Abstract
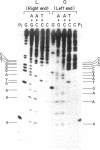
Phage phi 29 DNA cannot be phosphorylated with polynucleotide kinase and [gamma-32P]ATP because of the presence of a viral protein covalently linked to the 5' termini. The 5' ends can, however, be made susceptible to phosphorylation by treatment with alkali and alkaline phosphatase. Restriction fragments Hpa II C and Hpa II F, corresponding to the right and left ends of phi 29 DNA, respectively, were labeled at the 5' ends with polynucleotide kinase and [gamma-32P]ATP or at the 3' ends with terminal transferase and [alpha-32P]ATP or [alpha-32P]cordycepin 5'-triphosphate. After a secondary cleavage of the labeled fragments, the sequence of the first 150-180 nucleotides at the termini of phi 29 DNA was determined by the method of Maxam and Gilbert. The ends of phi 29 DNA are flush, and a six-nucleotides-long inverted terminal repetition was found. The functional implications of the sequences determined are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga H., Shimojo H., Hidaka S., Miura K. Specific cleavage of the terminal protein from the adenovirus 5 DNA under the condition of single-strand scission by nuclease S1. FEBS Lett. 1979 Nov 15;107(2):355–358. doi: 10.1016/0014-5793(79)80406-8. [DOI] [PubMed] [Google Scholar]
- Ariga H., Shimojo H. Initiation and termination sites of adenovirus 12 DNA replication. Virology. 1977 May 15;78(2):415–424. doi: 10.1016/0042-6822(77)90118-0. [DOI] [PubMed] [Google Scholar]
- Arrand J. R., Roberts R. J. The nucleotide sequences at the termini of adenovirus-2 DNA. J Mol Biol. 1979 Mar 15;128(4):577–594. doi: 10.1016/0022-2836(79)90294-8. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Osinga K. A., Van der Horst G., Borst P. Nucleotide sequence of the mitochondrial structural genes for cysteine-tRNA and histidine-tRNA of yeast. Nucleic Acids Res. 1979 Jul 25;6(10):3255–3266. doi: 10.1093/nar/6.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascosa J. L., Viñuela E., Salas M. Proteins induced in Bacillus subtilis infected with bacteriophage phi 29. Virology. 1973 Nov;56(1):291–299. [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz E., Shatkin A. J. Assignment of reovirus mRNA ribosome binding sites to virion genome segments by nucleotide sequence analyses. Nucleic Acids Res. 1980 Jan 25;8(2):337–350. doi: 10.1093/nar/8.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkema R., Dekker B. M. The inverted terminal repetition of the DNA of weakly oncogenic adenovirus type 7. Gene. 1979 Dec;8(1):7–15. doi: 10.1016/0378-1119(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Donelson J. E., Wu R. Nucleotide sequence analysis of deoxyribonucleic acid. VI. Determination of 3'-terminal dnucleotide sequences of several species of duplex deoxyribonucleic acid using Escherichia coli deoxyribonucleic acid polymerase I. J Biol Chem. 1972 Jul 25;247(14):4654–4660. [PubMed] [Google Scholar]
- Harding N. E., Ito J. DNA replication of bacteriophage phi 29: characterization of the intermediates and location of the termini of replication. Virology. 1980 Jul 30;104(2):323–338. doi: 10.1016/0042-6822(80)90337-2. [DOI] [PubMed] [Google Scholar]
- Harding N. E., Ito J., David G. S. Identification of the protein firmly bound to the ends of bacteriophage phi 29 DNA. Virology. 1978 Feb;84(2):279–292. doi: 10.1016/0042-6822(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Hawley L. A., Reilly B. E., Hagen E. W., Anderson D. L. Viral protein synthesis in bacteriophage phi 29-infected Bacillus subtilis. J Virol. 1973 Nov;12(5):1149–1159. doi: 10.1128/jvi.12.5.1149-1159.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso J. M., Salas M. Protein p3 is linked to the DNA of phage phi 29 through a phosphoester bond between serine and 5'-dAMP. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6425–6428. doi: 10.1073/pnas.77.11.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobom G., Grosschedl R., Lusky M., Scherer G., Schwarz E., Kössel H. Functional analysis of the replicator structure of lambdoid bacteriophage DNAs. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):165–178. doi: 10.1101/sqb.1979.043.01.023. [DOI] [PubMed] [Google Scholar]
- Inciarte M. R., Lázaro J. M., Salas M., Vińuela E. Physical map of bacteriophage phi29 DNA. Virology. 1976 Oct 15;74(2):314–323. [PubMed] [Google Scholar]
- Inciarte M. R., Salas M., Sogo J. M. Structure of replicating DNA molecules of Bacillus subtilis bacteriophage phi 29. J Virol. 1980 Apr;34(1):187–199. doi: 10.1128/jvi.34.1.187-199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. Bacteriophage phi29 terminal protein: its association with the 5' termini of the phi29 genome. J Virol. 1978 Dec;28(3):895–904. doi: 10.1128/jvi.28.3.895-904.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lechner R. L. The structure of replicating adenovirus DNA molecules: characterization of DNA-protein complexes from infected cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):721–728. doi: 10.1101/sqb.1979.043.01.080. [DOI] [PubMed] [Google Scholar]
- Lechner R. L., Kelly T. J., Jr The structure of replicating adenovirus 2 DNA molecules. Cell. 1977 Dec;12(4):1007–1020. doi: 10.1016/0092-8674(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantei N., Schwarzstein M., Streuli M., Panem S., Nagata S., Weissmann C. The nucleotide sequence of a cloned human leukocyte interferon cDNA. Gene. 1980 Jun;10(1):1–10. doi: 10.1016/0378-1119(80)90137-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McConnell D. J. The DNA sequence at the T7 C promoter. Nucleic Acids Res. 1979 Feb;6(2):525–544. doi: 10.1093/nar/6.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado R. P., Moreno F., Viñuela E., Salas M., Reilly B. E., Anderson D. L. Genetic analysis of bacteriophage phi 29 of Bacillus subtilis: integration and mapping of reference mutants of two collections. J Virol. 1976 Aug;19(2):495–500. doi: 10.1128/jvi.19.2.495-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado R. P., Peñalva M. A., Inciarte M. R., Salas M. The protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi 29 is involved in the initiation of DNA replication. Virology. 1980 Jul 15;104(1):84–96. doi: 10.1016/0042-6822(80)90367-0. [DOI] [PubMed] [Google Scholar]
- Messer W., Meijer M., Bergmans H. E., Hansen F. G., von Meyenburg K., Beck E., Schaller H. Origin of replication, oriC, of the Escherichia coli K12 chromosome: nucleotide sequence. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):139–145. doi: 10.1101/sqb.1979.043.01.020. [DOI] [PubMed] [Google Scholar]
- Moore D. D., Denniston-Thompson K., Kruger K. E., Furth M. E., Williams B. G., Daniels D. L., Blattner F. R. Dissection and comparative anatomy of the origins of replication of lambdoid phages. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):155–163. doi: 10.1101/sqb.1979.043.01.022. [DOI] [PubMed] [Google Scholar]
- Reiter T., Fütterer J., Weingärtner B., Winnacker E. L. Initiation of adenovirus DNA replication. J Virol. 1980 Sep;35(3):662–671. doi: 10.1128/jvi.35.3.662-671.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Bodnar J. W., Coombs D. H., Pearson G. D. Replicating adenovirus 2 DNA molecules contain terminal protein. Virology. 1979 Jul 15;96(1):143–158. doi: 10.1016/0042-6822(79)90180-6. [DOI] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Wells R. D. The synthesis and purification of (gamma-32P)-adenosine triphosphate with high specific activity. J Biol Chem. 1973 Dec 10;248(23):8319–8321. [PubMed] [Google Scholar]
- Shinagawa M., Padmanabhan R. Comparative sequence analysis of the inverted terminal repetitions from different adenoviruses. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3831–3835. doi: 10.1073/pnas.77.7.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa M., Padmanabhan R. Nucleotide sequence at the inverted terminal repetition of adenovirus type 2 DNA. Biochem Biophys Res Commun. 1979 Apr 13;87(3):671–678. doi: 10.1016/0006-291x(79)92011-4. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Inciarte M. R., Corral J., Viñuela E., Salas M. RNA polymerase binding sites and transcription map of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1979 Feb 5;127(4):411–436. doi: 10.1016/0022-2836(79)90230-4. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Rodeño P., Koller T., Viñuela E., Salas M. Comparison of the A-T rich regions and the Bacillus subtilis RNA polymerase binding sites in phage phi 29 DNA. Nucleic Acids Res. 1979 Sep 11;7(1):107–120. doi: 10.1093/nar/7.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergh P. H., Maat J., van Ormondt H., Sussenbach J. S. The nucleotide sequence at the termini of adenovirus type 5 DNA. Nucleic Acids Res. 1977 Dec;4(12):4371–4389. doi: 10.1093/nar/4.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergh P. H., Sussenbach J. S. The nucleotide sequence of the right-hand terminus of adenovirus type 5 DNA: implications for the mechanism of DNA replication. Gene. 1979 Aug;6(4):307–318. doi: 10.1016/0378-1119(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Bellett A. J. An adenovirus protein associated with the ends of replicating DNA molecules. Virology. 1979 Feb;93(1):69–79. doi: 10.1016/0042-6822(79)90276-9. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Kuijk M. G. Studies on the mechanism of replication of adenovirus DNA. V. The location of termini of replication. Virology. 1977 Mar;77(1):149–157. doi: 10.1016/0042-6822(77)90414-7. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Kuijk M. G. The mechanism of replication of adenovirus DNA. VI. Localization of the origins of the displacement synthesis. Virology. 1978 Feb;84(2):509–517. doi: 10.1016/0042-6822(78)90266-0. [DOI] [PubMed] [Google Scholar]
- Talkington C., Pero J. Distinctive nucleotide sequences of promoters recognized by RNA polymerase containing a phage-coded "sigma-like" protein. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5465–5469. doi: 10.1073/pnas.76.11.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Aleström P., Pettersson U. Sequence of inverted terminal repetitions from different adenoviruses: demonstration of conserved sequences and homology between SA7 termini and SV40 DNA. Cell. 1979 Jul;17(3):705–713. doi: 10.1016/0092-8674(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Yehle C. O. Genome-linked protein associated with the 5' termini of bacteriophage phi29 DNA. J Virol. 1978 Sep;27(3):776–783. doi: 10.1128/jvi.27.3.776-783.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Friedmann T., Ito J. Nucleotide sequences at the termini of phi 29 DNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1336–1340. doi: 10.1073/pnas.78.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., Kleppe K., Khorana H. G. Reversal of bacteriophage T4 induced polynucleotide kinase action. Biochemistry. 1973 Dec 4;12(25):5050–5055. doi: 10.1021/bi00749a004. [DOI] [PubMed] [Google Scholar]