Geological Survey. 2006 [Google Scholar]
- 62.Longnecker MP, Stram DO, Taylor PR, et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology. 1996;7(4):384–90. doi: 10.1097/00001648-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Takata Y, Morris JS, King IB, et al. Correlation between selenium concentrations and glutathione peroxidase activity in serum and human prostate tissue. Prostate. 2009;69(15):1635–42. doi: 10.1002/pros.21012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


