Abstract
For primates, as for many other vertebrates, copulation which results in ejaculation is a prerequisite for reproduction. The probability of ejaculation is affected by various physiological and social factors, for example reproductive state of male and female and operational sex-ratio. In this paper, we present quantitative and qualitative data on patterns of sexual behaviour in a captive group of hamadryas baboons (Papio hamadryas), a species with a polygynous–monandric mating system. We observed more than 700 copulations and analysed factors that can affect the probability of ejaculation. Multilevel logistic regression analysis and Akaike’s information criterion (AIC) model selection procedures revealed that the probability of successful copulation increased as the size of female sexual swellings increased, indicating increased probability of ovulation, and as the number of females per one-male unit (OMU) decreased. In contrast, occurrence of female copulation calls, sex of the copulation initiator, and previous male aggression toward females did not affect the probability of ejaculation. Synchrony of oestrus cycles also had no effect (most likely because the sample size was too small). We also observed 29 extra-group copulations by two non-adult males. Our results indicate that male hamadryas baboons copulated more successfully around the time of ovulation and that males in large OMUs with many females may be confronted by time or energy-allocation problems.
Keywords: Sexual behaviour, Copulation pattern, Papio hamadryas, Akaike’s Information Criterion
Introduction
Effective copulation that leads to conception is crucial for successful reproduction and hence for fitness. Successful copulation is affected by a number of factors, for example the quality and quantity of sperm (Birkhead and Fletcher 1995; Parker 1998) and timing in relation to ovulation (Hrdy and Whitten 1987; Dixson 1998). In primates, as in other mammals, copulation comprises mounting and intromission, at least (Dixson 1998). In any case, one decisive prerequisite for copulation to be effective is the occurrence of ejaculation. The ability of a male to provide sperm during copulation depends both on his own reproductive physiology and behaviour and on the reproductive status and behaviour of his mating partner. Most primate males can only copulate when females cooperate (Strum 1982; Bercovitch 1995). Copulation success can also be affected by the frequency with which males copulate given that sperm can be a limited resource in some circumstances, as proposed by Small (1988). Such a situation may emerge, for instance, in one-male groups if the operational sex-ratio is strongly female-biased by oestrus synchrony.
However, quantitative data on sexual behaviour and copulatory patterns are sparse or non-existent for many primate species. This includes hamadryas baboons (Papio hamadryas), despite decades of study and extensive data on their social system (Kummer 1968, 1995; Swedell 2002a, b; Schreier and Swedell 2009). Hamadryas baboons live in a multi-level society, of which the basic entity is the one-male unit (OMU), consisting of one adult male and one to several females and their offspring (Kummer 1968; Abegglen 1984; Schreier and Swedell 2009). Ascending social levels include the clan (an association of OMUs possibly linked by male kinship), the band (the ecological unit), and the troop (a temporary aggregation of bands at a sleeping site). Attached to some OMUs are one or two follower males. Also within a band one finds a number of solitary males that are not attached to a particular OMU (Pines et al. 2011). OMU leader males try to maintain exclusive social and sexual access to a set of females and they herd their females aggressively by use of neck bites and threats. Consequently, almost all mating takes place within OMUs, classifying the hamadryas mating system as polygynous and monandrous (Kummer 1968; Stammbach 1987; Swedell 2006). Although Kummer et al. (1974; cf. Sigg et al. 1982) argued that the potential for female mate choice is small, because of the ability of leader males to monopolize females in their OMUs, recent studies show that not all infants are offspring of OMU-leaders, which means that successful extra-group copulation occurs (Yamane et al. 2003; Zinner et al. 2006). Sexual interactions with subadult males, presumably capable of siring infants, have been observed in the wild (Kummer 1968; Swedell 2006).
Oestrus synchrony can lead to female–female competition for mating and conception but can also affect the ability of females to copulate with males other than their OMU leaders, thus increasing a female’s chances for mate choice. This may be because leader males might have time and energy-allocation problems when forced to herd several females at the same time (Zinner et al. 1994).
Furthermore, in contrast with males of other species with a single male multi-female systems (e.g. many Asian colobines, Kirkpatrick 2011; most guenons, Jaffe 2011; gorillas, Robbins 2011) copulation success in male hamadryas baboons may not only be affected by the operational sex-ratio within the one-male unit but also by the socionomic sex-ratio. As a consequence of the multi-level organisation of hamadryas society, hamadryas leader males live permanently in close spatial proximity to a number of adult male competitors, i.e. other OMU-leaders, follower males, and solitary males which do not have any females (Kummer 1968; Swedell 2006; Pines et al. 2011). At the same time, hamadryas females seem to have a lower tendency to stay close to their males than in other species living in one-male units (e.g. gorillas, Stewart and Harcourt 2007), forcing leader males to monitor and, if necessary, herd their females, even when the females are not sexually receptive. A hamadryas male may be caught in a situation where he has to consort a receptive female while at the same time he has to control and herd his other females.
The sexual behaviour of free-ranging hamadryas baboons was first described by Kummer (1968). Sexual interactions can be initiated either when females present to males or when males approach and touch the females with their hands to make them stand up or press or pull females into a copulatory positions. Copulation is characterized by mounts during which males grasp the females’ ankles with their feet (“double-foot clasp position”) and by intromission of the penis (Kummer 1968). Females sometimes give copulation calls (Swedell 2006).
In many species, proceptive females are responsible for initiating most copulation (Hrdy and Whitten 1987; Hashimoto and Furuichi 2006). Furthermore, female baboons, similar to some other catarrhine primates, provide males with some information about the probability of ovulation through anogenital swellings. In other baboon taxa, the probability of ovulation is highest on the last 1 or 2 days of maximum swelling before detumescence (Gillman and Gilbert 1946; Hendrickx and Kraemer 1969; Wildt et al. 1977; Shaikh et al. 1982). Male chacma baboons seem to be more attracted to females with bigger swellings (Girolami and Bielert 1987).
The pattern of copulation in hamadryas baboons has been classified as “multiple mount-to-ejaculation” (MME) or “multiple brief intromission”, in which ejaculation takes place only after a series of mounts and intromissions (Dewsbury 1972, 1989; Dixson 1998). Kummer (1968) assumed that single extra-group copulation is reproductively insignificant, because he found that single mounts did not lead to ejaculation, although more recent research calls this into question (Yamane et al. 2003; Zinner et al. 2006).
In our paper we report a quantitative analysis of hamadryas baboon sexual behaviour. In particular, we address the factors that determine whether a copulation leads to an ejaculation. Such factors include female reproductive condition, with swelling size used as a proxy for fecundity (probability of ovulation), socionomic and operational sex-ratios within OMUs, and male and female behaviour before copulation. Furthermore we were interested in the frequency of extra-group copulation in this species, because OMU leader males are thought to have reproductive monopolies.
Methods
Study site, animals and data collection
Observations were conducted at the hamadryas baboon colony of the Zoologischer Garten in Berlin, Germany from August to December 2005. The baboons were living in an outdoor enclosure (ca. 400 m2) with access to an indoor enclosure. Food was provided daily in the indoor part of the enclosure, and water was available permanently in a well outdoors. The outdoor enclosure consisted of an artificial rock with several places to hide. Around the rock, the ground consisted of sand banks with several trunks enriching the site.
The social organization and social structure of the colony was determined by analysis of dyadic grooming interactions. The colony had the typical hamadryas social system, as described for free-ranging and captive populations (Kummer 1968; Colmenares et al. 1994). It consisted of four OMUs with four adult males, 13 adult females, two subadult males, and several infants of both sexes. Because no information on the baboons’ exact ages was available, animals were categorized into age classes by size and fur colour according to Sigg et al. (1982).
We focussed our observations on females, using focal animal sampling (Altman 1974) to record the behaviour of receptive females (indicated by perineal swellings). When only one female was swollen, we observed her for as long as possible. If two or more females were swollen simultaneously, we systematically sampled each in turn. Thus, focal observation periods lasted from 6 min to 5 h (mean = 60 min, SD = 36 min), depending on the female’s reproductive status, sexual activity, and visibility. We recorded the occurrence, duration, and success of copulation, number of pelvic thrusts per copulation, and occurrence of female copulation calls. We also recorded all acts of male aggression to females in the context of copulation and noted whether males or females initiated copulation. The data reported here come from 262.8 h of observation. Three out of 13 females in the colony were excluded from the observation and analysis because they did not develop sexual swellings during the observation period, owing to pregnancy or lactational amenorrhoea. In addition to focal sampling, all sexual interactions noticed by the observer were recorded ad libitum.
Definitions
The most important distinction was made between successful (indicated by the presence of ejaculate) and unsuccessful copulation. Cases for which we could not determine copulation success within 2 min were termed “unknown outcome” copulation and were excluded from analysis.
Definitions of the variables describing copulations are given in Table 1. We were not able to record all variables for all copulation, because of lack of visibility. We excluded incomplete cases from the respective analyses. As a result, sample sizes differ, depending on the variable considered. All calculations of copulation rates (copulations per hour) were corrected for observation time.
Table 1.
Definitions of variables
| Variable | Definition |
|---|---|
| Copulation success | Ejaculation, indicated by clearly visible clues of fresh sperm on the male’s penis or the female’s perineum after copulation. When the outcome of a copulation could not be determined within 2 min after the copulation, the respective copulation was termed “unknown outcome” copulation |
| Copulation duration | Time (s) from insertion of the male’s penis until its extraction from the female’s vagina |
| Pelvic thrusts | Number of pelvic thrusts of the male during copulation |
| Copulation calls (call) | Female utters context-specific call while copulating |
| Overt male aggression (agg) | Biting, pulling, or grabbing of the female by the male before copulation |
| Initiation of copulation (ini) | Initiation by the female via presenting or by the male via approaching, pressing, or pulling of the female |
| Sex ratio | Number of adult females in an OMU |
| Synchronya (syn) | When more than one female of the same OMU had a large swelling (>50% of her maximum swelling size) on the same day |
| Swelling size | See text |
aSynchrony here does not imply any female strategy; it merely refers to the fact that two or more females are simultaneously oestrous
Estimation of relative swelling size
For baboons, the probability of ovulation is highest on the last day of maximum swelling and the first day of detumescence (Gillman and Gilbert 1946; Hendrickx and Kraemer 1969; Wildt et al. 1977). The size of the anogenital swelling thus indicates the likelihood of ovulation and, hence, the probability of conception, and males are expected to mate preferentially during periods of maximum swelling (Hrdy and Whitten 1987; Wallis 1992; Nunn 1999; Zinner et al. 2004). We therefore measured size to determine the reproductive status of females, using photographs of female swellings taken with a digital video camera on each day of the study period. Relative swelling size was estimated, by use of “Adobe Photoshop 10.0”, as the ratio of swelling width (sw) to the maximum length of the ischial callosity (cal) where width was defined as a line from the edge of the swelling at a central position of the ischial callosity to an imaginary central axis through the anal and vaginal orifices (Fig. 1). Relative swelling sizes for each female were calculated as percentages of the biggest swelling of the respective female. Swelling sizes were categorized into two states, defined as follows: small = 0–50% of maximum swelling size of the respective female; large = 51–100% of maximum swelling size of the respective female.
Fig. 1.
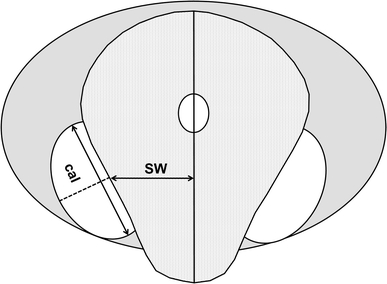
Schematic diagram of female hamadryas baboon anogenital swelling depicting sections of measurement used in the analysis: SW, swelling width at callosity midline; cal, ischial callosity length
Statistical analysis
We analysed the data on two sample size levels:
sample size based on the number of individual females; and
sample size based on the number of copulations.
For analyses on the first, inter-individual, level we used non-parametric tests (SPSS for Windows, Rel. 12.0; SPSS, Chicago, USA) with alpha set at 0.5. For analyses on the second, intra-individual, level we used multilevel logistic regression, which takes into account repeated observations of individuals by including female identity as a random factor in the model. We estimated the models using multilevel logistic regression analysis with maximum likelihood estimation to predict the probability of a successful copulation. Estimates are presented with bias-corrected accelerated bootstrap confidence intervals (bCI; Efron and Tibshirani 1993), which are robust against possible violation of the assumptions of logistic regression. Additionally, the final models were rerun with an interaction between the fixed factors and the random factor “female identity” (random slope). Almost no variance was estimated for these random effects and results changed only marginally. The assumptions of multilevel logistic regression were assessed by plotting predicted values against binned residuals (Gelman and Hill 2007). Similar multilevel linear regression analysis was used for analysis of the duration of copulation. Analyses were done using STATA 10.1 (StataCorp. 2007. Stata Statistical Software: Release 10; StataCorp LP, College Station, TX, USA).
We used Akaike information criterion (AIC) model selection to select from our candidate set of models the one that best approximated our data set (Burnham and Anderson 2002; Claeskens and Lid 2008). The AIC is a measure of the goodness of fit that includes a penalty for the number of variables estimated. AIC selects the best of several competing models as that which predicts best in a new data set. The best model is the one with the lowest AIC. Unlike model selection based on Null-hypothesis testing, AIC model selection enables an evaluation of the quality of other models by assessing AIC-related measures (Delta Δ AICi, Akaike’s weight and evidence ratio). Δ AICi is the difference in AIC between model i and the best model. As a rule of thumb, a Δ AICi value of <2 times the AICi of the best model has substantial support and should be considered with the best model. A Δ AICi value >4 times the AICi of the best model has substantially less support and models with a Δ AICi > 10 times the AICi of the best model can be omitted from further consideration (Burnham and Anderson 2002). Akaike’s weight is an estimate of the probability that model i is the best model among the candidate set of models. The evidence ratio for a given model i is the ratio of Akaike’s weights of the best model and of model i. Unlike Akaike’s weight, the evidence ratio of two models is independent of other candidate models.
Because of the small number of females and observations in our data set, our main model selection analysis is based on single-variable models. In a second step, we used the single best predictor and compared models with this variable and one of the remaining variables. As possible predictor variables, we assessed: synchrony (yes/no), male aggression (yes/no), female copulation calls (yes/no), initiation of copulation (male/female), OMU ID (equal to male ID), sex ratio, and relative swelling size.
Model selection was done using the complete case data set (n = 301). The parameters of the final best models were estimated using all available data of the selected variables (n = 347). Estimates differed only marginally between the two data sets.
Note on ethics
F.N. collected all data from the public viewing area in the zoo where the baboons were accustomed to people being present. Data collection required no direct contact with the animal subjects.
Results
Sexual behaviour of males and females was similar to that reported for free-ranging hamadryas baboons (Kummer 1968), including initiation of copulation by both sexes and the “double-foot clasp” copulation position. Harassment or interruption of copulating pairs by other females or males never occurred. Overall, we observed 734 copulations (482 during focal sampling, 252 during ad-libitum sampling). Of the copulations, 26.7% (196) were definitively successful and 29.0% (213) ceased without ejaculation. For 44.3% (325) of the copulations, whether ejaculation occurred was unknown.
Series of mounts consisted of a maximum of two unsuccessful copulations and one successful. On 46 occasions, two successive successful copulations were recorded without any additional mount between. This indicates that hamadryas males are capable of reaching ejaculation after only one intromission and multiple mounting is not required for ejaculation.
Sexual behaviour and swelling states
A total of 23 cycles was observed either in part (8) or completely (15). In 75% (198 h) of the total observation time (263 h), at least one female in the colony had a large swelling (>50% of her maximum swelling size). Of the copulations, 85% were observed when females had large swellings, and copulation rate (copulations per hour) was higher during this state of swelling than at times when females had smaller swellings (n = 10, median large = 2.11, median small = 0.13; Wilcoxon matched pairs test: z = 2.80, p = 0.005). We therefore based the following analyses on cases when females had large swellings (>50% of maximum swelling size) and when adult leader males were involved, which reduces the number of analysed copulations to 614.
Copulation of females with large swellings
Of the 614 copulations, 182 (29.6%) resulted in ejaculation, 165 (26.9%) were unsuccessful, and in 267 (43.5%) cases we were unable to decide whether the copulation was successful. Of the 347 copulations for which we identified success or lack of it, 52.4% were successful (Table 2). In 19.4% of the 614 cases, at least one additional female of the same OMU also had a swelling on the day the respective copulation was observed (synchronous). In 26.1% of copulations, females uttered copulation calls. In 491 cases we were able to observe whether or not the male was aggressive toward the female before copulation, and found they were in 13.0% of instances only. Of 489 cases in which we were able to observe whether males or females initiated the copulation, we found males initiated 57.3%.
Table 2.
Rates and proportions of sexual behaviour in females with a large swelling
| Number of copulations (n = 614) | Decided | Yes | % Yes (range between females) |
|---|---|---|---|
| Successful copulations | 347 | 182 | 52.4 (5.3–71.6) |
| Synchrony | 614 | 119 | 19.4 (0.0–66.7) |
| Female vocalization | 614 | 160 | 26.1 (0.0–79.1) |
| Male aggression | 491 | 64 | 13.0 (0.0–31.4) |
| Initiated by males | 489 | 280 | 57.3 (8.3–70.0) |
An average copulation lasted 6.2 s ± 1.9 s (mean ± SD, n = 487; range 2–12 s), during which the male on average performed 9.7 ± 3.3 pelvic thrusts (mean ± SD, n = 504; range 1–20 thrusts). Both variables were strongly correlated (n = 484; r = 0.77; p < 0.001).
Multilevel linear regression analysis showed that successful copulation was significantly longer than unsuccessful copulation (Fig. 2; mean difference = 1.26 s (95% bCI 0.87–1.65, z = 6.3, p < 0.0001).
Fig. 2.
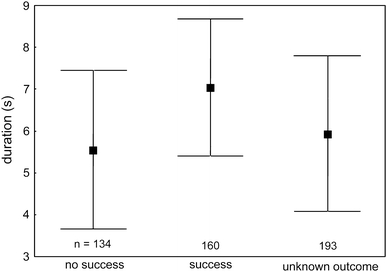
Average duration (s) of unsuccessful and successful copulation and of copulation of unknown outcome (mean ± SD)
Variation of copulation rate and successful copulation
Copulation rates per female ranged from 0.52 to 5.25 copulations per hour (n = 10, median = 2.03 copulations per hour, Table 3) and rates varied significantly among individual females (G-test, df = 9, G = 67, p < 0.001). This difference could not be explained by variation in the socionomic sex ratio, because we found no correlation between the sex ratio within OMUs and copulation rates (Spearman: n = 10, rs = −0.33, p = 0.35). There was, however, a negative correlation between the socionomic sex ratio and proportion of successful copulation (Spearman: n = 10, rs = −0.69, p < 0.05), suggesting that females in OMUs with a more female-biased sex ratio experienced lower copulation success.
Table 3.
Individual copulation frequencies and copulation success of females (relative swelling size >50%)
| Female | Sex | OMU | Sex ratio (females in OMU) | Observed number of copulations (decided copulations) | Copulations/h | Proportion of successful copulations (%) |
|---|---|---|---|---|---|---|
| Af1 | f | A | 2 | 153 (81) | 3.08 | 71.6 |
| Af2a | f | A | 2 | |||
| Bf1 | f | B | 2 | 103 (50) | 2.37 | 48.0 |
| Bf2 | f | B | 2 | 61 (48) | 5.25 | 70.8 |
| Cf1 | f | C | 3 | 10 (7) | 0.52 | 42.9 |
| Cf2 | f | C | 3 | 36 (25) | 1.42 | 60.0 |
| Cf3 | f | C | 3 | 80 (51) | 1.65 | 49.0 |
| Df1b | f | D | 6 | 48 (23) | 2.50 | 52.2 |
| Df2 | f | D | 6 | 59 (20) | 2.72 | 15.0 |
| Df3b | f | D | 6 | 35 (23) | 1.69 | 30.4 |
| Df4a | f | D | 6 | |||
| Df5a | f | D | 6 | |||
| Df6b | f | D | 6 | 29 (19) | 1.00 | 5.3 |
Because females were the focus of the study, we could not calculate the copulation rate for males
aFemales which did not show any sign of a swelling, because of pregnancy or lactational amenorrhea. They were excluded from the analysis
bFemales which experienced extra-group copulation
We did not find the expected effect of oestrus synchrony (operational sex ratio) on copulation success. However, sample size for testing the effect was rather small. Only 19.4% of copulation occurred when the mating female and an additional female of the same OMU were simultaneously oestrous. These cases concerned only 4 females of 2 OMUs. In all cases only one additional female in the respective OMUs was simultaneously in oestrus.
Determinants of successful copulation
The proportion of successful copulations ranged from 5% to 72% for females (n = 10, median = 49%, Table 3) and varied significantly among individuals (G-test: df = 9, G = 124, p < 0.001). The single-variable multilevel logistic regression models used to predict copulation success are compared in Table 4. Relative swelling size has the lowest AIC value and, therefore, best approximates the data set. The odds of a successful copulation increase by 1.029 (95% bca CI 1.001–1.049) with each 1% unit increase in swelling size (Fig. 3). However, the second best model, with sex ratio as a predictor, has only a slightly larger Δ AICi of 0.68 and is 1.4 times less likely to be the best model. There is almost no support for any other model to be a best model.
Table 4.
Rank of 7 models used to predict success of copulation using Akaike’s information criterion (AIC)
| Model | AIC | Δ AICi | Likelihood | Akaike’s weight | Evidence ratio |
|---|---|---|---|---|---|
| Syn | 400 | 11.13 | 0.004 | 0.002 | 261.1 |
| Agg | 398.02 | 9.15 | 0.010 | 0.005 | 97.0 |
| Call | 397.93 | 9.06 | 0.011 | 0.005 | 92.8 |
| Ini | 395.01 | 6.14 | 0.046 | 0.024 | 21.5 |
| OMU | 392.25 | 3.38 | 0.185 | 0.094 | 5.4 |
| Sex ratio | 389.55 | 0.68 | 0.712 | 0.362 | 1.4 |
| Swelling size | 388.87 | 0 | 1 | 0.508 | 1 |
Presented are AIC, Δ AICi, Akaike’s weight and evidence ratio (best model in comparison with model i). The best model is the model with the lowest AIC (relative swelling size) and, consequently, with a Δ AICi of 0
Fig. 3.
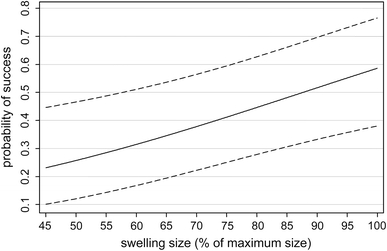
Best single-variable model: relationship between relative swelling size and predicted probability of successful copulation. Shown are predicted mean values and 95% confidence intervals. Model variables: relative swelling size: b = 0.028 (95% bCI: 0.010–0.046), exp(b) = 1.029 (95% bCI: 1.001–1.048), z = 3.02, p = 0.003; constant = −2.47 (95% bCI: −3.95 − [−0.94]). Random effects variable “Female identity”: SD = 1.03 (SE = 0.19)
Socionomic sex ratio correlates with OMU to a high degree, because the sex ratio within an OMU was constant during our observation period. The differences between the two variables are because sex ratio can be treated as a continuous variable whereas OMU is a nominal (categorical) variable. The apparent effect may simply be a spurious effect because of its correlation with OMU (or male ID, as OMU is confounded with male ID) and we therefore calculated the AIC of a model including OMU. This model has a Δ AICi of 3.38 and is less likely to be the best model by a factor of 5.4. Comparison of the AIC between models including either OMU or sex ratio shows that the AICi of the model with sex ratio is smaller by a factor of 2.7. The model with OMU is a factor of 3.9 less likely to be a better model than that with sex ratio as a predictor. Therefore, socionomic sex ratio predicts success of ejaculation better than OMU (and/or male ID).
In the second step of our model selection analysis, we calculated the AIC and AIC-related measures for 5 models with two variables, each of which used relative swelling size and one of the 5 remaining variables and compared these models with each other and with the best single-variable model (relative swelling size) (Table 5). The model including sex ratio and relative swelling size has the lowest AIC and therefore best approximates the data set. Comparison of the AIC-related measures shows that none of the other models is truly competitive with the best model. The next two models, which include (a) swelling size and initiation and (b) swelling size alone, both have a Δ AICi of 9.18 and are less likely to be the best model by a factor of 98.5.
Table 5.
Rank of 5 two-variable models including the best single-variable model to predict success of copulation using Akaike’s information criterion (AIC)
| Model | AIC | Δ AICi | Likelihood | Akaike’s weight | Evidence ratio |
|---|---|---|---|---|---|
| Swelling size syn | 389.69 | 10 | 0.007 | 0.007 | 148.4 |
| Swelling size agg | 390.84 | 11.15 | 0.004 | 0.004 | 263.7 |
| Swelling size call | 390.85 | 11.16 | 0.004 | 0.004 | 265.1 |
| Swelling size ini | 388.87 | 9.18 | 0.010 | 0.010 | 98.5 |
| Swelling size | 388.87 | 9.18 | 0.010 | 0.010 | 98.5 |
| Swelling size sex ratio | 379.69 | 0 | 1 | 0.967 | 1 |
Presented are AIC, Δ AICi, Akaike’s weight and evidence ratio (best model in comparison with model i). The best model is the model with the lowest AIC (sex ratio and relative swelling size) and, consequently, with a Δ AICi of 0
According to the best two-variable model, copulation success was positively related to relative swelling size and negatively related to the sex ratio. With relative swelling size constant, the odds of a successful copulation decreased by 0.60 (95% bCI 0.53–0.73) with each unit increase in sex ratio. The odds of a successful copulation increased by 1.03 (95% bCI 1.011–0.1.046) for a 1% increase in relative swelling size, if sex ratio is kept constant. The relationship between the predicted probability of successful copulation and relative swelling sizes is plotted for three sex ratios (2, 3, and 6 females) in Fig. 4.
Fig. 4.
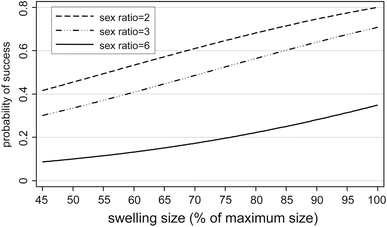
Best two-variables model: relationship between relative swelling size and predicted probability of successful copulation if the sex ratio is 2, 3, and 6 females. Shown are predicted mean values. Model variables: relative swelling size: b = 0.03 (95% bca C.I.: 0.011–0.045), exp(b) = 1.03 (95% bCI: 1.011–0.1.046), z = 3.36, p = 0.001; sex ratio: b = −0.49 (95% bca CI: −0.63 − [−0.31]), exp(b) = 0.60 (95% bCI: 0.530–0.730), z = −4.19, p < 0.001; constant = −0.88 (95% bCI: −2.14 − 0.43). Random effects variable “Female identity”: SD = 0.42, SE = 1.32
Extra-group copulation
Four females from two OMUs were observed to copulate with males other than their leader male on 29 occasions (4.0% of 734 observed copulations). Twenty-four cases involved copulation between juvenile males and four females from two OMUs, and five involved a subadult male copulating with three females from two OMUs. OMU leader males were never seen to participate in any sexual interaction with females of an OMU other than their own.
All extra-group copulation was initiated by females and they occurred on the periphery of the group only, out of the sight of the leader male. Both participants looked frequently in the direction of the leader male during the encounter. Of the copulations with juvenile males, 14 were definitely not successful and in 10 cases the outcome was unknown. In 9 of these 24 cases, the female had a large swelling. One copulation of the subadult male definitely resulted in ejaculation, three were not successful, and the outcome of the fifth case was unknown. The successful copulation was accomplished when the female had a large swelling. In the other four cases the swellings were small. In 27 (23 juvenile male, 4 subadult male) of the 29 cases, females were members of the OMU with the most female-biassed sex ratio (OMU D which had 6 females); this included the one successful copulation by the subadult male. In none of the subadult male’s copulations and in only 5 of the juvenile male’s copulations was the criterion for “synchrony” fulfilled.
Discussion
Our study is based on two questions about the copulation patterns of hamadryas baboons:
what determines the success of a copulation; and
does extra-group copulation occur?
Results from our AIC model selection approach showed that relative swelling size was the most important single determinant of copulation success. In the two-variable approach, relative swelling size with sex ratio was the best predictor of copulation success.
The phase of maximum receptivity for a baboon female is supposed to be when she has a maximally swollen perineum (Wildt et al. 1977). For this reason, males are expected to use swelling size as an indicator of female receptivity, and to find swollen females more attractive than females with none or smaller swellings (Girolami and Bielert 1987). Our results are consistent with these expectations, because the probability of success was more than twice as high when females had large swellings than when they had swellings smaller than half of the maximum swelling. Additionally, females generally copulated at higher rates when they had large swellings. It is therefore likely that males use the swollen perineum as a signal of higher probability of ovulation. Proximately hamadryas males may simply find larger swellings more attractive therefore copulate and ejaculate more often, similarly to chacma baboon males in the study by Bielert and van der Walt (1982).
Variation in the sex ratio of OMUs was almost as good as swelling size at explaining variation in copulation success. Furthermore, a model that included both sex ratio and relative swelling size performed substantially better than models that included relative swelling size or sex ratio alone. Both factors therefore independently help to explain variation in copulation success.
Males have a limited budget of sperm, time, and energy (Nakatsuru and Kramer 1982; Dunbar 1988, 1992). Therefore, when several females are simultaneously in oestrus, males may have to divide these resources, possibly by reducing the number of successful copulations per female. Contrary to our expectation, oestrus synchrony had no effect on copulation success. In our study, however, overall synchrony was rather low (<20% and in all cases only one additional female within the OMU was simultaneously in oestrus). We cannot, therefore, exclude the possibility that oestrus synchrony affects copulation success in large OMUs when more than two females are simultaneously oestrous. We know that synchrony (or asynchrony) can be part of a female reproductive strategy (e.g. Lemur katta, Pereira 1991). However, synchrony can occur by chance, as was demonstrated for hamadryas baboons by Tobler et al. (2010).
In contrast with the operational sex-ratio, the socionomic sex ratio has a clear effect on copulation success in our study colony. This, however, might be an effect of the particular hamadryas social system, in which male leaders are most likely to have to invest more time and energy in monitoring and herding their females because of the permanent close spatial presence of several male competitors (Kummer 1968; Swedell 2006; Swedell and Schreier 2009; Pines et al. 2011).
In other species which live in single-male groups, leader males are normally not permanently surrounded by a large number of male competitors (Kirkpatrick 2011; Jaffe 2011; Robbins 2011) and females might have an interest in staying close to the male or to other, mostly related, females in the single-male group.
Hamadryas females are assumed not to be related and their social network is relatively weak (Kummer 1968; Swedell 2006), leading to a reduced tendency to form a cohesive group with other females, which may impose additional monitoring and herding costs on the male. In geladas (Theropithecus gelada), which have a multi-level system superficially similar to that of hamadryas baboons, females within an OMU are related and have a strong tendency to remain as a cohesive group, even if the leader male changes (Grueter and Zinner 2004; le Roux et al. 2011).
If the time and energy costs for monitoring and herding females for hamadryas leader males are actually higher than in other species, and if these costs increase with increasing number of females within an OMU, it seems reasonable that males in smaller OMUs (those with fewer females) can copulate successfully with the same female more often than males in bigger OMUs. In our study females in bigger OMUs tended to have fewer successful copulations, with the odds of successful copulation decreasing by 0.60 with each unit increase in sex ratio (Fig. 4).
Study of AIC-related model assessment criteria did not provide any noteworthy support for additional factors affecting the probability of successful copulation. Neither the occurrence of female copulation calls, sex of the copulation initiator, nor male aggression toward females significantly affected the probability that copulation was successful.
Although copulation calls may contribute to the success of copulations in other species (Barbary macaques, Pfefferle et al. 2008), we did not observe this in our study (see also Swedell 2006). In our study, copulation calls only occurred in a small number of copulations (26.1% of all cases) in comparison with observations of chacma baboons, for which female copulatory vocalizations are much more frequent (84%: Saayman 1970, 97%: O’Connell and Cowlishaw 1994). It therefore seems that copulation calls have, at best, only a minor effect on male copulatory behaviour in hamadryas baboons, which is in agreement with the two hypothesized immediate functions of copulation calls: to encourage mating attempts by other males and to increase mate guarding by the consort male (Pradhan et al. 2006). In hamadryas baboons, chances of copulation with other males are very rare because of already intensive mate guarding by the leader male.
Female baboons present to males particularly often when they have swellings, to show their willingness to mate (Saayman 1970; Hrdy and Whitten 1987). Therefore, we might expect males to ejaculate more often when females initiate copulations, because female proceptivity may indicate a high probability of conception. On the other hand, males might initiate more successful copulations when females are receptive compared with non-reproductive times so they use their energy devoted to gamete production efficiently. However, neither of these hypotheses was supported here, because the sex of the initiator of copulation did not affect the likelihood of copulation success.
Aggression toward females, for example holding, pulling, and biting, is an important part of the hamadryas baboon males’ herding behaviour (Kummer 1968; Swedell and Schreier 2009) and could also be involved in a sexual context. However, we found no evidence that pre-copulatory male aggression increased the probability that copulation was successful. In fact, this form of male aggression was observed in only 13% of all copulation.
Our second main question deals with the occurrence of extra-group copulation in hamadryas baboons. The ability of females to choose mates is supposed to have a positive effect on female fitness (Andersson 1994). In hamadryas baboon, the leader male and his females build stable pair bonds, sustained by the aggressive herding behaviour of the male. This leaves other adult males little chance to copulate (Kummer et al. 1974; Sigg et al. 1982) and thus limits female opportunity for mate choice. Juvenile and adolescent males, however, sometimes copulate with females (Kummer 1968; Sigg et al. 1982; Swedell 2006) and the adolescents are able to inseminate them (Zinner et al. 2006). We observed several extra-group copulations with juvenile and subadult males. However, such copulation was infrequent and rarely successful. All extra-group copulation was initiated by females, showing that females were willing to interact sexually with these males. Most extra-group copulation involved females from the OMU with the lowest sex ratio, which might be an indication that male control is not as efficient in relatively large OMUs. However, we found no effect of oestrus synchrony on either the occurrence or success of extra-group copulation, although the small sample size means that conclusions about the effect of sex ratio and synchrony on extra-group copulation are preliminary.
Hamadryas baboons have been regarded as multiple mount ejaculators (MME) since Kummer (1968: 39) stated that: “True copulation among hamadryas consists of a series of mountings occurring at intervals of three to eight minutes. Ejaculation occurs only after several such mountings.” Several authors adopted Kummer’s description (Dewsbury 1972, 1989; Dixson 1998), although subsequent observations showed that hamadryas males are capable of ejaculation with only single mounts (Nystrom 1992). Our data confirm this finding and raise the question of whether a simple dichotomy exists between SME (single-mount ejaculator) and MME species.
Whether copulation in hamadryas baboons is successful—i.e. whether it leads to ejaculation—depends primarily on whether females have large swellings, i.e. whether the probability of ovulation and conception is high. A second important effect seems to be the number of females in an OMU, with decreasing probability of success as the number of females increases. Our results suggest that male hamadryas baboons concentrate their successful copulation on times when the probability of ovulation, and hence conception, is high, i.e. when females have their largest swellings. Furthermore, males in larger OMUs with many females may face a time and/or energy-allocation problem in controlling all their females and at the same time spending time/energy on successful copulations.
Acknowledgments
The authors are very grateful to the Zoologischer Garten Berlin for giving us the opportunity to conduct this study. We especially thank Dr P. Rahn from the Zoologischer Garten for his kind support, and Brandon Wheeler and Peter Henzi for constructive comments on an earlier version of the paper. We also thank David Watts and an anonymous reviewer for their helpful comments.
References
- Abegglen JJ. On socialization in hamadryas baboons. Lewisburg: Bucknell University Press; 1984. [Google Scholar]
- Altman J. Observational studies of behaviour: sampling methods. Behaviour. 1974;49:227–265. doi: 10.1163/156853974X00534. [DOI] [PubMed] [Google Scholar]
- Andersson M. Sexual selection. Princeton: Princeton University Press; 1994. [Google Scholar]
- Bercovitch F. Female cooperation, consortship maintenance, and male mating success in savanna baboons. Anim Behav. 1995;50:137–149. doi: 10.1006/anbe.1995.0227. [DOI] [Google Scholar]
- Bielert C, Walt LA. Male chacma baboon (Papio ursinus) sexual arousal; mediation by visual cues from female conspecifics. Psychoneuroendocrinology. 1982;7:31–48. doi: 10.1016/0306-4530(82)90053-1. [DOI] [PubMed] [Google Scholar]
- Birkhead T, Fletcher F. Ejaculate quality and the success of extra-pair copulations in the zebra finch. Nature. 1995;377:422–423. doi: 10.1038/377422a0. [DOI] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretical approach. 2. New York: Springer; 2002. [Google Scholar]
- Claeskens G, Lid N. Model selection and model averaging. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Colmenares F, Lozano MG, Torres P. Harem social structure in a multiharem colony of baboons (Papio spp.): a test of the hypothesis of the “star-shaped” sociogram. In: Roeder JJ, Thierry B, Anderson JR, Herrenschmidt N, editors. Current primatology, vol. ii: Social development, learning and behaviour. Strasbourg: Univ. Louis Pasteur; 1994. pp. 93–101. [Google Scholar]
- Dewsbury DA. Patterns of copulation behavior in male mammals. Quart Rev of Biol. 1972;47:1–33. doi: 10.1086/407097. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Copulatory patterns of primates as viewed in broad mammalian perspective. Am J Primatol. 1989;17:51–72. doi: 10.1002/ajp.1350170106. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Primate sexuality. Oxford: Oxford University Press; 1998. [Google Scholar]
- Dunbar RIM. Primate social systems. Ithaca: Cornell University Press; 1988. [Google Scholar]
- Dunbar RIM. Time: a hidden constraint on the behavioural ecology of baboons. Behav Ecol Sociobiol. 1992;31:35–49. doi: 10.1007/BF00167814. [DOI] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Gillman J, Gilbert C. The reproductive cycles of the chacma baboon (Papio ursinus) with special reference to the problems of menstrual irregularities as assessed by the behaviour of the sex skin. S Afr J Med Sci. 1946;11:1–54. [PubMed] [Google Scholar]
- Girolami L, Bielert C. Female perineal swelling and its effects on male sexual arousal: an apparent sexual releaser in the chacma baboon (Papio ursinus) Int J Primatol. 1987;8:651–661. doi: 10.1007/BF02735782. [DOI] [Google Scholar]
- Grueter C, Zinner D. Nested societies. Convergent adaptations of baboons and snub-nosed monkeys? Primate Rep. 2004;70:1–98. [Google Scholar]
- Hashimoto C, Furuichi T. Comparison of behavioral sequence of copulation between chimpanzees and bonobos. Primates. 2006;47:51–55. doi: 10.1007/s10329-005-0144-x. [DOI] [PubMed] [Google Scholar]
- Hendrickx AG, Kraemer DC. Observations on the menstrual cycle, optimal mating time and pre-implantation embryos of the baboon, Papio anubis and Papio cyncocephalus. J Reprod Fertil. 1969;6:119–128. [Google Scholar]
- Hrdy SB, Whitten PL. Patterning of sexual activity. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago: University of Chicago Press; 1987. pp. 370–384. [Google Scholar]
- Jaffe KE. The Guenons: polyspecific associations in socioecological perspective. In: Campbell CJ, Fuentes A, MacKinnon KC, Bearder SK, Stumpf RM, editors. Primates in perspective. New York: Oxford University Press; 2011. pp. 277–300. [Google Scholar]
- Kirkpatrick RC. The Asian colobines: diversity among leaf-eating monkeys. In: Campbell CJ, Fuentes A, MacKinnon KC, Bearder SK, Stumpf RM, editors. Primates in perspective. New York: Oxford University Press; 2011. pp. 189–202. [Google Scholar]
- Kummer H. Social organization of hamadryas baboons: a field study. Chicago: The University of Chicago Press; 1968. [Google Scholar]
- Kummer H. In quest of the sacred baboon. Princeton: Princeton University Press; 1995. [Google Scholar]
- Kummer H, Goetz W, Angst W. Triadic differentiation: an inhibitory process protecting pair bonds in baboons. Behaviour. 1974;49:62–87. doi: 10.1163/156853974X00408. [DOI] [PubMed] [Google Scholar]
- le Roux A, Beehner JC, Bergman TJ. Female philopatry and dominance patterns in wild geladas. Am J Primatol. 2011;73:422–430. doi: 10.1002/ajp.20916. [DOI] [PubMed] [Google Scholar]
- Nakatsuru K, Kramer DL. Is sperm cheap? Limited male fertility and female choice in lemon tetra (Pisces, Characidae) Science. 1982;216:753–755. doi: 10.1126/science.216.4547.753. [DOI] [PubMed] [Google Scholar]
- Nunn C. The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav. 1999;58:229–246. doi: 10.1006/anbe.1999.1159. [DOI] [PubMed] [Google Scholar]
- Nystrom P (1992) Mating Success of Hamadryas, Anubis and Hybrid Male Baboons in a Mixed Social Group in the Awash National Park, Ethiopia. PhD thesis, Washington University, St Louis
- O’Connell S, Cowlishaw G. Infanticide avoidance, sperm competition and mate choice: the function of copulation calls in female baboons. Anim Behav. 1994;48:687–694. doi: 10.1006/anbe.1994.1288. [DOI] [Google Scholar]
- Parker GA. Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead TR, Møller AP, editors. Sperm competition and sexual selection. London: Academic Press; 1998. pp. 3–54. [Google Scholar]
- Pereira M. Asynchrony within estrous synchrony among ringtailed lemurs (Primates: Lemuridae) Physiol Behav. 1991;49:47–52. doi: 10.1016/0031-9384(91)90228-G. [DOI] [PubMed] [Google Scholar]
- Pfefferle D, Brauch K, Heistermann M, Hodges JK, Fischer J. Female Barbary macaque (Macaca sylvanus) copulation calls do not reveal the fertile phase but influence mating outcome. Proc R Soc Lond B. 2008;275:571–578. doi: 10.1098/rspb.2007.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines M, Saunders J, Swedell L (2011) Alternative routes to the leader male role in a multi-level society: follower vs. solitary male strategies and outcomes in hamadryas baboons. Am J Primatol 73:679–691 [DOI] [PubMed]
- Pradhan GR, Engelhardt A, Schaik CP, Maestripieri D. The evolution of female copulation calls in primates: a review and a new model. Behav Ecol Sociobiol. 2006;59:333–343. doi: 10.1007/s00265-005-0075-y. [DOI] [Google Scholar]
- Robbins MM. Gorillas: diversity in ecology and behaviour. In: Campbell CJ, Fuentes A, MacKinnon KC, Bearder SK, Stumpf RM, editors. Primates in perspective. New York: Oxford University Press; 2011. pp. 326–339. [Google Scholar]
- Saayman GS. The menstrual cycle and sexual behaviour in a troop of free ranging chacma baboons (Papio ursinus) Folia Primatol. 1970;12:81–110. doi: 10.1159/000155283. [DOI] [PubMed] [Google Scholar]
- Schreier A, Swedell L. The fourth level of social structure in a multi-level society: ecological and social functions of clans in hamadryas baboons. Am J Primatol. 2009;71:948–955. doi: 10.1002/ajp.20736. [DOI] [PubMed] [Google Scholar]
- Shaikh AA, Celaya CL, Gomez I, Shaikh SA. Temporal relationship of hormonal peaks to ovulation and sex skin deturgescence in the baboon. Primates. 1982;23:444–452. doi: 10.1007/BF02381326. [DOI] [Google Scholar]
- Sigg H, Stolba A, Abegglen JJ, Dasser V. Life history of hamadryas baboons: physical development, infant mortality, reproductive parameters and family relationships. Primates. 1982;23:473–487. doi: 10.1007/BF02373959. [DOI] [Google Scholar]
- Small MF. Female primate sexual behavior and conception. Are there really sperm to spare? Curr Anthropol. 1988;29:81–99. doi: 10.1086/203615. [DOI] [Google Scholar]
- Stammbach E. Desert, forest and montane baboons: multilevel societies. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: The University of Chicago Press; 1987. pp. 112–120. [Google Scholar]
- Stewart KJ, Harcourt AH. Gorilla society: conflict, compromise and cooperation between the sexes. Chicago: The University of Chicago Press; 2007. [Google Scholar]
- Strum SC. Agonistic dominance in male baboons: an alternative view. Int J Primatol. 1982;3:175–202. doi: 10.1007/BF02693494. [DOI] [Google Scholar]
- Swedell L. Affiliation among females in wild hamadryas baboons (Papio hamadryas hamadryas) Int J Primatol. 2002;23:1205–1226. doi: 10.1023/A:1021170703006. [DOI] [Google Scholar]
- Swedell L. Ranging behavior, group size and behavioral flexibility in Ethiopian hamadryas baboons (Papio hamadryas hamadryas) Folia Primatol. 2002;73:95–103. doi: 10.1159/000064787. [DOI] [PubMed] [Google Scholar]
- Swedell L. Strategies of sex and survival in hamadryas baboons: through a female lens. Upper Saddle River: Pearson Prentice Hall; 2006. [Google Scholar]
- Swedell L, Schreier A. Male aggression toward female hamadryas baboons: conditioning, coercion, and control. In: Muller MN, Wrangham RW, editors. Sexual coercion in primates and humans. an evolutionary perspective on male aggression against females. Cambridge: Harvard University Press; 2009. pp. 244–268. [Google Scholar]
- Tobler R, Pledger S, Linklater W. No evidence for ovarian synchrony or asynchrony in hamadryas baboons. Anim Behav. 2010;80:829–837. doi: 10.1016/j.anbehav.2010.07.018. [DOI] [Google Scholar]
- Wallis J. Chimpanzee genital swelling and its role in the pattern of sociosexual behavior. Am J Primatol. 1992;28:101–113. doi: 10.1002/ajp.1350280203. [DOI] [PubMed] [Google Scholar]
- Wildt DE, Doyle U, Stone SC, Harrison RM. Correlation of perineal swelling with serum ovarian hormone levels, vaginal cytology and ovarian follicular development during the baboon reproductive cycle. Primates. 1977;18:261–270. doi: 10.1007/BF02383104. [DOI] [Google Scholar]
- Yamane A, Shotake T, Mori A, Boug A, Iwamoto T. Extra-unit paternity of hamadryas baboons (Papio hamadryas) in Saudi Arabia. Ethol Ecol Evol. 2003;15:379–387. doi: 10.1080/08927014.2003.9522664. [DOI] [Google Scholar]
- Zinner D, Schwibbe M, Kaumanns W. Cycle synchrony and probability of conception in female hamadryas baboons (Papio hamadryas) Behav Ecol Sociobiol. 1994;35:175–183. doi: 10.1007/BF00167957. [DOI] [Google Scholar]
- Zinner D, Nunn CL, Schaik CP, Kappeler PM. Sexual selection and exaggerated sexual swellings of female primates. In: Kappeler PM, Schaik CP, editors. Sexual selection in primates. New and comparative perspectives. Cambridge: Cambridge University Press; 2004. pp. 71–89. [Google Scholar]
- Zinner D, Krebs E, Schrod A, Kaumanns W. Early sexual maturity in male hamadryas baboons (Papio hamadryas hamadryas) and its reproductive implications. Am J Phys Anthropol. 2006;129:584–590. doi: 10.1002/ajpa.20344. [DOI] [PubMed] [Google Scholar]


