Abstract
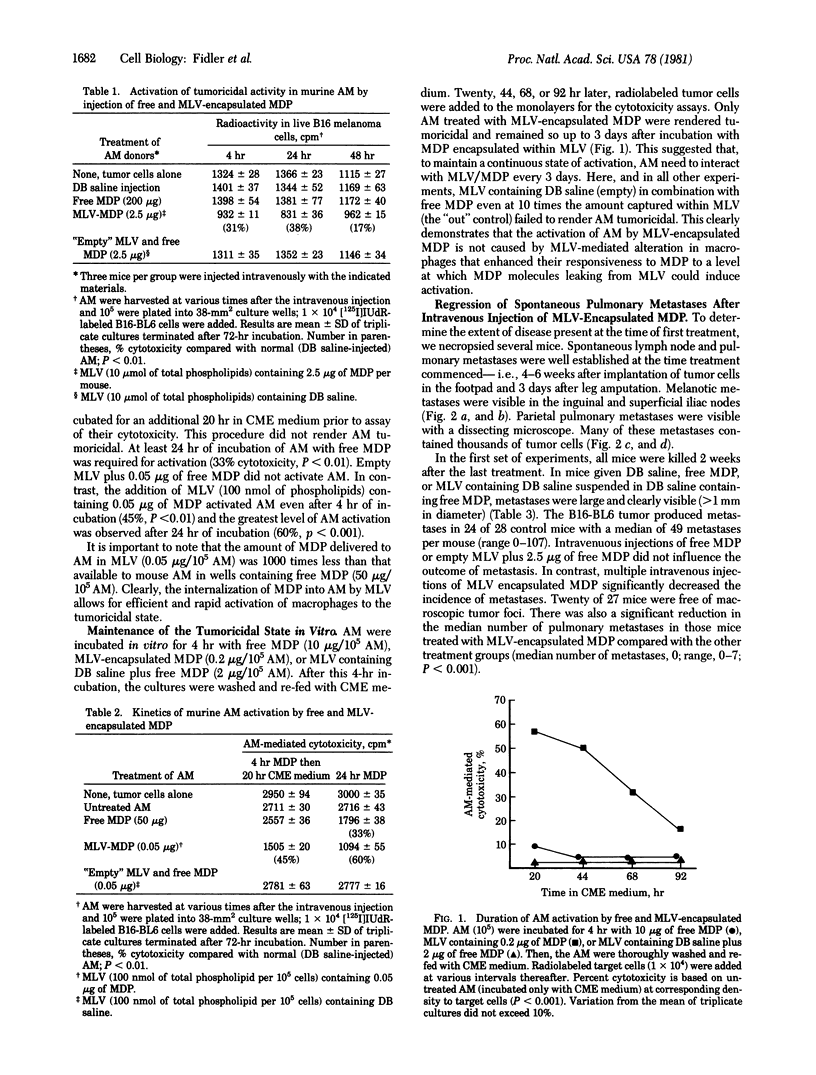
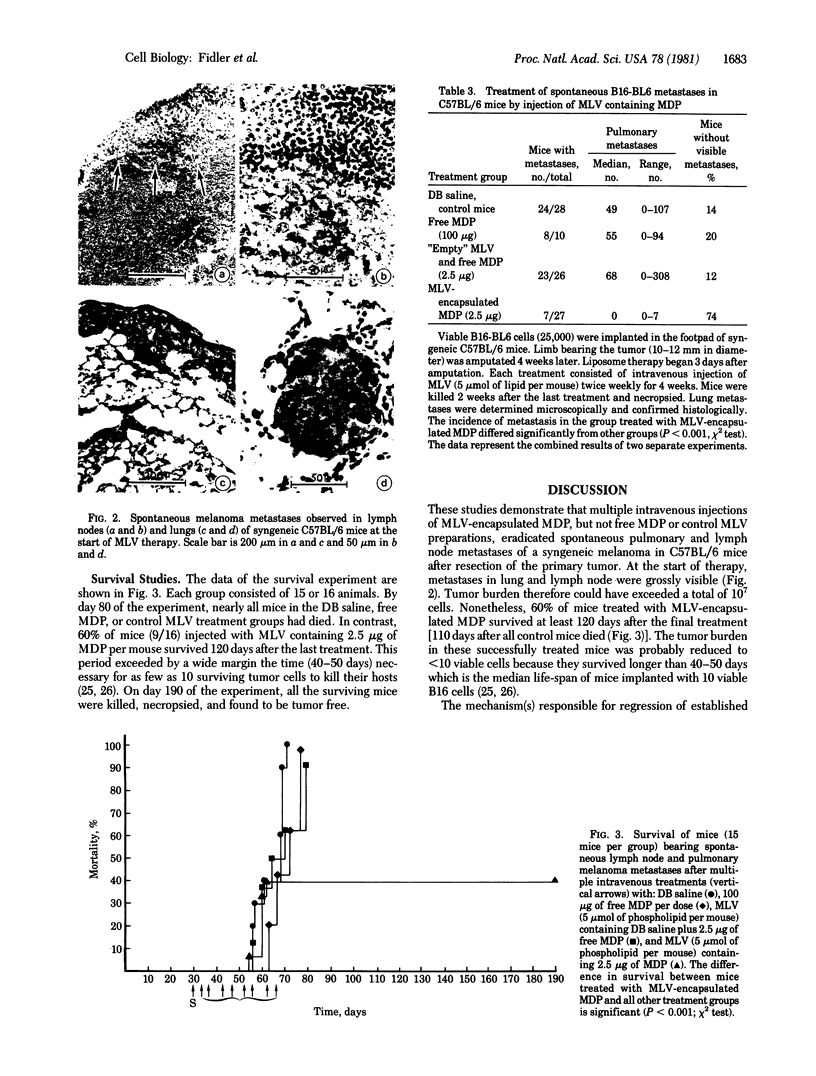
The multiple systemic administration of multilamellar liposomes composed of phosphatidylserine and phosphatidylcholine (molar ratio 3:7) that contained water-soluble muramyl dipeptide (MDP) activated alveolar macrophages to become tumoricidal and eradicated established spontaneous pulmonary and lymph node metastases. Spontaneously metastasizing melanoma cells were injected into the footpads of mice. After 4-5 weeks, the tumors were resected by a midfemoral amputation; 3 days later, twice-weekly injections of liposomes were initiated and continued for 4 weeks. In some experiments the mice were killed 2 weeks after the final treatment. Seventy-four percent of animals injected with liposomes containing MDP were free of visible metastases. In a separate life-span experiment, 60% of mice treated with liposome-encapsulated MDP were tumor-free 120 days after the last liposome treatment or 110 days after all control mice treated with free MDP or control liposome preparations had died of disseminated cancer. These data suggest that the systemic administration of liposomes containing MDP, or similar compounds that produce macrophage activation, may provide an additional useful approach to the therapeutic regimens currently used to eradicate cancer metastases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mode of action of immunological adjuvants. J Reticuloendothel Soc. 1979 Dec;26(Suppl):619–630. [PubMed] [Google Scholar]
- Audibert F., Chédid L., Lefrancier P., Choay J. Distinctive adjuvanticity of synthetic analogs of mycobacterial water-soluble components. Cell Immunol. 1976 Feb;21(2):243–249. doi: 10.1016/0008-8749(76)90053-8. [DOI] [PubMed] [Google Scholar]
- Chedid L., Carelli C., Audibert F. Recent developments concerning muramyl dipeptide, a synthetic immunoregulating molecule. J Reticuloendothel Soc. 1979 Dec;26(Suppl):631–641. [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Activation in vitro of mouse macrophages by syngeneic, allogeneic, or xenogeneic lymphocyte supernatants. J Natl Cancer Inst. 1975 Nov;55(5):1159–1163. doi: 10.1093/jnci/55.5.1159. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Inhibition of pulmonary metastasis by intravenous injection of specifically activated macrophages. Cancer Res. 1974 May;34(5):1074–1078. [PubMed] [Google Scholar]
- Fidler I. J., Kripke M. L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977 Aug 26;197(4306):893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Raz A., Fogler W. E., Kirsh R., Bugelski P., Poste G. Design of liposomes to improve delivery of macrophage-augmenting agents to alveolar macrophages. Cancer Res. 1980 Dec;40(12):4460–4466. [PubMed] [Google Scholar]
- Fidler I. J. Recognition and destruction of target cells by tumoricidal macrophages. Isr J Med Sci. 1978 Jan;14(1):177–191. [PubMed] [Google Scholar]
- Fidler I. J. Therapy of spontaneous metastases by intravenous injection of liposomes containing lymphokines. Science. 1980 Jun 27;208(4451):1469–1471. doi: 10.1126/science.7384789. [DOI] [PubMed] [Google Scholar]
- Griswold D. P., Jr Consideration of the subcutaneously implanted B16 melanoma as a screening model for potential anticancer agents. Cancer Chemother Rep 2. 1972 Nov;3(1):315–324. [PubMed] [Google Scholar]
- Hadden J. W., Englard A., Sadlik J. R., Hadden E. M. The comparative effects of isoprinosine, levamisole, muramyl dipeptide and SM1213 on lymphocyte and macrophage proliferation and activation in vitro. Int J Immunopharmacol. 1979;1(1):17–27. doi: 10.1016/0192-0561(79)90026-2. [DOI] [PubMed] [Google Scholar]
- Hart I. R. The selection and characterization of an invasive variant of the B16 melanoma. Am J Pathol. 1979 Dec;97(3):587–600. [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S. Implications of immunological heterogeneity of tumours. Nature. 1979 Aug 2;280(5721):358–360. doi: 10.1038/280358a0. [DOI] [PubMed] [Google Scholar]
- Kotani S., Kinoshita F., Morisaki I., Shimono T., Okunaga T., Takada H., Tsujimoto M., Watanabe Y., Kato K., Shiba T. Immunoadjuvant activities of synthetic 6-O-acyl-N-acetylmuramyl-L-alanyl-D-isoglutamine with special reference to the effect of its administration with liposomes. Biken J. 1977 Dec;20(3-4):95–103. [PubMed] [Google Scholar]
- Kripke M. L., Budmen M. B., Fidler I. J. Production of specific macrophage activating factor by lymphocytes from tumor-bearing mice. Cell Immunol. 1977 May;30(2):341–352. doi: 10.1016/0008-8749(77)90077-6. [DOI] [PubMed] [Google Scholar]
- Lederer E. Synthetic immunostimulants derived from the bacterial cell wall. J Med Chem. 1980 Aug;23(8):819–825. doi: 10.1021/jm00182a001. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Gattozzi C., Kleinerman J., Saidel G. Reduction of tumour cell entry into vessels by BCG-activated macrophages. Br J Cancer. 1977 Nov;36(5):639–641. doi: 10.1038/bjc.1977.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. A., Schwartzman S. M., Horner B. L., Jones G. H., Moffatt J. G., Nestor J. J., Jr, Tegg D. Regression of tumors in guinea pigs after treatment with synthetic muramyl dipeptides and trehalose dimycolate. Science. 1980 Apr 25;208(4442):415–416. doi: 10.1126/science.7189295. [DOI] [PubMed] [Google Scholar]
- Nagao S., Tanaka A. Muramyl dipeptide-induced adjuvant arthritis. Infect Immun. 1980 May;28(2):624–626. doi: 10.1128/iai.28.2.624-626.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Togawa A., Chedid L., Mizel S. Components of mycobacteria and muramyl dipeptide with adjuvant activity induce lymphocyte activating factor. Cell Immunol. 1980 Mar 1;50(1):71–81. doi: 10.1016/0008-8749(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L., Yapo A., Petit J. F., Lederer E. Fate of the synthetic immunoadjuvant, muramyl dipeptide (14C-labelled) in the mouse. Int J Immunopharmacol. 1979;1(1):35–41. doi: 10.1016/0192-0561(79)90028-6. [DOI] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Poste G., Kirsh R., Fogler W. E., Fidler I. J. Activation of tumoricidal properties in mouse macrophages by lymphokines encapsulated in liposomes. Cancer Res. 1979 Mar;39(3):881–892. [PubMed] [Google Scholar]
- Sone S., Poste G., Fidler I. J. Rat alveolar macrophages are susceptible to activation by free and liposome-encapsulated lymphokines. J Immunol. 1980 May;124(5):2197–2202. [PubMed] [Google Scholar]
- Taniyama T., Holden H. T. Direct augmentation of cytolytic activity of tumor-derived macrophages and macrophage cell lines by muramyl dipeptide. Cell Immunol. 1979 Dec;48(2):369–374. doi: 10.1016/0008-8749(79)90131-x. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]



