Abstract
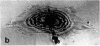
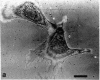
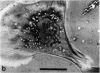
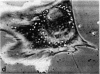
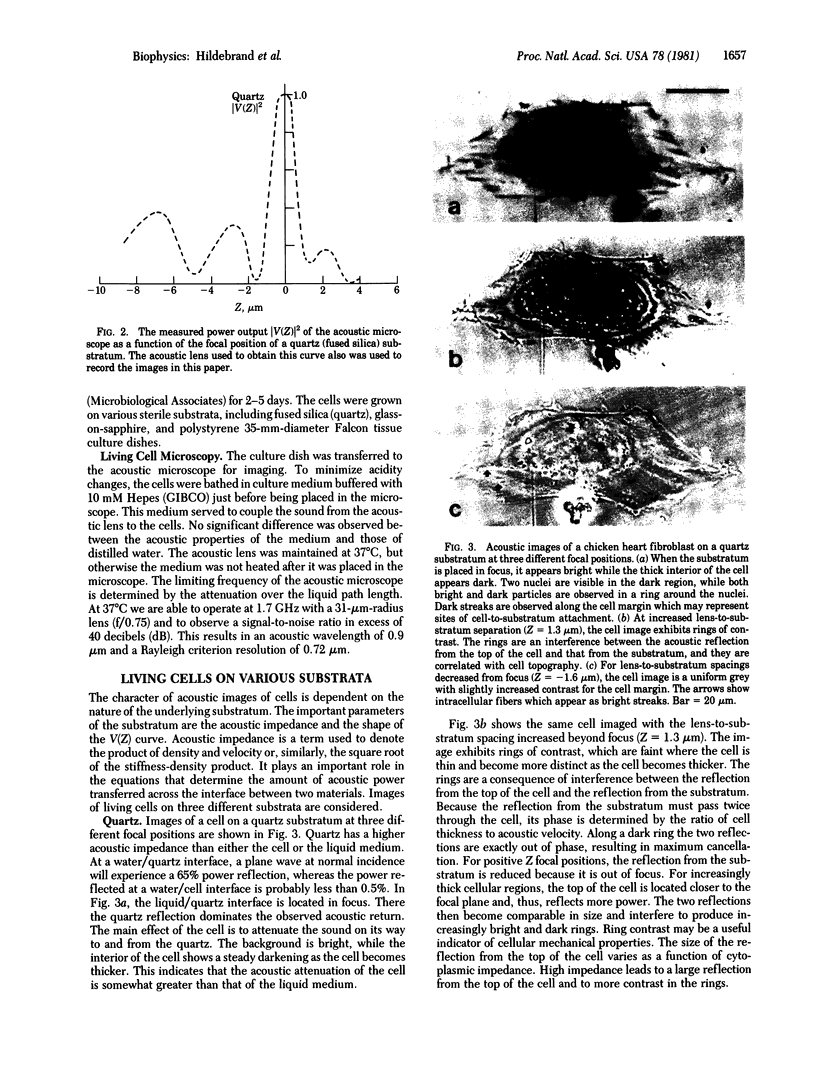
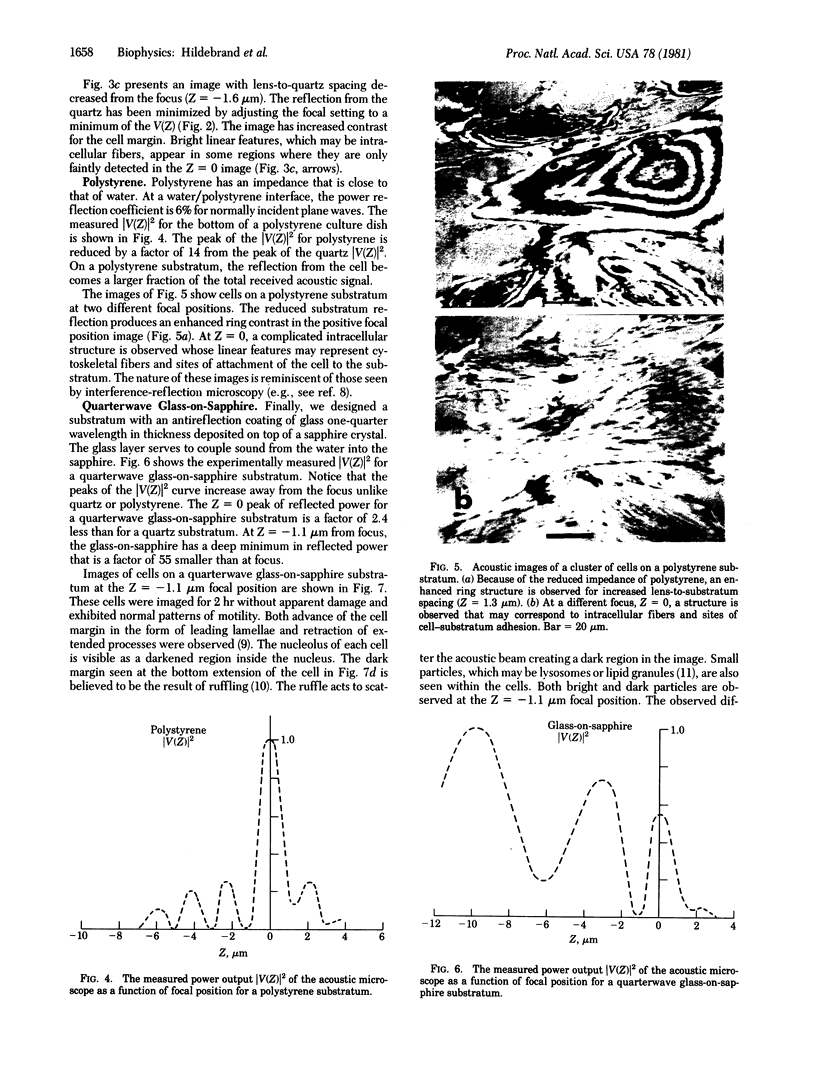
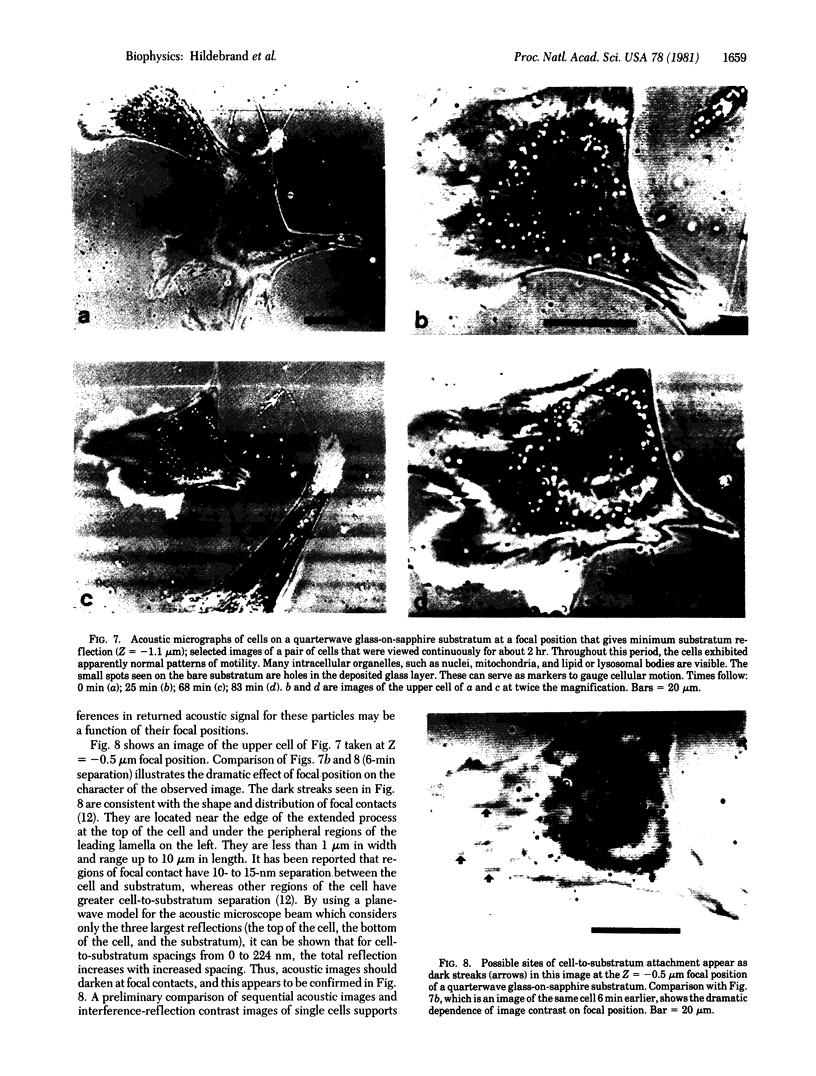
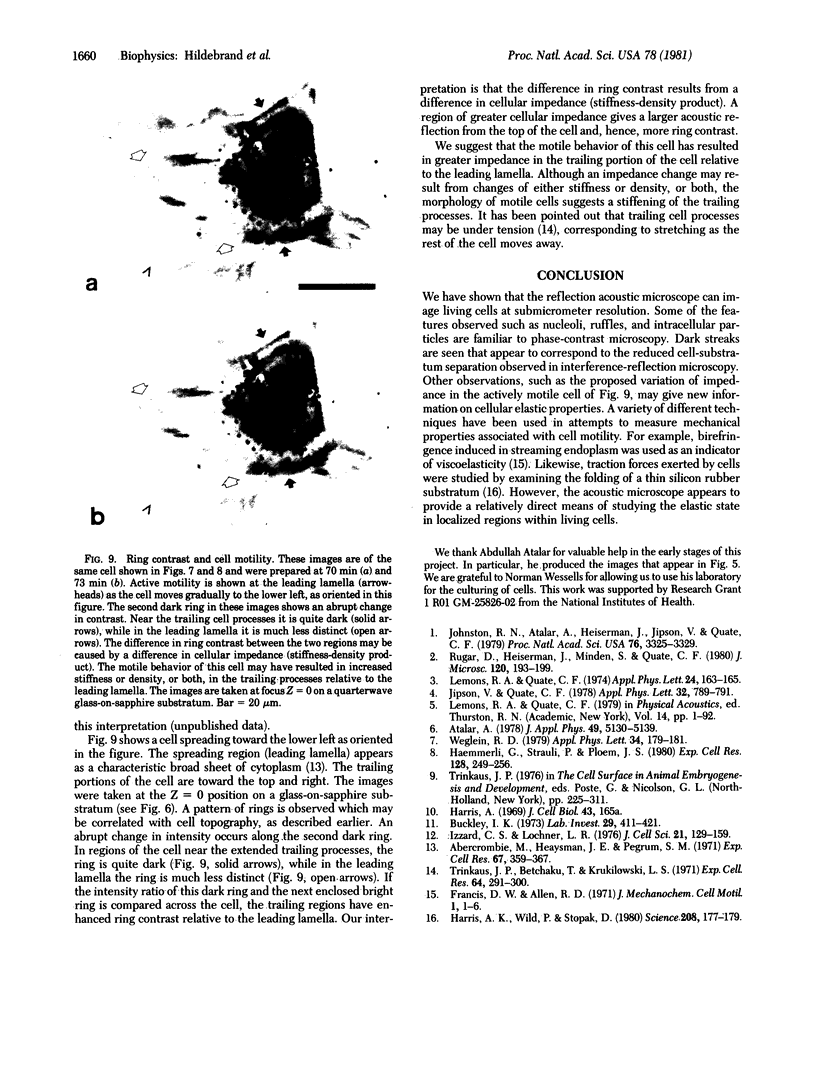
This paper reports preliminary results of the observation by acoustic microscopy of living cells in vitro. The scanning acoustic microscope uses high-frequency sound waves to produce images with submicrometer resolution. The contrast observed in acoustic micrographs of living cells depends on the acoustic properties (i.e., density, stiffness, and attenuation) and on the topographic contour of the cell. Variation in distance separating the acoustic lens and the viewed cell also has a profound effect on the image. When the substratum is located at the focal plane, thick regions of the cell show a darkening that can be related to cellular acoustic attenuation (a function of cytoplasmic viscosity). When the top of the cell is placed near the focal plane, concentric bright and dark rings appear in the image. The location of the rings can be related to cell topography, and the ring contrast can be correlated to the stiffness and density of the cell. In addition, the character of the images of single cells varies dramatically when the substratum upon which they are grown is changed to a different material. By careful selection of the substratum, the information content of the acoustic images can be increased. Our analysis of acoustic images of actively motile cells indicates that leading lamella are less dense or stiff than the quiescent trailing processes of the cells.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Heaysman J. E., Pegrum S. M. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971 Aug;67(2):359–367. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- Buckley I. K. The lysosomes of cultured chick embryo cells. A correlated light and electron microscopic study. Lab Invest. 1973 Oct;29(4):411–421. [PubMed] [Google Scholar]
- Haemmerli G., Sträuli P., Ploem J. S. Cell-to-substrate adhesions during spreading and locomotin of carcinoma cells. A study by mcrocinematography and reflection contrast microscopy. Exp Cell Res. 1980 Aug;128(2):249–256. doi: 10.1016/0014-4827(80)90061-0. [DOI] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J Cell Sci. 1976 Jun;21(1):129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Johnston R. N., Atalar A., Heiserman J., Jipson V., Quate C. F. Acoustic microscopy: resolution of subcellular detail. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3325–3329. doi: 10.1073/pnas.76.7.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugar D., Heiserman J., Minden S., Quate C. F. Acoustic microscopy of human metaphase chromosomes. J Microsc. 1980 Nov;120(Pt 2):193–199. doi: 10.1111/j.1365-2818.1980.tb04135.x. [DOI] [PubMed] [Google Scholar]
- Trinkaus J. P., Betchaku T., Krulikowski L. S. Local inhibition of ruffling during contact inhibition of cell movement. Exp Cell Res. 1971 Feb;64(2):291–300. doi: 10.1016/0014-4827(71)90079-6. [DOI] [PubMed] [Google Scholar]