Summary
Background
Findings of small studies have suggested that short treatments with anti-CD3 monoclonal antibodies that are mutated to reduce Fc receptor binding preserve β-cell function and decrease insulin needs in patients with recent-onset type 1 diabetes. In this phase 3 trial, we assessed the safety and efficacy of one such antibody, teplizumab.
Methods
In this 2-year trial, patients aged 8–35 years who had been diagnosed with type 1 diabetes for 12 weeks or fewer were enrolled and treated at 83 clinical centres in North America, Europe, Israel, and India. Participants were allocated (2:1:1:1 ratio) by an interactive telephone system, according to computer-generated block randomisation, to receive one of three regimens of teplizumab infusions (14-day full dose, 14-day low dose, or 6-day full dose) or placebo at baseline and at 26 weeks. The Protégé study is still underway, and patients and study staff remain masked through to study closure. The primary composite outcome was the percentage of patients with insulin use of less than 0.5 U/kg per day and glycated haemoglobin A1c (HbA1C) of less than 6.5% at 1 year. Analyses included all patients who received at least one dose of study drug. This trial is registered with ClinicalTrials.gov, number NCT00385697.
Findings
763 patients were screened, of whom 516 were randomised to receive 14-day full-dose teplizumab (n=209), 14-day low-dose teplizumab (n=102), 6-day full-dose teplizumab (n=106), or placebo (n=99). Two patients in the 14-day full-dose group and one patient in the placebo group did not start treatment, so 513 patients were eligible for efficacy analyses. The primary outcome did not differ between groups at 1 year: 19·8% (41/207) in the 14-day full-dose group; 13·7% (14/102) in the 14-day low-dose group; 20·8% (22/106) in the 6-day full-dose group; and 20·4% (20/98) in the placebo group. 5% (19/415) of patients in the teplizumab groups were not taking insulin at 1 year, compared with no patients in the placebo group at 1 year (p=0·03). Across the four study groups, similar proportions of patients had adverse events (414/417 [99%] in the teplizumab groups vs 98/99 [99%] in the placebo group) and serious adverse events (42/417 [10%] vs 9/99 [9%]). The most common clinical adverse event in the teplizumab groups was rash (220/417 [53%] vs 20/99 [20%] in the placebo group).
Interpretation
Findings of exploratory analyses suggest that future studies of immunotherapeutic intervention with teplizumab might have increased success in prevention of a decline in β-cell function (measured by C-peptide) and provision of glycaemic control at reduced doses of insulin if they target patients early after diagnosis of diabetes and children.
Introduction
In type 1 diabetes mellitus, pancreatic insulin-secreting β cells are progressively destroyed by autoreactive CD4+ and CD8+ lymphocytes.1 When clinical hyperglycaemia occurs, about 30% of β-cell function remains intact, but these cells are not fully functional because of inflam-mation and glucotoxicity.2,3 Residual endogenous insulin secretion synergises with exogenous insulin therapy to create an interim period with fewer hypoglycaemic events and markedly lower overall glycaemia.4 Immunotherapy aims to preserve endogenous insulin secretion, by attenuation of the activated, autoreactive T cells that probably mediate β-cell killing, to prolong this interim period and lessen complications.4 However, in view of the long experience with exogenous insulin therapy and the slow appearance of serious complications, new inter ventions should have reasonably low systemic toxic effects.
Regimens of chronic immunosuppression—eg, ciclo-sporin—have shown promise for attenuation of the loss of insulin secretion in new-onset disease, but have unacceptable toxic effects (potential risk of infections and tumours from continuous immunosuppression and nephrotoxicity). Antigen-specific therapies to restore β-cell tolerance have shown low toxic effects but little efficacy.5,6 Non-antigen-specific short-course therapies, such as anti-CD3 and anti-CD20, have had more success.7,8 Of these, anti-CD3 had a durable effect, with efficacy up to 4 years after one 1-week treatment in a pilot study, and longlasting efficacy in non-obese diabetic mice.9,10
Teplizumab is a humanised, anti-CD3 monoclonal antibody that has been mutated to greatly reduce Fc receptor and complement binding.11 In an early trial of anti-CD3 antibody,12 24 patients with recent-onset diabetes were randomised equally to receive open-label teplizumab (34 mg cumulative dose for one 14-day course in a 70 kg individual) or no antibody for 14 days, with daily dose based on previous transplantation trials. At 12 months, C-peptide response to a mixed meal was maintained in 60% of treated patients versus 8% of controls (p<0·03).
In a trial of otelixizumab,13 another monoclonal anti-CD3 antibody with reduced binding to the Fc receptor, β-cell function was preserved in patients receiving otelixizumab and their insulin needs were decreased up to 48 months after treatment. Adverse events, including Epstein-Barr virus reactivation, were more frequent than in the teplizumab trial,12 which is consistent with the higher cumulative dose.14 A much lower dose of 3·1 mg otelixizumab was subsequently used in a phase 3 trial, but the primary efficacy outcome of change in C-peptide at month 12 was not met.15
We undertook a phase 3, multicentre, randomised study (Protégé) to assess the safety and efficacy of teplizumab, and we report results at 1 year. By contrast with previous studies of one dose cycle, our study included a second dose cycle at 6 months.
Methods
Patients
The Protégé study was undertaken in 83 academic centres, hospitals, and clinics in North America (USA, Canada, and Mexico), India, Israel, and Europe (Czech Republic, Estonia, Germany, Latvia, Poland, Romania, Spain, Sweden, and Ukraine; webappendix pp 1–3). The study procedures in effect during the Protégé study are described most completely in protocol version 8; the statistical analysis was revised shortly before unblinding for analysis of the primary outcome and is described in protocol version 9. Participants were eligible if they met the following criteria: aged 8–35 years; body mass of at least 36 kg; type 1 diabetes mellitus diagnosed for 12 weeks or fewer, according to American Diabetes Association criteria,16 with need for injected insulin therapy; detectable fasting or stimulated C-peptide; and positive autoantibody titre against an islet-cell antigen (ICA-512/IA-2), glutamic acid decarboxylase (GAD 65), or insulin, within 2 weeks of initiating insulin treatment. Exclusion criteria focused on medical disorders that would potentially confound results or interfere with safe completion of the trial, including serious cardiovascular disorders, active infections, recent participation in a clinical trial, vaccination, or pregnancy (webappendix p 3).
The research protocol was approved by institutional review boards, and all participants or guardians gave written informed consent.
Randomisation and masking
An independent third party, United BioSource Corporation (San Francisco, CA, USA), generated the allocation schedule by computer with block random-isation (block size of five), and managed the distribution and assignment of study drugs at all sites via a controlled access interactive telephone system. Randomisation was stratified by country and age group (8–11, 12–17, and 18–35 years). Patients were randomly assigned (2:1:1:1 ratio) to receive treatment in one of four parallel groups: a 14-day course of escalating doses of intravenous teplizumab, with a total cumulative dose of about 9034 μg/m2 (14-day full-dose group); a 14-day course of escalating doses of intravenous teplizumab, with a total cumulative dose of about 2985 μg/m2 (14-day low-dose group); a 6-day course of escalating doses of intravenous teplizumab plus 8 days of intravenous placebo, with a total cumulative dose of about 2426 μg/m2 (6-day full-dose group); or a 14-day course of intravenous placebo (placebo group). All treatments were repeated at week 26. Further details about randomisation and dosing are provided in webappendix pp 3–4.
Dosing was double blind and double dummy, with use of two vials for each dose (full dose and low dose, or matching placebos) and numbered codes to conceal allocation. All patients and study personnel were masked to the treatment codes, block sizes, and laboratory measurements that might reveal allocation (such as serum teplizumab concentrations and T-cell proteins), with a few exceptions for safety issues, laboratory validation, and drug supply.
Procedures
From February, 2007, groups of about ten patients in each age stratum received open-label teplizumab according to the 14-day full-dose regimen to assess safety and tolerability before randomisation of patients in the double-blind study. Enrolment was staggered: patients aged 8–11 and 12–17 years were enrolled after the independent data monitoring committee reviewed and approved results from the initial dosing regimen of the age 18–35 group. Treatment was repeated at 26 weeks and patients were followed up for a total of 24 months, with the last patient’s visit in March, 2010. Data for the open-label phase are not presented here.
We did not prespecify regimens to adjust insulin use, but investigators were instructed to aggressively treat diabetes, attempt to keep HbA1C at 6·5% or lower, and maintain an insulin dose of at least 0·25 U/kg per day. Patients recorded insulin use in diary cards at screening and for 3 days before each visit at days 91, 140, 364, 546, and 728; daily doses in U/kg were calculated from these data and weight measurements from the corresponding office visits. Use of inhaled insulin, exenatide, or other agents that stimulate pancreatic β-cell regeneration or insulin secretion were not permitted during the study.
Blood samples were used to measure HbA1C and area under the curve (AUC) of C-peptide during 4 h after a mixed meal2 (webappendix p 5). Epstein-Barr virus (EBV) and cytomegalovirus (CMV) seropositivity were documented by measurement of anti-CMV IgG, anti-EBV IgG, or anti-EBV IgM at screening and days 28, 91, 140, 210, 273, 364, and 728. For seropositive patients, viral load was measured by use of semi-quantitative PCR (webappendix p 5).
Investigators reported abnormal laboratory values and other adverse events that were coded according to the Medical Dictionary for Regulatory Activities and graded according to the Common Terminology Criteria for Adverse Events (version 3.0). Clinically significant hypoglycaemic events, requiring assistance by another individual for parenteral dextrose, glucagon, or equivalent intervention, were reported as adverse events. Patients permanently discontinued treatment if they had protocol-defined dose-stopping events, including pregnancy, anaphylaxis, abnormal liver function tests, abnormal blood cell counts, or certain adverse events rated grade 3 or higher (webappendix pp 3–4). Many of the patients who stopped dosing continued to participate in the study. Patients received a non-steroidal anti-inflammatory drug for at least the first 5 days of the treatment cycle, or longer if the investigator judged it to be appropriate to prevent adverse events, and concomitant need for an antihistamine was decided by the investigator.
The trial is planned to last for 2 years, but we report results for the first year because analyses of primary and main secondary outcomes were prespecified to occur at 1 year. After the 1-year review by the data monitoring committee, the study was changed to continue safety assessments, but minimise blood draws for biomarker measurement. The primary composite outcome was the percentage of patients from each treatment group with insulin use of less than 0·5 U/kg per day and HbA1C of less than 6·5% at 1 year. A second primary outcome was mean change from baseline in HbA1C. Secondary outcomes at 1 year were mean change from baseline in AUC of C-peptide, and a composite of insulin use of less than 0·5 U/kg per day and HbA1C of less than 7%.
Statistical analysis
Statistical analyses were done in accordance with a prespecified analysis plan (webappendix p 6). The second primary analysis, secondary analyses, and post-hoc analyses were regarded as exploratory and hypothesis generating because they were originally planned only to be assessed if the primary outcome was significant. Patients with missing composite outcome data were counted as non-responders. Composite outcome analyses were done with Cochran-Mantel-Haenszel tests, stratified by age-group, to compare teplizumab groups with placebo. The target sample sizes were 200 patients for the 14-day full-dose group and 100 patients for each of the other three groups. With the planned sample sizes and an overall type 1 error rate of 0·05, the power for the primary composite outcome with a binomial test, which is more conservative than Cochran-Mantel-Haenszel test, was more than 99% to detect group differences of 50% versus 5% and about 90% for differences of 30% versus 5%; these differences were based on a previous study.12
ANCOVA models, adjusted for age-group and baseline values, were used to compare C-peptide and HbA1C values in teplizumab groups with the placebo group; missing values were imputed by use of last observation carried forward at the request of regulators. Safety analyses of adverse events and exploratory efficacy analyses of dichotomous outcomes were done with Fisher’s exact test. Analyses of treatment or region differences with respect to baseline characteristics were done with Cochran-Mantel-Haenszel tests for categorical outcomes or ANOVA tests for continuous outcomes. If normality assumptions relating to the ANOVA or ANCOVA models were not met, then post-hoc exploratory analyses used non-parametric Wilcoxon rank-sum tests to detect differences between treatment groups.
Two-sided testing was done at an α level of 0·05. All efficacy analyses controlled for multiple treatment group comparisons with placebo.17 However, because the primary outcome did not differ, subsequent analyses compared the 14-day full-dose regimen with placebo. These analyses were done for hypothesis generation, therefore no adjustment for multiple comparisons was made. Primary and secondary efficacy analyses included all patients who received at least one dose of study drug, and safety analyses included all randomly assigned patients. The statistical analysis plan predefined subgroup analyses of the primary and main secondary outcomes based on age group (8–11, 12–17, and >17 years), geographical region (USA, India, Europe and Israel, and Mexico and Canada), and time from diagnosis (defined as first physician visit related to diabetes symptoms) to randomisation (≤6 weeks and >6 weeks). SAS (version 9.2) was used for all analyses.
This trial is registered with ClinicalTrials.gov, number NCT00385697.
Role of the funding source
The study was designed by MacroGenics, with substantial input from advisers and site investigators. MacroGenics had direct oversight or participation in every stage of the study, including pharmacovigilance, data collection, data interpretation, and writing of the report. Data analyses were done by MacroGenics and an independent academic centre (Data Analysis Center, Department of Biostatistics and Medical Informatics, University of Wisconsin, Madison, WI, USA); the centre provided data to the independent data monitoring committee, which was supplemented by information from MacroGenics as requested. After initiation of the trial, Eli Lilly partnered with MacroGenics for development of teplizumab. The Juvenile Diabetes Research Foundation and Eli Lilly did not participate in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to the data, and the corresponding author had final responsibility for the decision to submit for publication.
Results
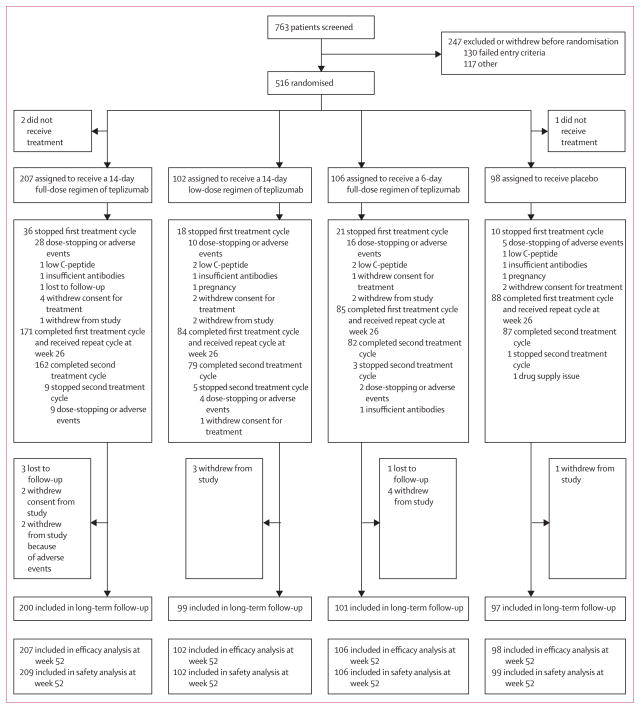
Between July, 2007, and June, 2009, 763 patients were screened for the double-blind study, of whom 516 were randomised (figure 1). All randomised patients were included in safety analyses, and 513 patients who received treatment were eligible for primary and secondary efficacy analyses. In the first treatment cycle, 85 patients stopped treatment because of protocol-defined dose-stopping events, adverse events, or other reasons (withdrawal from treatment or study, low C-peptide at day 140, insufficient autoantibodies at study entry, pregnancy, or loss to follow-up). In the second treatment cycle at week 26, 18 of 428 patients stopped treatment because of dose-stopping events, adverse events, or other reasons (withdrawal of dosing, insufficient autoantibodies at study entry, or drug supply issue). 1-year follow-up was completed by August, 2010. Many patients who stopped or withdrew from treatment continued to participate; 12 patients withdrew completely from study participation and four patients were lost to follow-up, leaving 497 patients who continued long-term follow-up after 1 year.
Figure 1.
Trial profile
Baseline characteristics were well balanced across treatment groups (table 1), but varied by region (webappendix p 7). Most notably, patients in India had higher HbA1C and insulin use, lower AUC of C-peptide (characteristic of later disease stage), and lower frequency of anti-ICA512 antibodies than did those in other regions.
Table 1.
Demographic and clinical characteristics at baseline
| 14-day full-dose group (n=207) | 14-day low-dose group (n=102) | 6-day full-dose group (n=106) | Placebo group (n=98) | |
|---|---|---|---|---|
| Age (years) | 18·9 (7·6) | 17·9 (6·1) | 18·1 (6·9) | 18·2 (7·3) |
| 8–11 | 31 (15%) | 15 (15%) | 16 (15%) | 15 (15%) |
| 12–17 | 81 (39%) | 42 (41%) | 44 (42%) | 38 (39%) |
| 18–35 | 95 (46%) | 45 (44%) | 46 (43%) | 45 (46%) |
|
| ||||
| Male sex | 130 (63%) | 62 (61%) | 72 (68%) | 61 (62%) |
|
| ||||
| White | 146 (71%) | 74 (73%) | 75 (71%) | 69 (70%) |
|
| ||||
| Time from diagnosis to randomisation (weeks) | 8·4 (2·6) | 8·4 (2·6) | 9·0 (4·5) | 8·3 (2·6) |
|
| ||||
| History of ketoacidosis | 76 (37%) | 37 (36%) | 45 (42%) | 33 (34%) |
|
| ||||
| Number of positive autoantibodies* | ||||
| 1 | 18 (9%) | 13 (13%) | 15 (14%) | 11 (11%) |
| 2 | 91 (44%) | 38 (37%) | 45 (43%) | 45 (46%) |
| 3 | 98 (47%) | 51 (50%) | 45 (43%) | 42 (43%) |
|
| ||||
| Antibody type | ||||
| GAD 65 (≥0·5 U/mL) | 194 (94%) | 90 (88%) | 91 (86%) | 89 (91%) |
| Human insulin (≥2·0 U/mL) | 187 (90%) | 94 (92%) | 89 (84%) | 88 (90%) |
| Islet cell 512 (≥0·8 U/mL) | 113 (55%) | 58 (57%) | 60 (57%) | 53 (54%) |
|
| ||||
| HbA1C (%) | 8·3% (2·0) | 8·4% (2·1) | 8·1% (1·8) | 8·2% (2·0) |
|
| ||||
| AUC of C-peptide (nmol/L per min) | 0·65 (0·54) | 0·69 (0·45) | 0·68 (0·40) | 0·65 (0·44) |
|
| ||||
| Total insulin dose (U/kg per day) | 0·63 (0·42) | 0·68 (0·41) | 0·63 (0·39) | 0·65 (0·32) |
Data are mean (SD) or number (%). HbA1C=glycated haemoglobin A1C. AUC=area under the curve.
Data are missing for one patient in the 6-day full-dose group.
HbA1C and insulin use decreased after patients entered the study, and then increased after day 91 (webappendix pp 8–9). Mean changes in HbA1C from baseline were essentially identical in the 14-day full-dose and placebo groups at all times. The 14-day full-dose group had numerically larger decreases in mean insulin use relative to the placebo group, and the difference was maintained at all times (webappendix pp 8–9). Differences between the 14-day full-dose and placebo groups were not significant for the primary and secondary outcomes (table 2). The amount of missing data for these outcomes was generally small. Results were largely unchanged in sensitivity analyses in which alternative methods were used for imputation of missing data.
Table 2.
Primary, secondary, and post-hoc exploratory outcomes at 1 year
| 14-day full-dose group (n=207) | 14-day low-dose group (n=102) | 6-day full-dose group (n=106) | Placebo group (n=98) | p value* | |
|---|---|---|---|---|---|
|
Primary outcomes
| |||||
| Composite of HbA1c <6·5% and insulin dose <0·5 U/kg per day | 19·8% (n=41) | 13·7% (n=14) | 20·8% (n=22) | 20·4% (n=20) | 0·904 |
| Change in HbA1c from baseline (%) | −0·41% (2·3) | −0·33% (2·2) | −0·36% (2·1) | −0·40% (2·7) | 0·659 |
|
| |||||
|
Secondary outcomes
| |||||
| Change in AUC of C-peptide from baseline (nmol/L per min) | −0·06 (0·36) | −0·14 (0·30) | −0·07 (0·39) | −0·09 (0·42) | 0·382 |
| Composite of HbA1c <7% and insulin dose <0·5 U/kg per day | 29·0% (n=60) | 20·6% (n=21) | 26·4% (n=28) | 24·5% (n=24) | 0·404 |
|
| |||||
|
Post-hoc exploratory outcomes
| |||||
| HbA1c (%) | 7·9% (2·3) | 8·0% (2·2) | 7·7% (1·9) | 7·8% (2·4) | 0·978 |
| Change in insulin use from baseline (U/kg per day) | −0·04 (0·58); −0·07 (0·38)† | −0·07 (0·35) | −0·02 (0·35) | 0·01 (0·31) | 0·601 |
| Median change in AUC of C-peptide from baseline (nmol/L per min; IQR) | −0·06 (−0·25 to 0·12) | −0·13 (−0·33 to 0·01) | −0·08 (−0·31 to 0·11) | −0·14 (−0·30 to 0·02) | 0·046 |
| Composite of HbA1c <7·0% and insulin dose <0·25 U/kg per day | 13·0%(n=27) | 8·8% (n=9) | 9·4% (n=10) | 3·1% (n=3) | 0·006 |
Data are percentage (n) or mean (SD), unless otherwise indicated. HbA1C=glycated haemoglobin A1C. AUC=area under the curve.
14-day full-dose group versus placebo group.
Excludes one outlier with insulin use of 8·5 U/kg per day.
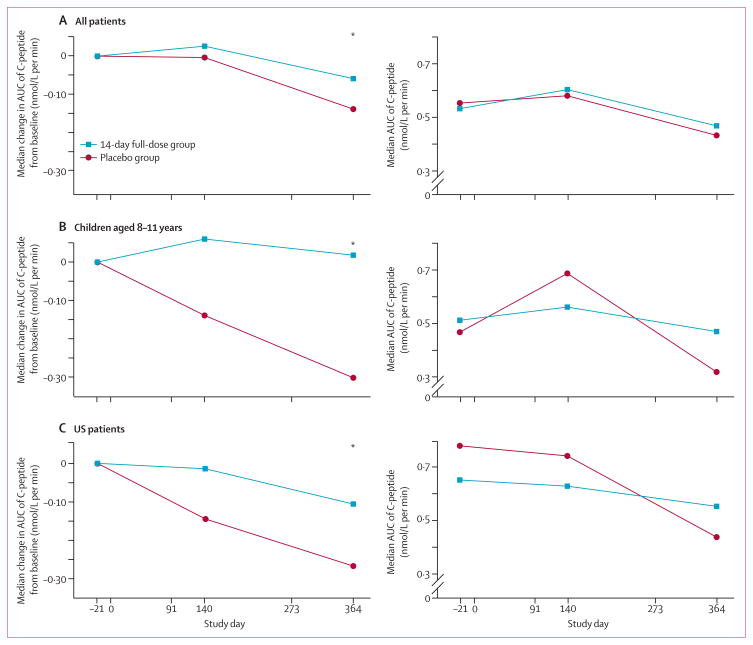
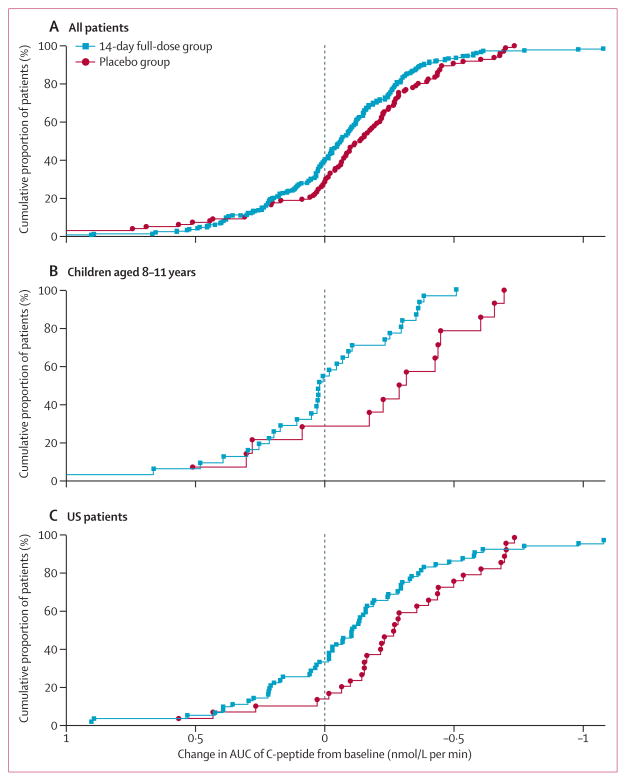
AUC of C-peptide was analysed with non-parametric methods because data were not normally distributed. Median change from baseline to 1 year showed less decline in the 14-day full-dose group than in the placebo group (figure 2A, table 2). The shift between treatment groups is evident from the empirical cumulative distribution curves in the overall population, in which 40% of the 14-day full-dose group had a preservation or increase in AUC of C-peptide relative to baseline compared with 28% of the placebo group (figure 3A).
Figure 2. Change in AUC of C-peptide from baseline over time and AUC of C-peptide over time.
(A) All patients. (B) Children aged 8–11 years. (C) US patients. Statistical testing was not done on absolute AUC values. AUC=area under the curve. *p<0·05 with Wilcoxon rank-sum test.
Figure 3. Cumulative distribution of change in AUC of C-peptide from baseline at 1 year.
(A) All patients (n=298). (B) Children aged 8–11 years. (C) US patients. Because of violations of normality assumptions, inferential comparisons were made with non-parametric methods (Wilcoxon rank-sum test). AUC=area under the curve.
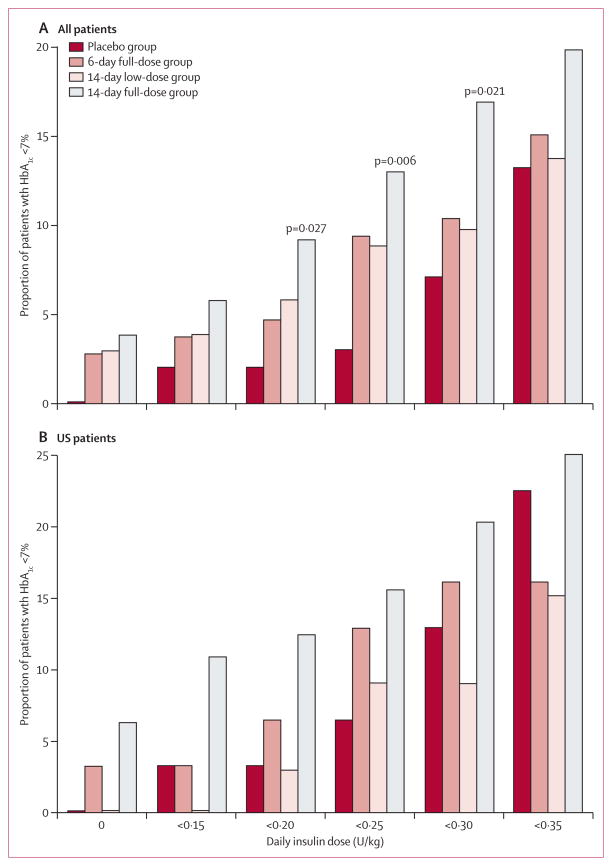
Change in insulin use from baseline did not differ between the 14-day full-dose group and placebo (table 2). However, at any given HbA1C threshold, a greater percentage of patients in the 14-day full-dose group achieved the threshold at lower insulin dose cutoffs than in the placebo group (figure 4A, webappendix p 10). This effect was most notable at insulin doses of lower than 0·5 U/kg per day. 5% (19/415) of patients receiving teplizumab were not taking insulin at 1 year compared with no patients in the placebo group (p=0·03), and 15 of these 19 patients had HbA1C of less than 7%. In the Diabetes Prevention Trial,5 insulin was given to the relatives of patients with type 1 diabetes at a dose of 0·25 U/kg per day because this dose did not increase the occurrence of hypoglycaemia.5 We therefore used the dose of 0·25 U/kg per day as a cutoff in subsequent analyses. The proportion of patients who achieved HbA1C of less than 7% and insulin use of less than 0·25 U/kg per day was greater in the 14-day full-dose group than in the placebo group for the study overall (table 2, figure 4A, webappendix p 10) and at each study visit (figure 5). A similar effect was seen at HbA1C of less than 6·5% and insulin use of less than 0·25 U/kg per day (p=0·02).
Figure 4. Proportion of patients with HbA1c <7% by daily insulin dose cutoffs.
(A) All patients. (B) US Patients. Reported p values are for the 14-day full-dose group versus the placebo group, and were calculated with Fisher’s exact test; p values are not shown when p>0·05. Patients with missing values are counted as not meeting criteria. HbA1c=glycated haemoglobin A1c.
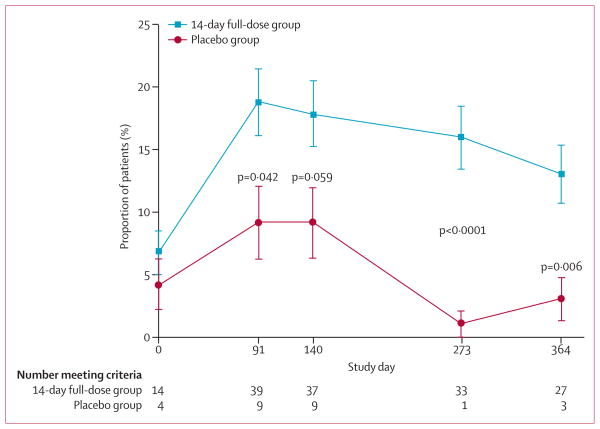
Figure 5. Proportion of patients who met the post-hoc exploratory outcome of HbA1C <7% and insulin use <0·25 U/kg per day at each study visit.
HbA1c=glycated haemoglobin A1c.
The lower reduction in median AUC of C-peptide in the 14-day full-dose group than in the placebo group was evident in three predefined subgroups (table 3): children aged 8–11 years (figure 2B, figure 3B), the US region (figure 2B, figure 3C), and patients randomised at 6 weeks or fewer after diagnosis (webappendix p 11). Because of violations of normality assumptions, inferen tial comparisons were made with non-parametric methods.
Table 3.
Non-parametric summary statistics and subgroup analysis for change in AUC of C-peptide from baseline to year 1
| 14-day full-dose group (n=207) | 14-day low-dose group (n=102) | 6-day full-dose group (n=106) | Placebo group (n=98) | p value | |
|---|---|---|---|---|---|
|
Region
| |||||
| USA | −0·10 (n=63; −0·30 to 0·16) | −0·17 (n=32; −0·29 to 0·02) | −0·18 (n=30; −0·43 to 0·11) | −0·27 (n=30; −0·50 to −0·14) | 0·015 |
| India | −0·00 (n=55; −0·15 to 0·24) | −0·09 (n=27; −0·35 to 0·00) | −0·06 (n=27; −0·15 to 0·42) | −0·04 (n=27; −0·13 to 0·31) | 0·824 |
| Europe and Israel | −0·11 (n=70; −0·25 to 0·03) | −0·15 (n=37; −0·39 to 0·01) | −0·05 (n=36; −0·26 to 0·16) | −0·14 (n=34; −0·27 to 0·01) | 0·422 |
| Canada and Mexico | 0·01 (n=13; −0·02 to 0·21) | −0·02 (n=4; −0·31 to 0·36) | −0·09 (n=9; −0·38 to 0·04) | 0·03 (n=5; −0·19 to 0·23) | 0·657 |
|
| |||||
|
Age-group (years)
| |||||
| 8–11 | 0·02 (n=31; −0·25 to 0·20) | −0·17 (n=15; −0·34 to 0·01) | −0·22 (n=14; −0·38 to −0·03) | −0·30 (n=14; −0·45 to 0·09) | 0·047 |
| 12–17 | −0·09 (n=78; −0·27 to 0·03) | −0·22 (n=40; −0·39 to −0·02) | −0·014 (n=42; −0·40 to 0·04) | −0·20 (n=38; −0·29 to −0·02) | 0·114 |
| 18–35 | −0·06 (n=92; −0·22 to 0·13) | −0·05 (n=45; −0·26 to 0·09) | −0·00 (n=46; −0·12 to 0·30) | −0·07 (n=44; −0·27 to 0·10) | 0·959 |
|
| |||||
|
Time from diagnosis to randomisation (weeks)
| |||||
| ≤6 | 0·02 (n=41; −0·13 to 0·39) | −0·04 (n=20; −0·27 to 0·11) | 0·17 (n=14; −0·11 to 0·30) | −0·15 (n=16; −0·38 to 0·09) | 0·020 |
| >6 | −0·09 (n=160; −0·27 to 0·06) | −0·17 (n=80; −0·37 to 0·01) | −0·10 (n=88; −0·35 to 0·05) | −0·14 (n=80; −0·28 to 0·02) | 0·332 |
Data are median (n; IQR) and are in units of nmol/L per min. Missing values were imputed with last observation carried forward. 14 patients (six in the 14-day full-dose group, two in the 14-day low-dose group, four in the 6-day full-dose group, and two in the placebo group) were not included in the last-observation-carried-forward analyses because no C-peptide data were obtained. AUC=area under the curve.
14-day full-dose group versus placebo group; Wilcoxon rank-sum test.
In the subgroup of the US region, the proportion of patients who achieved an HbA1c of less than 7% at each low dose insulin cutoff was higher in the 14-day full-dose group than in the placebo group (figure 4B). A similar pattern was seen in children aged 8–11 years and patients randomised at no more than 6 weeks after diagnosis, but data are not shown because sample sizes were small. Furthermore, in the subgroups of children aged 8–11 years, the US region, and patients randomised at 6 weeks or fewer after diagnosis, the proportion of patients who achieved HbA1C of less than 7% and insulin use of less than 0·25 U/kg per day was greater in the 14-day full-dose group than in the placebo group at all timepoints (webappendix pp 12–14).
In the safety analyses, the proportion of patients who had adverse events (512/516 [99%]) and serious adverse events (51/516 [10%]) was similar across the four study groups (table 4). The proportion of patients with infection did not differ between the four treatment groups (table 4). Although the study population had a high occurrence of anti-EBV IgG at baseline (441/513 [86%]), 22 of 415 (5%) patients receiving teplizumab became positive for anti-EBV IgG or IgM during the study compared with seven of 98 (7%) receiving placebo, and only one patient (teplizumab group) had a small transient increase in EBV viral load. 77% (318/415) of patients treated with teplizumab (150/207 [72%] in the 14-day full-dose group, 78/102 [76%] in the 14-day low dose group, and 90/106 [85%] in the 6-day full-dose group) and 13% (13/98) of those receiving placebo developed anti-drug antibodies, defined as a titre of more than 1:100 when samples were obtained at 28 or 56 days.
Table 4.
All serious adverse events, adverse events occurring in 10% or more of patients in any treatment group, and adverse events of special interest
| 14-day full-dose group (n=209) | 14-day low-dose group (n=102) | 6-day full-dose group (n=106) | Placebo group (n=99) | |
|---|---|---|---|---|
|
Any adverse event
| ||||
| Total adverse events | 207 (99%) | 101 (99%) | 106 (100%) | 98 (99%) |
| Blood and lymphatic system disorders | 181 (87%)* | 88 (86%)* | 85 (80%)* | 51 (52%) |
| Lymphopenia | 153 (73%)* | 70 (69%)* | 79 (75%)* | 19 (19%) |
| Leukopenia | 98 (47%)* | 49 (48%)* | 50 (47%)* | 23 (23%) |
| Neutropenia | 76 (36%)* | 39 (38%)* | 21 (20%) | 20 (20%) |
| Anaemia | 30 (14%) | 13 (13%) | 10 (9%) | 13 (13%) |
| Thrombocytopenia | 21 (10%) | 8 (8%) | 15 (14%) | 10 (10%) |
| Gastrointestinal disorders | 71 (34%) | 31 (30%) | 44 (42%)* | 26 (26%) |
| Nausea | 41 (20%) | 16 (16%) | 21 (20%) | 11 (11%) |
| Vomiting | 30 (14%)* | 8 (8%) | 14 (13%)* | 5 (5%) |
| General disorders and administration site conditions | 89 (43%) | 41 (40%) | 44 (42%) | 36 (36%) |
| Pyrexia | 44 (21%) | 18 (18%) | 28 (26%) | 20 (20%) |
| Fatigue | 22 (11%) | 9 (9%) | 15 (14%)* | 5 (5%) |
| Chills | 20 (10%)* | 5 (5%) | 13 (12%)* | 2 (2%) |
| Hepatobiliary disorders | 24 (12%) | 10 (10%) | 9 (9%) | 9 (9%) |
| Immune system disorders | 18 (9%) | 3 (3%) | 9 (9%) | 3 (3%) |
| Cytokine release syndrome† | 12 (6%)* | 2 (2%) | 8 (8%)* | 0 |
| Infections and infestations | 94 (45%) | 53 (52%) | 55 (52%) | 54 (55%) |
| Upper respiratory tract infection | 26 (12%) | 19 (19%) | 21 (20%) | 15 (15%) |
| Nasopharyngitis | 21 (10%) | 9 (9%) | 13 (12%) | 11 (11%) |
| Acute mononucleosis-like syndrome† | 15 (7%) | 4 (4%) | 5 (5%) | 8 (8%) |
| Injury, poisoning and procedural complications | 22 (11%) | 8 (8%) | 12 (11%) | 8 (8%) |
| Laboratory investigations | 190 (91%) | 93 (91%) | 99 (93%)* | 84 (85%) |
| Blood bicarbonate decreased | 83 (40%) | 57 (56%)* | 38 (36%) | 36 (36%) |
| Haemoglobin decreased | 66 (32%) | 32 (31%) | 38 (36%) | 30 (30%) |
| Aspartate aminotransferase increased | 72 (34%) | 25 (25%) | 35 (33%) | 30 (30%) |
| White blood cell count decreased | 79 (38%)* | 27 (27%) | 34 (32%)* | 18 (18%) |
| Alanine aminotransferase increased | 72 (34%)* | 25 (25%) | 31 (29%)* | 16 (16%) |
| Lymphocyte count decreased | 46 (22%)* | 16 (16%) | 25 (24%)* | 11 (11%) |
| Neutrophil count decreased | 41 (20%) | 17 (17%) | 25 (24%) | 14 (14%) |
| Blood alkaline phosphatase increased | 28 (13%)* | 18 (18%) | 16 (15%) | 25 (25%) |
| Blood sodium decreased | 37 (18%) | 17 (17%) | 14 (13%) | 15 (15%) |
| Platelet count decreased | 32 (15%) | 12 (12%) | 15 (14%) | 9 (9%) |
| Blood calcium decreased | 26 (12%) | 12 (12%) | 11 (10%) | 9 (9%) |
| γ-glutamyltransferase increased | 9 (4%)* | 7 (7%) | 5 (5%) | 10 (10%) |
| Metabolism and nutrition disorders | 133 (64%) | 64 (63%) | 69 (65%) | 64 (65%) |
| Hyponatraemia | 66 (32%) | 38 (37%) | 39 (37%) | 33 (33%) |
| Hypocalcaemia | 55 (26%) | 21 (21%) | 29 (27%) | 24 (24%) |
| Hypoalbuminaemia | 20 (10%) | 9 (9%) | 13 (12%) | 8 (8%) |
| Hypokalaemia | 16 (8%) | 7 (7%) | 9 (9%) | 11 (11%) |
| Hyperkalaemia | 20 (10%) | 11 (11%) | 12 (11%) | 11 (11%) |
| Musculoskeletal and connective tissue disorders | 29 (14%) | 17 (17%) | 14 (13%) | 8 (8%) |
| Nervous system disorders | 67 (32%) | 30 (29%) | 30 (28%) | 23 (23%) |
| Headache | 53 (25%)* | 25 (25%) | 26 (25%) | 15 (15%) |
| Renal and urinary disorders | 26 (12%) | 19 (19%) | 18 (17%) | 9 (9%) |
| Proteinuria | 24 (12%) | 17 (17%) | 12 (11%) | 9 (9%) |
| Respiratory, thoracic, and mediastinal disorders | 43 (21%) | 17 (17%) | 14 (13%) | 20 (20%) |
| Oropharyngeal pain | 18 (9%) | 7 (7%) | 8 (8%) | 11 (11%) |
| Skin and subcutaneous tissue disorders | 117 (56%)* | 58 (57%)* | 61 (58%)* | 21 (21%) |
| Rash (of special interest)† | 109 (52%)* | 55 (54%)* | 56 (53%)* | 20 (20%) |
| Any rash | 67 (32%)* | 44 (43%)* | 37 (35%)* | 11 (11%) |
| Pruritus | 32 (15%)* | 10 (10%) | 11 (10%) | 4 (4%) |
|
| ||||
|
Any serious adverse event
| ||||
| Total serious adverse events | 19 (9%) | 11 (11%) | 12 (11%) | 9 (9%) |
| Neutropenia | 1 (<1%) | 0 | 1 (1%) | 1 (1%) |
| Lymphopenia | 1 (<1%) | 0 | 0 | 0 |
| Eye disorders (corneal erosion, contact subcapsular) | 1 (<1%) | 0 | 1 (1%) | 0 |
| Abdominal pain | 1 (<1%) | 0 | 0 | 0 |
| Coeliac disease | 0 | 0 | 1 (1%) | 0 |
| Gastritis | 0 | 1 (1%) | 0 | 0 |
| Intestinal obstruction | 0 | 0 | 1 (1%) | 0 |
| Vomiting | 0 | 1 (1%) | 0 | 0 |
| Non-cardiac chest pain | 0 | 0 | 0 | 1 (1%) |
| Fever | 1 (<1%) | 0 | 0 | 0 |
| Hepatobiliary disorders | 1 (<1%) | 0 | 1 (1%) | 0 |
| Immune system disorders (hypersensitivity) | 1 (<1%) | 0 | 0 | 0 |
| Gastroenteritis, viral | 1 (<1%) | 0 | 1 (1%) | 0 |
| Anal abscess | 0 | 0 | 1 (1%) | 0 |
| Appendicitis | 1 (<1%) | 0 | 0 | 0 |
| Bronchitis | 0 | 1 (1%) | 0 | 0 |
| Cellulitis | 0 | 0 | 0 | 1 (1%) |
| Gastritis viral | 0 | 0 | 1 (1%) | 0 |
| Gastroenteritis | 0 | 0 | 1 (1%) | 0 |
| Infection | 0 | 0 | 1 (1%) | 0 |
| Paronychia | 0 | 0 | 0 | 1 (1%) |
| Pneumonia | 0 | 1 (1%) | 0 | 0 |
| Pulmonary tuberculosis | 0 | 1 (1%) | 0 | 0 |
| Tuberculosis | 0 | 0 | 0 | 1 (1%) |
| Injury, poisoning, and procedural complications | 1 (<1%) | 0 | 0 | 1 (1%) |
| Abnormal nuclear MRI of brain | 0 | 0 | 1 (1%) | 0 |
| Diabetic ketoacidosis | 5 (2%) | 3 (3%) | 1 (1%) | 0 |
| Hyperglycaemia | 1 (<1%) | 1 (1%) | 1 (1%) | 1 (1%) |
| Diabetes mellitus inadequate control | 1 (<1%) | 2 (2%) | 0 | 0 |
| Hypoglycaemic seizure | 1 (<1%) | 1 (1%) | 1 (1%) | 0 |
| Hypoglycaemic unconsciousness | 0 | 0 | 1 (1%) | 1 (1%) |
| Dehydration | 1 (<1%) | 0 | 0 | 0 |
| Ketosis | 0 | 0 | 0 | 1 (1%) |
| Metastatic malignant melanoma | 0 | 1 (1%) | 0 | 0 |
| Hypoglycaemic coma | 1 (<1%) | 0 | 0 | 0 |
| Complication of pregnancy | 0 | 0 | 0 | 1 (1%) |
| Depression | 0 | 1 (1%) | 1 (1%) | 0 |
| Epididymitis | 0 | 0 | 1 (1%) | 0 |
| Subclavian vein thrombosis | 1 (<1%) | 0 | 0 | 0 |
Data are number of patients (%). See webappendix pp 15–17 for actual blood count and liver function changes over time. No significant differences in serious adverse events were noted for any of the intervention groups versus placebo. Other adverse events occurring in less than 10% of patients in any treatment group were: cardiac disorders (including palpitations, bradycardia, cardiomyopathy), ear and labyrinth disorders (including ear pain, vertigo, tinnitus), eye disorders (including conjunctivitis), endocrine disorders (including goitre), neoplasms (benign, malignant, and unspecified, including cysts and polyps), psychiatric disorders (including anxiety, insomnia, depression), vascular disorders (including flushing), pregnancy, puerperium, and perinatal conditions, and reproductive system and breast disorders.
p<0·05 dose versus placebo.
Prespecified adverse event of special interest.
Rash, the most common clinical adverse event in the teplizumab groups, occurred in a higher proportion of patients than in the placebo group (table 4). With median onset at day 6 (IQR 5–11), rash was usually mild to moderate (218/220 [99%]), self-limited in all but one patient, most often maculopapular (132/220 [60%]), and sometimes pruritic 56/220 (25%). Mild cytokine release syndrome was infrequent in the teplizumab groups (22/417 [5%]) and was not recorded in the placebo group (table 4). The safety profile was characterised by transient, small increases in aminotransferases, and mild, transient decreases in the neutrophil and leucocyte concentrations (webappendix pp 15–17). A more profound, but transient drop occurred in the lymphocyte concentration (nadir at day 6), as reported in previous studies12,13 (webappendix pp 15–17). The proportion of patients who were not able to complete all drug doses because of lymphopenia and protocol-defined stopping rules for alanine or aspartate aminotransferase increases, neutropenia, and reduced platelet counts was higher in the teplizumab groups (39/415 [9%]) than in the placebo group (2/98 [2%]).
Discussion
Our study of immune therapy in recent-onset type 1 diabetes did not show any significant between-group differences in the primary and secondary outcomes (panel). The primary composite outcome has not been used or validated previously, but was used to ensure that low insulin use would be accompanied by adequate glycaemic control. The exact parameters chosen (HbA1C <6·5% and daily insulin use <0·5 U/kg) were derived from a previous unblinded, uncontrolled study of teplizumab,12 and might not translate to our study population, which was older (mean age of 19 vs 14 years), recruited from different regions of the world (vs USA only), masked to treatment, and treated later after diagnosis (<12 weeks vs <6 weeks). Moreover, the patients recruited into this study might differ from previous cohorts. For example, 20% of the placebo group met the primary outcome criteria in our study compared with 5% in the previous trial.12 The differences might be due to improved overall treatment practices for type 1 diabetes in the 9 years between the studies, and to demographic differences.
Exploratory analyses in all patients who received treatment and in subgroups showed effects similar to those seen in previous, smaller studies of anti-CD3 antibody: C-peptide secretion was preserved, allowing glycaemic control to be achived at a lower insulin dose in the teplizumab groups than in the placebo group. A greater proportion of patients in the teplizumab groups was able to discontinue or use very low doses of insulin than in the placebo group. Investigators were instructed to maintain an insulin dose of more than 0·25 U/kg per day, which might have diminished the effect of teplizumab on insulin use. Although discontinuation of insulin was never a study objective and was driven entirely by glycaemic status, reduced insulin requirement with maintenance of glycaemic control supports a biological effect of teplizumab. Studies of other immune modulators have not consistently achieved this outcome.8 During the course of Protégé, accumulating evidence suggested that C-peptide concentration might be a more reliable outcome because C-peptide is a more direct indicator of endogenous insulin secretion than is HbA1c or insulin use, or both, and it is independent of manipulation by patients or physicians. Our results support this view, showing a treatment effect on C-peptide changes in exploratory analyses.
The effect of teplizumab was only seen with the highest dose (two courses of 17 mg, 6 months apart, in a 70 kg individual on the basis of a cumulative dose of 90343μg/m2 and a body surface area of 1·92 m2), yielding a cumulative dose per cycle that was half that in the previous study of teplizumab12 (34 mg cumulative dose for one 14-day course in a 70 kg individual). Otelixizumab has a similar specificity to the ε-subunit of CD3, but is produced by different methods, and has different affinity and structural features from teplizumab.20 The teplizumab dose seems to be lower than the dose used in the phase 2 trial of otelixizumab (48 mg cumulative dose),13 but higher than the dose in the phase 3 trial (3·1 mg cumulative dose).21 The dose regimen we used was not associated with severe reactions or biological signs of EBV reactivation, which was a problem in the otelixizumab trial, and, thus, had an acceptable safety profile in view of the seriousness of type 1 diabetes.14 More research is needed to establish whether higher teplizumab doses (eg, closer to that in the previous study12) might improve efficacy without impairing safety, and whether repeated dosing improves efficacy or is countered by the development of teplizumab antibodies and late timing after diagnosis.
Panel: Research in context.
Systematic review
We searched PubMed Clinical Queries up to June 2, 2011, with the search term “type 1 diabetes immunotherapy”, and identified a systematic review and meta-analysis18 and a comprehensive, non-systematic review.19 Findings of several small (n<100) phase 1–2 trials showed some success with antigen-specific and non-specific immune interventions between 6 months and 2 years. Among these trials, anti-CD3 monoclonal antibody showed promise in terms of strength and duration of efficacy balanced by a reasonable safety profile.
Interpretation
This phase 3 trial of immunotherapy in recent-onset type 1 diabetes is unique because it has a large sample size (n>500), is multinational, and used two courses of therapy. The primary outcome was not met, but treatment of type 1 diabetes with immunotherapy is an evolving area and a consensus outcome does not exist. Findings of exploratory analyses suggested that teplizumab could help preserve pancreatic β-cell secretion of insulin (as measured by C-peptide), and might decrease the amount of exogenous insulin needed for glycaemic control, particularly in subgroups such as children. These findings could help guide the design of future studies that are needed to assess the short-term and long-term efficacy of this therapeutic strategy.
Limitations of this study include the failure to achieve the primary outcome, necessitating the use of post-hoc (hypothesis-generating) analyses, and a need for additional follow-up to examine long-term safety and efficacy; 2-year results are forthcoming soon. Another limitation was the lack of extensive data for hypoglycaemia, which was not captured on diary cards. Future studies could benefit from documenting hypoglycaemic events more thoroughly, particularly because hypoglycaemia is an important clinical problem.
The study also identified subgroups that might be more likely to respond to treatment. Larger treatment effects were associated with younger age, recruitment from the USA, and earlier treatment. The reasons for these differences might be as much a reflection of the behaviour of the study subpopulations as the effect of the drug, but this possibility does not detract from their potential importance. This finding underscores the heterogeneity in type 1 diabetes and could have implications for showing efficacy of immune interventions in future trials. For example, the rate of β-cell decline is faster in children than in adults. Immunotherapy might, therefore, be particularly effective for improvement of clinical management of the disease in children—an important finding because the management of diabetes in children is particularly challenging, with higher rates of both hyperglycaemia and hypoglycaemia.4,7 Also, US patients generally had higher C-peptide concentrations, lower insulin use, and lower HbA1C at baseline than did patients in other regions, suggesting a more advanced stage of disease progression in these other regions, especially India. The increased response in patients from the USA and those treated within 6 weeks of diagnosis suggests that there is an advantage to treating early in the course of disease. Future studies might benefit from use of metabolic entry criteria that indicate a fairly early stage of disease.
Findings of this study showed that teplizumab had a treatment effect on C-peptide and insulin use while maintaining glycaemic control, particularly in selected, prespecified subgroups, and exemplifies the risks of developing a new outcome without previous validation. The data suggest that future studies intending to examine the effects of CD3 therapy might benefit from recruitment of a population enriched with young patients who are treated early after diagnosis.
Acknowledgments
Funding MacroGenics, the Juvenile Diabetes Research Foundation, and Eli Lilly.
MacroGenics, the Juvenile Diabetes Research Foundation, and Eli Lilly provided financial support for the clinical trial. Philip D Ross (MedStrat Communications) provided medical writing services, which were funded by MacroGenics. George Eisenbarth, Janet Wittes, and William Tamborlane provided substantial input and advice during study design, and Janet Wittes contributed to planning of protocol-stated analyses and post-hoc analyses; William Tamborlane and Janet Wittes were paid consultants to MacroGenics on the report topic at times during the past 3 years. The following MacroGenics personnel provided logistical and other study support: David Parker, Matthew Kovalsky, Michelle Greene, Susan Brann, Andrew McGrath, Maria Petkoski, Kristan Phillips, J Steven Wilkinson, Hua Li, Wei Chen, Laura Fellows, Young Wang, Michael Kadan, Joan Brandt, Mark Bowe, and Valentina Ciccarone.
Footnotes
See Online for webappendix
For the Protégé study protocol see http://www.macrogenics.com/content/file/protocol_v8.pdf and http://www.macrogenics.com/content/file/protocol_v9.pdf
Contributors
SK, EB, KES, KCH, RLW, SJ, SP, DC, WH, and JL contributed to the study design. All authors contributed to study implementation and supervision of data collection at the sites. AGD, DC, CH, KCH, NS, and SP3 contributed to planning of protocol-stated analyses and post-hoc analyses. DC and CH designed and did the statistical analysis and verified its accuracy. KLK, CH, DC, and AGD contributed to compiling of the official clinical study report. All authors had full access to the data, helped draft the report or critically revise the draft, contributed to data interpretation, and reviewed and approved the final version of the report.
Conflicts of interest
CH, DC, KLK, EB, SJ, KES, SK, and AGD are employees of MacroGenics and potentially own shares or options in the company. NS, JL, SA, BB, JW, and RJF received research and travel support for this study from MacroGenics. JL has received advisory board support from Johnson & Johnson. RJF has received research support within the past 3 years from Tolerx (to study otelixizumab for recent-onset type 1 diabetes); unrelated research support from the US National Institutes of Health (grants R21 HD059292 and T35 DK007405), Gabrielle’s Angel Foundation, Eli Lilly, Diamyd, Pfizer, and Novo Nordisk; and unrestricted research support from Le Bonheur Foundation (Memphis TN, USA). WH chairs the data safety and monitoring board for the BHT-3021-01 insulin plasmid trial (Bayhill Pharmaceuticals). KCH has received research support from MacroGenics, and support from the Juvenile Diabetes Foundation (grant 2008-502) for laboratory studies on patients’ samples from Protégé. RLW is a former employee of MacroGenics and holds restricted stock in the company, and is now an employee of Parexel International, the lead contract research organisation that coordinated the Protégé trial globally. SA has received speaking honoraria from Eli Lilly. SP was formerly employed by MacroGenics and received stock options while employed there. SMJ declares that he has no conflicts of interest.
References
- 1.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 2.Steele C, Hagopian WA, Gitelman S, et al. Insulin secretion in type 1 diabetes. Diabetes. 2004;53:426–33. doi: 10.2337/diabetes.53.2.426. [DOI] [PubMed] [Google Scholar]
- 3.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on β-cell turnover in type 1 diabetes. Diabetes. 2006;55:3238–45. doi: 10.2337/db05-1034. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–91. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 6.Ludvigsson J, Faresjö M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359:1909–20. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein J, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 8.Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we at and where should we be going? Immunity. 2010;32:488–99. doi: 10.1016/j.immuni.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–23. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 10.Chatenoud L. Immune therapy of autoimmune diabetes: what is unique with CD3 antibodies? Nat Rev End. 2010;6:149–57. doi: 10.1038/nrendo.2009.275. [DOI] [PubMed] [Google Scholar]
- 11.Xu D, Alegre M-L, Varga SS, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cellular Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 12.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–98. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 13.Keymuelen B, Vandemeulebroucke E, Zeigler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 14.Keymeulen B, Candon S, Fafi-Kremer S, et al. Transient Epstein-Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood. 2009;115:1145–55. doi: 10.1182/blood-2009-02-204875. [DOI] [PubMed] [Google Scholar]
- 15.PR Newswire. Tolerx and GlaxoSmithKline announce phase 3 defend-1 study of otelixizumab in type 1 diabetes did not meet its primary endpoint. [(accessed June 9, 2011).]; http://www.prnewswire.com/news-releases/tolerx-and-glaxosmithkline-announce-phase-3-defend-1-study-of-otelixizumab-in-type-1-diabetes-did-not-meet-its-primary-endpoint-117795473.html.
- 16.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29:S43–48. [PubMed] [Google Scholar]
- 17.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 18.Gandhi GY, Murad MH, Flynn DN, et al. Immunotherapeutic agents in type 1 diabetes: a systematic review and meta-analysis of randomized trials. Clin Endocrinol (Oxf) 2008;69:244–52. doi: 10.1111/j.1365-2265.2008.03179.x. [DOI] [PubMed] [Google Scholar]
- 19.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 20.Bolt S, Routledge E, Lloyd I, et al. The generation of a humanized, non-mitogenic CD3 monoclonal antibody which retains in vitro immunosuppressive properties. Eur J Immunol. 1993;23:403–11. doi: 10.1002/eji.1830230216. [DOI] [PubMed] [Google Scholar]
- 21.EvaluatePharma. Tolerx presents otelixizumab phase 2 dose optimization data at the IDF’s 20th World Diabetes Congress. [accessed June 20, 2011]; http://www.evaluatepharma.com/Universal/View.aspx?type=Story&id=224944.