Abstract
The enteric bacterium Proteus mirabilis is a common cause of complicated urinary tract infections. In this study, microarrays were used to analyze P. mirabilis gene expression in vivo from experimentally infected mice. Urine was collected at 1, 3, and 7 days postinfection, and RNA was isolated from bacteria in the urine for transcriptional analysis. Across nine microarrays, 471 genes were upregulated and 82 were downregulated in vivo compared to in vitro broth culture. Genes upregulated in vivo encoded mannose-resistant Proteus-like (MR/P) fimbriae, urease, iron uptake systems, amino acid and peptide transporters, pyruvate metabolism enzymes, and a portion of the tricarboxylic acid (TCA) cycle enzymes. Flagella were downregulated. Ammonia assimilation gene glnA (glutamine synthetase) was repressed in vivo, while gdhA (glutamate dehydrogenase) was upregulated in vivo. Contrary to our expectations, ammonia availability due to urease activity in P. mirabilis did not drive this gene expression. A gdhA mutant was growth deficient in minimal medium with citrate as the sole carbon source, and loss of gdhA resulted in a significant fitness defect in the mouse model of urinary tract infection. Unlike Escherichia coli, which represses gdhA and upregulates glnA in vivo and cannot utilize citrate, the data suggest that P. mirabilis uses glutamate dehydrogenase to monitor carbon-nitrogen balance, and this ability contributes to the pathogenic potential of P. mirabilis in the urinary tract.
INTRODUCTION
The urinary tract is a common site of infection (for a recent review see reference 57). Recent strides have been made in understanding the pathogenesis of uropathogenic Escherichia coli (UPEC), the most common cause of urinary tract infection (UTI) in otherwise healthy individuals. There are now studies defining UPEC transcription during experimental UTI in mice (74), asymptomatic bacteriuria in humans (70), and human UTI (22). However, far less is known about bacterial agents of complicated UTI, which occurs in catheterized patients or patients with structural or functional abnormalities of the urinary tract and is frequently polymicrobial (28). UTI is the most common type of nosocomial infection, causing an estimated 424,000 cases and 13,000 deaths in U.S. hospitals in 2002 (34). Proteus mirabilis is one of the major causes of complicated UTI (81, 82) and is associated with particularly severe disease due to its ability to cause nephrolithiasis (kidney stones) and block urinary catheters (32, 41, 54).
P. mirabilis, a member of the Enterobacteriaceae, is well known for its ability to swarm across solid surfaces (55, 64). Urease, which mediates the hydrolysis of urea into ammonia and carbon dioxide, is perhaps the most potent virulence factor of P. mirabilis. Urease activity increases the pH of urine, leading to the precipitation of minerals and stone formation (53); it is required for P. mirabilis virulence during experimental UTI (33). P. mirabilis carries genes that encode 17 putative fimbrial operons (65), 5 of which have been named: MR/P (mannose-resistant Proteus-like) (9, 60), PMF (P. mirabilis fimbriae) (50), UCA (uroepithelial cell adhesin) (15, 85), PMP (P. mirabilis P-like pili) (11), and ATF (ambient-temperature fimbria) (47, 48). Two of these, MR/P (8) and PMF (49, 86), have been shown to play a role in virulence during experimental UTI and have been the focus of vaccine efforts (39, 66). Other known virulence factors for this organism include flagella (3, 51), toxins (HpmAB) (77, 80), proteases (Zap [83] and Lon [13]), autotransporters (Pta [2] and AipA and TaaP [1]), iron (27, 42) and zinc (59) uptake, and capsule (16). Signature-tagged mutagenesis studies have also highlighted the importance of central metabolism in the disease process (12, 26). Recent microarray studies examined total gene transcription during swarming (64) and iron restriction (27).
In this article, we investigate for the first time the transcriptome of P. mirabilis during infection, using a mouse model of ascending UTI. This is also the first study that investigates global transcription over time during UTI. The results have many parallels with UPEC gene expression during murine and human UTI, but also some important differences. Probing the distinctions in P. mirabilis and UPEC gene expression led to the discovery of an unexpected novel role for glutamate dehydrogenase (GDH) in P. mirabilis metabolism.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
P. mirabilis HI4320, isolated from the urine of an elderly, long-term-catheterized woman, has been previously described (54), sequenced, and annotated (65). E. coli CFT073 was isolated from the blood and urine of a patient with acute pyelonephritis (52) and has also been sequenced and annotated (84). The P. mirabilis ureC mutant has been previously reported (33). E. coli DH5α was used as the host for cloning mutational constructs. Bacteria were routinely cultured at 37°C with aeration in LB broth (10 g/liter tryptone, 5 g/liter yeast extract, 0.5 g/liter NaCl) or on LB solidified with 1.5% agar. The urease mutant was also cultured in filter-sterilized, pooled human urine from healthy adult volunteers. For studies on the effect of carbon source on P. mirabilis growth and gene transcription, minimal A medium (10) was utilized with 1% glucose, glycerol, citrate, or acetate as the carbon source.
Mouse model of ascending UTI.
To investigate in vivo gene transcription by P. mirabilis during UTI, 30 female CBA/J mice were transurethrally inoculated with approximately 1 × 107 CFU of P. mirabilis HI4320, using a modification (32) of a previously published method (23). Briefly, an overnight culture of wild-type P. mirabilis HI4230 was adjusted to an estimated density of 2 × 108 CFU/ml (optical density at 600 nm [OD600] of 0.2). Six- to 8-week-old female CBA/J mice were inoculated transurethrally with 50 μl of the suspension (i.e., 1 × 107 CFU) via a sterile polyethylene catheter, using an infusion pump (Harvard Apparatus). At 1, 3, and 7 days postinfection, urine was collected from mice directly into 14-ml polypropylene round-bottom tubes (Falcon, Becton-Dickinson) containing RNA Protect (Qiagen). At least 4 ml urine was collected per time point per experiment. Two milliliters of RNA Protect was used per ml of urine collected. The RNA-stabilized urine was placed on ice for at least 1 h, after which the fluid was separated from a crystalline precipitate that had formed (74). The fluid was centrifuged (10 min, 7,000 × g, 4°C), and the bacterial pellet was stored at −20°C before RNA extraction. The University of Michigan University Committee on Use and Care of Animals approved all mouse protocols.
RNA extraction and microarray analysis.
RNA was isolated with the RNeasy minikit (Qiagen). One column was used per ml of urine collected. RNA was treated with DNase (Ambion) and concentrated with an RNeasy MinElute column (Qiagen). The entire RNA sample (2 to 4 μg) was converted to cDNA and labeled with Cy5 CyDye (GE Healthcare) as previously reported (64). As a comparison condition, RNA was extracted from a mid-logarithmic-phase (OD600 of 1.0) P. mirabilis HI4320 LB broth culture, converted to cDNA, and labeled with Cy3. The in vivo and in vitro cDNA samples were hybridized with a P. mirabilis HI4320-specific microarray as previously described (64). Slides were scanned with a ScanArray Express microarray scanner (Perkin Elmer) at 10-μm resolution and quantified using ScanArray Express software. The resulting data were normalized by total intensity, and median spot intensities were identified by using MIDAS (v. 2.22) software (71). The statistical analysis of microarray (SAM) algorithm (79) was conducted using the MeV program (v. 4.5.1) (71). Genes were considered to be significantly differentially regulated in vivo compared to in vitro if they were identified by SAM, had a median fold change value of at least 2-fold, and had a median spot intensity above background fluorescence. Genes that were differentially regulated over time were identified by using the Bayesian estimation of temporal regulation (BETR) algorithm (6) in MeV.
qRT-PCR.
RNA was converted to cDNA by using the Superscript first-strand synthesis system (Invitrogen) according to the manufacturer's protocol. The resulting cDNA was confirmed to be free of chromosomal DNA contamination by PCR on samples with or without reverse transcriptase (RT) treatment. Samples were adjusted to a uniform concentration (4 or 6 ng/μl) by using a Nanodrop ND-1000 spectrophotometer. Each quantitative RT-PCR (qRT-PCR) was set up in duplicate and consisted of 30 ng cDNA template, 150 nM each primer, and 12.5 μl 2× SYBR green PCR master mix (Stratagene). Target genes were amplified with an Mx3000P thermal cycler (Stratagene). Melting curve analysis was used to confirm a lack of primer dimers, and no-template controls were used to ensure there was no genomic DNA contamination of reagents. Data were normalized to rpoA (RNA polymerase A) or rpmC (encodes 50S ribosomal protein L29) as specified and analyzed by the threshold cycle (2−ΔΔCT) method (43). Statistical analysis, including t tests and linear regression, was performed on log2-transformed data by using Prism software (GraphPad). Primer sequences will be provided upon request.
Mutant construction.
An isogenic gdhA mutant was constructed by insertion of a kanamycin resistance cassette into the gdhA (PMI3008) coding sequence by the Targetron method (Sigma) as previously described (63). Basically, a group II intron was retargeted by PCR to specifically insert into gdhA. Intron insertion in gdhA was confirmed by PCR. The mutant was maintained on LB agar supplemented with kanamycin (25 μg/ml).
Preparation of crude protein extracts.
P. mirabilis cells were grown in 1-liter flasks under the specified conditions for each experiment, harvested by centrifugation at 6,000 × g for 15 min, and resuspended in 5 ml buffer (for urease assays, 20 mM sodium phosphate buffer [pH 7.0], 1 mM EDTA, and 5 mM β-mercaptoethanol; for GDH and GS assays, 50 mM sodium phosphate buffer [pH 7.0]). Cells were disrupted by two 20,000-lb/in2 passages through a French pressure cell press (American Instruments Company) and immediately placed on ice. The sample was cleared by centrifugation at 30,000 × g for 30 min at 4°C to remove insoluble material. The resulting cell extract was used as the enzyme source for activity measurements. Total protein concentration was determined using the bicinchoninic acid (BCA) assay (Thermo Scientific) according to the manufacturer's instructions, using bovine serum albumin as the standard.
Enzyme assays.
Urease (EC 3.5.1.5) activity was determined by measuring released ammonia by the Berthelot reaction (24). Calibration curves used NH4Cl as a standard. The reaction mixture contained 30 mM urea, 1 mM EDTA, 10 mM β-mercaptoethanol, and 20 mM sodium phosphate buffer (pH 7.5), in a total volume of 240 μl. The reaction was initiated by addition of 10-μl aliquots of various enzyme concentrations. After allowing the reaction to proceed for 5 to 10 min at room temperature, it was stopped by addition of 500 μl phenol-nitroprusside solution, 500 μl alkaline hypochlorite, and 2.5 ml of water, and color was allowed to develop for 5 min at room temperature. One unit of urease activity was defined as the amount of enzyme required to produce 1 μmol NH3/min/ml. Jack bean urease (Sigma) was used as a positive control. Crude extracts of the P. mirabilis ΔureC strain grown under the same conditions and similarly processed were used to measure background activity. Absorbance at 570 nm was measured with a BioSpec-1601 spectrophotometer (Shimadzu).
Glutamine synthetase (GS; EC 6.3.1.2) activity was assayed using the γ-glutamyltransferase assay as previously described (75). Activity was measured in imidazole buffer both in the presence of 0.4 mM Mn2+ at pH 7.38 (both adenylylated and unadenylylated enzymes are active under these conditions) and in the presence of 60 mM Mg2+ and 0.4 mM Mn2+ at pH 7.2 (only unadenylylated enzyme is active). The reaction buffer contained 0.15 mM glutamine, 4.1% neutral NH2OH, 0.4 mM Na+-ADP, and 2% KAsO4 (pH 7.0) in 160 mM imidazole buffer (pH 7.4), and the divalent cation(s) in the concentrations specified above. The pH of the assay mix was adjusted according to the reaction. Briefly, 490-μl reactions were initiated by addition of 10-μl dilutions of crude P. mirabilis protein extract and allowed to proceed for 30 min at 37°C. The reaction was halted by addition of a stop mixture (3.3% FeCl3, 2% trichloroacetic acid [TCA], 0.25 N HCl), and samples were briefly centrifuged at 4,000 × g to remove any precipitate. The production of γ-glutamylhydroxamate was determined at 540 nm in a Shimadzu BioSpec-1601 spectrophotometer. One unit of enzyme activity was defined as the amount of enzyme producing 1 μmol γ-glutamylhydroxamate/min/mg protein in the transfer reaction. Glutamine synthetase from E. coli (Sigma) was used as a positive control.
Glutamate dehydrogenase (EC 1.4.1.4) activity was measured in the forward (reductive amination activity) direction as described elsewhere (72). The reaction followed the oxidation of NADPH2 spectrophotometrically at 340 nm and was monitored for 3 min at room temperature in a Genesys 10-S spectrophotometer (Thermo Scientific). The reaction mixture contained 100 mM Tris-HCl (pH 8.0), 100 mM NH4Cl, 10 mM α-ketoglutarate, 0.1 mM NADPH2, and appropriate dilutions of cell extracts in a total volume of 1 ml. Specific enzyme activity was calculated by using the NADPH2 extinction coefficient of 6220 cm−1 M−1. One unit of enzyme activity was defined as 1 μmol of coenzyme oxidized/min/ml. Crude extracts of the P. mirabilis gdhAΩkan strain grown under the same conditions and similarly processed were used to measure background activity. Glutamate dehydrogenase from bovine liver (Sigma) was used as a positive control.
In vivo cochallenge.
Overnight cultures of wild-type P. mirabilis HI4320 and the isogenic gdhA mutant strain were individually adjusted to an estimated density of 2 × 108 CFU/ml (OD600 of 0.2). The gdhA mutant culture was mixed in a 1:1 ratio with the wild-type parent strain. Mice were transurethrally infected with a 50-μl suspension of the mixture (i.e., 1 × 107 CFU) as described above. At 7 days postinoculation, bladders, kidneys, and spleens were removed from euthanized mice, homogenized in 3 ml phosphate-buffered saline (PBS) using an OMNI mechanical homogenizer (OMNI International), and plated on LB medium or LB medium supplemented with kanamycin by using a spiral plater (Autoplate 4000; Spiral Biotech) to assess bacterial burden. Colony counts were enumerated using a Qcount (Spiral Biotech). Wild-type HI4320 infection was determined by subtracting the number of colonies on the kanamycin plate from the number of colonies on the plain LB plate. Statistical significance was assessed using the two-tailed Wilcoxon matched-pairs test. The competitive index (CI) was calculated by dividing the ratio of mutant to wild-type output CFU to the ratio of mutant to wild-type input CFU. CI values below 1 indicate outcompetition of the mutant by the wild-type parent.
Growth curves.
Growth of bacterial strains was assessed with a Bioscreen C growth curve analyzer (Growth Curves, Ltd.). The optical density at 600 nm for triplicate cultures was measured at 15-min intervals. Cultures were maintained at 37°C with continuous shaking.
Microarray data accession number.
Microarray data have been deposited in the Gene Expression Omnibus (GEO) database under series GSE25977.
RESULTS
Transcriptome of Proteus mirabilis in the murine urinary tract.
Mice were transurethrally inoculated with P. mirabilis, and the infection was allowed to progress for 1 week. At 1, 3, and 7 days postinfection, transcription of bacteria in pooled voided mouse urine was measured by microarray and compared to transcription by P. mirabilis cultured to the logarithmic phase in LB broth. LB broth was selected for the in vitro condition, despite its complexity, for three reasons. LB broth represents a common laboratory medium for culture of enteric bacteria, the use of this medium allowed comparison to the only other in vivo transcriptome of a uropathogen, E. coli (74), and culture of P. mirabilis in urine ex vivo is cidal for the urease-producing bacterium.
At 7 days postinfection, there is a typical median of 1 × 106 to 1 × 107 CFU/ml in urine. Three independent microarrays were analyzed for each time point. To understand the overall pattern of gene expression in vivo, all nine microarrays were analyzed together. There were 471 genes significantly upregulated in vivo compared to in vitro, and there were 82 genes significantly downregulated in vivo. Table 1 lists the top 50 genes upregulated in vivo, Table 2 lists the 50 nonribosomal genes most highly expressed in vivo, and Table 3 lists the 50 genes most downregulated in vivo. The complete lists of differentially regulated genes are found in Tables S1 and S2 in the supplemental material. The genes encoding mannose-resistant Proteus-like (MR/P) fimbriae were very highly expressed in vivo; the nine genes comprising the mrp operon (40) were the nine most upregulated genes in vivo (Table 1). Indeed, mrpA, the gene encoding the major structural subunit of MR/P fimbriae, was the ninth most highly expressed nonribosomal gene in vivo (Table 2). Major classes of genes upregulated in vivo encode amino acid and carbohydrate transporters, energy production and conversion proteins, and ion transport (predominantly iron) proteins (Table 4). Genes encoding cell motility proteins were the most highly downregulated genes in vivo; 19 of the 50 most downregulated genes encoded flagellar components and chemotaxis proteins (Table 3). Major classes of genes downregulated in vivo, of which cell motility genes are the largest defined category, are depicted in Table 4.
Table 1.
The 50 genes most highly upregulated in vivo in this study
| ORFc | Gene | Annotation | Ranka |
Fold changeb | |
|---|---|---|---|---|---|
| In vivo | In vitro | ||||
| PMI0263 | mrpA | Major mannose-resistant/Proteus-like fimbrial protein | 37 | 3,040 | 1,3806.33 |
| PMI0264 | mrpB | Fimbrial subunit | 138 | 3,474 | 1,672.09 |
| PMI0266 | mrpD | Fimbrial chaperone protein | 146 | 3,496 | 1,358.22 |
| PMI0268 | mrpF | Fimbrial subunit | 218 | 2,905 | 1,227.47 |
| PMI0271 | mrpJ | Fimbrial operon regulator | 191 | 2,688 | 904.14 |
| PMI0269 | mrpG | Fimbrial subunit | 255 | 2,804 | 693.49 |
| PMI0267 | mrpE | Fimbrial subunit | 272 | 3,018 | 616.84 |
| PMI0265 | mrpC | Fimbrial outer membrane usher protein | 230 | 3,124 | 598.01 |
| PMI0270 | mrpH | Fimbrial adhesin | 458 | 2,891 | 514.01 |
| PMI3233 | Putative membrane protein | 350 | 2,194 | 344.22 | |
| PMI1706 | dmsB | Anaerobic dimethyl sulfoxide reductase chain B | 530 | 2,818 | 250.99 |
| PMI0173 | Hypothetical protein | 547 | 2,892 | 232.47 | |
| PMI2673 | gntK | Thermoresistant gluconokinase | 537 | 3,125 | 220.23 |
| PMI1205 | Putative anaerobic dimethyl sulfoxide reductase chain B | 533 | 2,487 | 210.95 | |
| PMI3234 | Putative membrane protein | 558 | 2,280 | 204.00 | |
| PMI1688 | Putative di-/tripeptide transporter | 546 | 2,490 | 183.19 | |
| PMI2969 | Major facilitator superfamily transporter | 503 | 2,286 | 167.77 | |
| PMI0730 | Conserved hypothetical protein | 514 | 1,973 | 161.35 | |
| PMI0249 | betU | Putative secondary glycine betaine transporter | 738 | 2,723 | 153.81 |
| PMI0361 | Putative phosphodiesterase | 645 | 3,614 | 153.01 | |
| PMI2134 | agaA | N-Acetylglucosamine-6-phosphate deacetylase | 652 | 3,233 | 148.09 |
| PMI1705 | dmsA | Dimethyl sulfoxide reductase chain A | 881 | 2,851 | 140.24 |
| PMI0391 | Putative sigma 54 modulation protein | 493 | 2,108 | 131.78 | |
| PMI3232 | Putative peptidase | 403 | 1,924 | 122.47 | |
| PMI0973 | Putative heat shock protein | 930 | 2,728 | 122.19 | |
| PMI0706 | focA | Probable formate transporter | 568 | 1,898 | 122.12 |
| PMI0187 | dsdA | d-Serine dehydratase | 909 | 1,990 | 121.54 |
| PMI1568 | Putative exported protease | 620 | 2,266 | 119.64 | |
| PMI1406 | gadC | Probable glutamate/γ-aminobutyrate antiporter | 413 | 1,871 | 118.51 |
| PMI2946 | ABC transporter, permease protein | 1,133 | 2,874 | 113.19 | |
| PMI3515 | Phosphotransferase system IIBC component | 1,003 | 3,455 | 108.08 | |
| PMI2672 | gntU | Low-affinity gluconate transporter | 797 | 2,149 | 102.07 |
| PMI2948 | Probable methyltransferase | 661 | 2,215 | 100.26 | |
| PMI0287 | Putative amidohydrolase/metallopeptidase | 764 | 2,288 | 100.05 | |
| PMI2192 | LuxR family transcriptional regulator | 697 | 2,632 | 99.95 | |
| PMI0272 | Probable transport protein | 1,008 | 2,662 | 98.42 | |
| PMI3674 | Probable aminohydrolase | 813 | 2,423 | 97.48 | |
| PMI1601 | Putative sodium:alanine symporter | 664 | 2,278 | 96.94 | |
| PMI1725 | Putative phage holin (lysis protein) | 1,063 | 3,227 | 96.93 | |
| PMI3675 | Putative C4-dicarboxylate transporter | 931 | 2,866 | 92.96 | |
| PMI2292 | ptsG | Phosphotransferase system, glucose-specific IIBC component | 479 | 2,153 | 88.71 |
| PMI2970 | Putative membrane protein | 854 | 2,925 | 88.61 | |
| PMI1707 | dmsC | Anaerobic dimethyl sulfoxide reductase chain C | 655 | 2,641 | 87.94 |
| PMI2846 | dppB | Dipeptide ABC transporter, permease protein | 759 | 2,116 | 87.06 |
| PMI2466 | Putative plasmid-related protein | 1,286 | 3,103 | 83.09 | |
| PMI0144 | Putative ATP-binding protein | 1,204 | 2,462 | 82.19 | |
| PMI2847 | dppA | Dipeptide ABC transporter, substrate-binding protein | 1,241 | 2,463 | 81.34 |
| PMI1945 | ireA | Putative TonB-dependent ferric siderophore receptor | 722 | 2,667 | 78.99 |
| PMI0434 | gltL | Glutamate/aspartate ABC transporter, ATP-binding protein | 1,048 | 2,580 | 74.90 |
| PMI2529 | rpiB | Putative ribose 5-phosphate isomerase | 159 | 1,396 | 72.28 |
Median expression rank across nine microarrays (including ribosomal genes): 1 is most highly expressed, and 3,719 is most weakly expressed.
Median fold change in vivo compared to LB broth across nine microarrays.
ORF, open reading frame.
Table 2.
The 50 nonribosomal genes most highly expressed in vivo in this study
| ORFc | Gene | Annotation | Ranka |
Fold changeb | |
|---|---|---|---|---|---|
| In vivo | In vitro | ||||
| PMI1035 | Translation initiation factor IF-3 | 9 | 3 | −1.33 | |
| PMI0765 | ompF | Outer membrane porin | 13 | 3 | −1.25 |
| PMI3251 | tufB | Elongation factor Tu | 13 | 6 | −1.23 |
| PMI3275 | secY | Preprotein translocase SecY subunit | 23 | 20 | 1.03 |
| PMI3280 | rpoA | DNA-directed RNA polymerase alpha chain | 26 | 19 | −1.32 |
| PMI2792 | tufB | Elongation factor Tu | 28 | 29 | −1.16 |
| PMI0856 | Conserved hypothetical protein | 29 | 36 | −1.85 | |
| PMI0862 | acpP | Acyl carrier protein | 33 | 16 | −1.66 |
| PMI0263 | mrpA | Major mannose-resistant/Proteus-like fimbria | 37 | 3,040 | 13,806.33 |
| PMI0785 | ompA | Outer membrane protein A | 37 | 50 | 1.21 |
| PMI1676 | cspC | Cold shock-like protein | 39 | 9 | −2.09 |
| PMI2771 | hupA | DNA-binding protein HU-alpha (HU-2) | 52 | 60 | −1.39 |
| PMI3375 | priB | Primosomal replication protein N | 52 | 34 | −1.61 |
| PMI2793 | fusA | Elongation factor G (EF-G) | 52 | 80 | 1.17 |
| PMI0716 | ihfB | Integration host factor beta-subunit | 57 | 53 | −1.25 |
| PMI0384 | rimM | 16S rRNA processing protein | 59 | 59 | −1.28 |
| PMI2284 | tsf | Elongation factor Ts | 59 | 75 | −1.00 |
| PMI3059 | atpE | ATP synthase C chain | 59 | 57 | −1.47 |
| PMI1213 | ahpC | Alkyl hydroperoxide reductase subunit C | 62 | 165 | 6.14 |
| PMI3062 | atpA | ATP synthase alpha chain | 62 | 68 | −1.07 |
| PMI3415 | secG | Protein export membrane protein | 66 | 71 | −1.05 |
| PMI0073 | ahpC | Putative alkyl hydroperoxide reductase | 67 | 104 | 1.83 |
| PMI3063 | atpG | ATP synthase gamma chain | 68 | 70 | −1.14 |
| PMI0385 | trmD | tRNA (guanine-N1)-methyltransferase | 69 | 65 | −1.69 |
| PMI0585 | pal | Peptidoglycan-associated lipoprotein | 71 | 46 | −2.60 |
| PMI3060 | atpF | ATP synthase B chain | 71 | 60 | −1.39 |
| PMI1504 | gapA | Glyceraldehyde 3-phosphate dehydrogenase A | 72 | 81 | 1.33 |
| PMI0118 | hupB | DNA-binding protein HU-beta | 75 | 76 | −1.70 |
| PMI3064 | atpD | ATP synthase beta chain | 75 | 69 | −1.18 |
| PMI3065 | atpC | ATP synthase epsilon chain | 78 | 84 | −1.22 |
| PMI3061 | atpH | ATP synthase delta chain | 81 | 83 | −1.15 |
| PMI2544 | groS | 10-kDa chaperonin | 82 | 66 | −1.26 |
| PMI3057 | atpI | ATP synthase protein I | 82 | 85 | −1.35 |
| PMI3296 | Conserved hypothetical protein | 83 | 96 | −1.11 | |
| PMI0418 | cspE | Putative cold shock protein | 84 | 69 | −2.52 |
| PMI0861 | fabG | 3-Oxoacyl-[acyl-carrier protein] reductase | 85 | 79 | −1.67 |
| PMI1039 | pheT | Phenylalanyl-tRNA synthetase beta chain | 93 | 103 | 1.06 |
| PMI2784 | rpoC | DNA-directed RNA polymerase beta′ subunit | 93 | 95 | −1.15 |
| PMI1898 | grcA | Autonomous glycyl radical cofactor | 95 | 788 | 19.18 |
| PMI3622 | fis | DNA-binding protein Fis | 96 | 90 | −1.37 |
| PMI1034 | thrS | Threonyl-tRNA synthetase | 96 | 90 | −1.27 |
| PMI0114 | tig | Trigger factor | 98 | 98 | −1.15 |
| PMI1828 | ptsH | Phosphotransferase system phosphocarrier protein | 100 | 683 | 16.77 |
| PMI1488 | hns | DNA-binding protein (histone-like structuring protein) | 100 | 93 | −1.41 |
| PMI3364 | miaA | tRNA delta(2)-isopentenylpyrophosphate transferase | 102 | 137 | −1.11 |
| PMI2833 | Putative endoribonuclease | 103 | 124 | −1.02 | |
| PMI0842 | TonB-dependent receptor | 104 | 913 | 17.18 | |
| PMI3058 | atpB | ATP synthase A chain | 106 | 77 | −1.66 |
| PMI0243 | fbaA | Fructose-bisphosphate aldolase class II | 110 | 144 | 1.61 |
| PMI0691 | infA | Translation initiation factor IF-1 | 111 | 101 | −1.60 |
Median expression rank across nine microarrays (including ribosomal genes): 1 is most highly expressed, and 3,719 is most weakly expressed.
Median fold change in vivo compared to LB broth across nine microarrays.
ORF, open reading frame.
Table 3.
The 50 genes most highly downregulated in vivo in this study
| ORFc | Gene | Annotation | Ranka |
Fold changeb | |
|---|---|---|---|---|---|
| In vivo | In vitro | ||||
| PMI1629 | fliE | Flagellar hook-basal body complex protein | 2,748 | 492 | −88.25 |
| PMI0994 | Putative lipoprotein | 2,785 | 506 | −70.63 | |
| PMI1654 | flgB | Flagellar basal-body rod protein | 2,254 | 465 | −47.91 |
| PMI1618 | fliA | RNA polymerase sigma factor for flagellar operon | 1,448 | 136 | −39.10 |
| PMI1651 | flgE | Flagellar hook protein | 2,232 | 439 | −35.30 |
| PMI1877 | pmfA | Major fimbrial subunit | 671 | 66 | −29.64 |
| PMI1650 | flgF | Flagellar basal-body rod protein | 1,943 | 435 | −23.85 |
| PMI1096 | 2-Oxoglutarate and Fe(II)-dependent oxygenase superfamily protein | 1,794 | 243 | −21.27 | |
| PMI1636 | fliL | Flagellar protein FliL | 2,049 | 503 | −20.33 |
| PMI1649 | flgG | Flagellar basal-body rod protein (distal rod protein) | 1,716 | 502 | −20.03 |
| PMI0993 | Putative lipoprotein | 2,330 | 540 | −19.98 | |
| PMI1653 | flgC | Flagellar basal-body rod protein | 2,525 | 703 | −19.72 |
| PMI1644 | flgL | Flagellar hook-associated protein 3 (hook-filament junction protein) | 2,323 | 674 | −19.41 |
| PMI0488 | Putative phage membrane protein | 1,245 | 394 | −19.10 | |
| PMI1941 | Conserved hypothetical protein | 2,460 | 1,084 | −18.60 | |
| PMI2109 | mgtE | Magnesium transporter | 1,769 | 412 | −15.85 |
| PMI1638 | fliN | Flagellar motor switch protein | 2,409 | 710 | −15.41 |
| PMI1637 | fliM | Flagellar motor switch protein | 2,410 | 679 | −15.38 |
| PMI2814 | RpiR-family transcriptional regulator | 2,364 | 873 | −15.02 | |
| PMI3177 | kbl | 2-Amino-3-ketobutyrate coenzyme A ligase | 1,871 | 471 | −12.99 |
| PMI1631 | fliG | Flagellar motor switch protein | 2,449 | 704 | −12.48 |
| PMI1469 | fim8A | Fimbrial subunit | 1,787 | 593 | −11.97 |
| PMI0022 | Putative cell killing protein | 1,859 | 441 | −11.88 | |
| PMI1595 | pagP | Putative antimicrobial peptide resistance and lipid A acylation protein | 1,662 | 550 | −10.92 |
| PMI1622 | fliS | Flagellar protein FliS | 1,825 | 905 | −10.83 |
| PMI0291 | treB | Phosphotransferase system, trehalose-specific IIBC component | 2,792 | 941 | −10.65 |
| PMI0596 | Putative membrane protein | 1,338 | 174 | −10.58 | |
| PMI1656 | flgM | Negative regulator of flagellin synthesis | 1,614 | 718 | −10.24 |
| PMI3178 | tdh | l-Threonine 3-dehydrogenase | 1,464 | 482 | −10.21 |
| PMI1943 | Major facilitator superfamily transporter | 1,415 | 207 | −10.15 | |
| PMI1620 | flaA/fliC1 | Flagellin 1 | 750 | 108 | −9.59 |
| PMI1666 | cheD | Methyl-accepting chemotaxis protein | 2,481 | 936 | −9.53 |
| PMI1645 | flgK | Flagellar hook-associated protein 1 | 2,057 | 807 | −9.29 |
| PMI0044 | Putative outer membrane protein | 1,442 | 363 | −8.33 | |
| PMI1621 | flaD/fliD | Flagellar hook-associated protein 2 | 2,306 | 1,261 | −8.29 |
| PMI3211 | glpF | Glycerol uptake facilitator protein | 1,171 | 217 | −7.52 |
| PMI1506 | mipA | MltA-interacting protein precursor | 566 | 107 | −7.37 |
| PMI1879 | pmfD | Fimbrial chaperone protein | 1,504 | 659 | −6.98 |
| PMI1489 | wbnF | Probable nucleotide sugar epimerase | 1,730 | 387 | −6.98 |
| PMI1617 | fliZ | FliZ protein | 979 | 240 | −5.85 |
| PMI1061 | fim5A | Fimbrial protein | 1,945 | 752 | −5.51 |
| PMI2203 | Putative isochorismatase | 1,828 | 799 | −5.42 | |
| PMI0580 | Putative thioesterase | 1,432 | 550 | −4.99 | |
| PMI2093 | speB | Agmatinase | 853 | 172 | −4.98 |
| PMI2094 | speA | Biosynthetic arginine decarboxylase | 898 | 285 | −4.93 |
| PMI0582 | tolR | Biopolymer transport protein | 1,280 | 379 | −4.77 |
| PMI1958 | Putative DNA-damage-inducible protein | 1,436 | 658 | −4.31 | |
| PMI2930 | glpD/glyD | Aerobic glycerol-3-phosphate dehydrogenase | 458 | 97 | −3.96 |
| PMI0682 | artP | Arginine ABC transporter, ATP-binding protein | 470 | 187 | −3.74 |
| PMI1286 | osmB | Osmotically inducible lipoprotein | 1,692 | 925 | −3.68 |
Median expression rank across nine microarrays (including ribosomal genes): 1 is most highly expressed, and 3,719 is most weakly expressed.
Median fold change in vivo compared to LB broth across nine microarrays.
ORF, open reading frame.
Table 4.
Categories of genes differentially regulated in vivo
| Category | No. of genes: |
|
|---|---|---|
| Upregulated in vivo | Downregulated in vivo | |
| Amino acid transport and metabolism | 58 | 7 |
| Carbohydrate transport and metabolism | 37 | 4 |
| Cell cycle control, cell division, and chromosome partitioning | 6 | 0 |
| Cell motility | 0 | 19 |
| Cell wall/membrane/envelope biogenesis | 19 | 9 |
| Coenzyme transport and metabolism | 17 | 3 |
| Defense mechanisms | 1 | 0 |
| Energy production and conversion | 50 | 4 |
| Fimbriae | 10 | 4 |
| Function unknown | 57 | 12 |
| General function prediction only | 42 | 4 |
| Inorganic ion transport and metabolism | 41 | 2 |
| Intracellular trafficking, secretion, and vesicular transport | 2 | 1 |
| Lipid transport and metabolism | 6 | 0 |
| Nucleotide transport and metabolism | 17 | 0 |
| Phage and mobile element | 20 | 1 |
| Posttranslational modification, protein turnover, and chaperones | 24 | 2 |
| Replication, recombination, and repair | 19 | 1 |
| Secondary metabolite biosynthesis, transport, and catabolism | 4 | 1 |
| Signal transduction mechanisms | 5 | 0 |
| Transcription | 25 | 3 |
| Translation, ribosomal structure, and biogenesis | 12 | 5 |
Virulence gene expression.
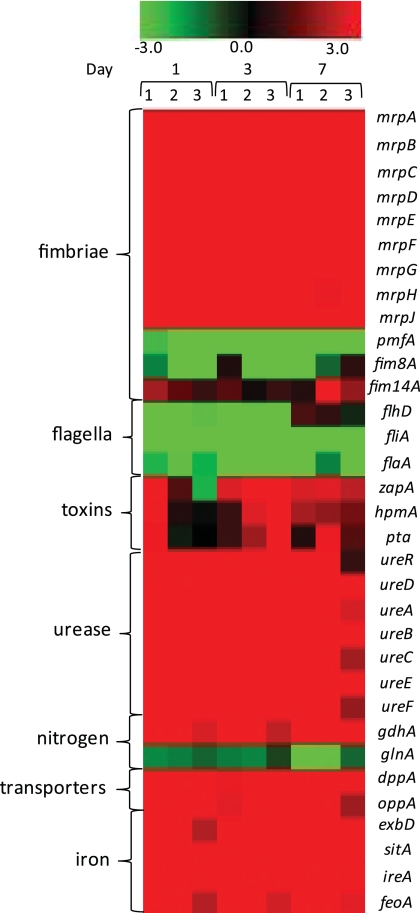
Regulation of genes previously identified in P. mirabilis virulence was specifically examined (Fig. 1). As mentioned, the mrp operon is both highly upregulated (Fig. 1) and highly expressed in vivo. However, pmfA, which encodes the major structural subunit of Proteus mirabilis fimbriae (PMF), was downregulated in vivo despite having a role in bladder (49, 86) and kidney (86) colonization in mice. Genes coding for two other fimbriae, previously identified as virulence factors by signature-tagged mutagenesis (12, 26), were either downregulated (fim8A) or upregulated (fim14A), although neither was highly expressed in vivo. Genes encoding three P. mirabilis toxins, zapA (44, 83), hemolysin hpmA (77), and pta (Proteus toxic agglutinin) (2), were expressed at approximately background levels in vivo and were inconsistently upregulated. The entire urease operon, which is induced in the presence of urea, was both highly expressed and highly upregulated in vivo. Peptide transporter genes dppA and oppA, which have been implicated in virulence for both P. mirabilis (64) and UPEC (4), were upregulated in vivo; iron uptake genes (exbBD, sitABC, hmuST, ireA, nrpY, feoAB, PMI0331, PMI0363, PMI0842, PMI1424, PMI1437, and PMI2957 to PMI2960) were similarly upregulated.
Fig. 1.
Heat map of expression data for specific virulence-associated genes, depicting the ratio of expression in LB broth versus in vivo. The legend at the top indicates the color associated with log2 fold change: red, upregulated in vivo; green, downregulated in vivo; black, not differentially regulated.
Other genes of interest that were upregulated in vivo included pyruvate metabolism genes (aceEF, pta, ackA, poxB, pflB, and dsdA), genes likely involved in osmoprotection (betU, putP, gltS, gltL, and gadC), and eight genes within the previously identified mobile integrated and conjugative element ICEPm1 (PMI2568, PMI2573, PMI2595, PMI2598, PMI2608, PMI2627, PMI2628, and PMI2629) (18).
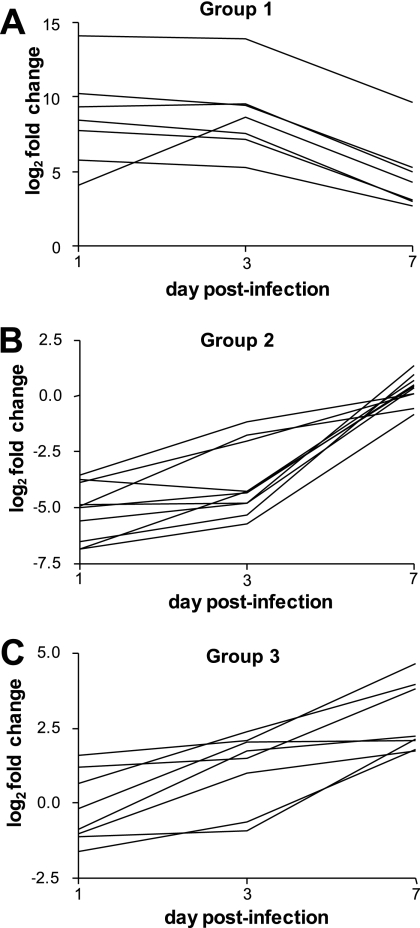
Temporal gene regulation.
Microarrays were also analyzed separately at each time point (1, 3, and 7 days postinfection), and some temporal regulation was noted. At 1 day postinfection, 93 genes were significantly upregulated in vivo; 5 genes were downregulated. At 3 days postinfection, 294 genes were upregulated and 6 were downregulated. At 7 days postinfection, 204 genes were upregulated and 1 gene was downregulated (data not shown). The BETR algorithm identified three groups of genes that were differentially regulated over time (Fig. 2). The first group consists of genes that were highly induced in vivo at day 1 but by day 7 were less upregulated (Fig. 2A). This group of seven genes included three genes from the mrp operon (mrpA, mrpE, and mrpH). Three other genes in this group are predicted to encode transporters (PMI0272 and PMI2947), including a putative di-/tripeptide transporter (PMI1688). The second group of genes was repressed at day 1 but by day 7 had similar expression compared to genes from cells cultured in LB broth (Fig. 2B). Of the 10 genes in group 2, 5 encode flagellar components (fliN, flgD, fliH, flhD, and flgL). Three genes in this group encode putative lipoproteins (PMI0977, PMI0994, and PMI1840). The third group consisted of genes that were either slightly repressed or not differentially regulated at day 1 but were induced at day 7 (Fig. 2C). There were eight genes in this group, including the transcriptional regulator hexA.
Fig. 2.
P. mirabilis genes are temporally regulated in vivo. Nine microarrays were analyzed for differential regulation of genes over time using the BETR algorithm. (A) Group 1 genes were highly induced in vivo at day 1 but less highly expressed by day 7. (B) Group 2 genes were repressed in vivo at day 1 but were not differentially expressed compared to in vitro at day 7. (C) Group 3 genes were either slightly repressed or not differentially regulated at day 1, but were induced at day 7.
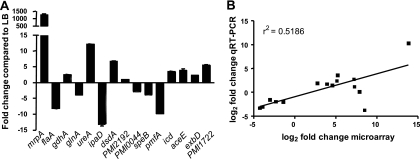
Validation of the microarrays by qRT-PCR.
Expression of 15 genes that were found to be differentially regulated by microarray in vivo compared to LB was also measured by qRT-PCR (Fig. 3A). Urine collected from mice 3 days postinfection was used as the in vivo source of RNA. Microarray and qRT-PCR data were in agreement for 13 of the 15 genes, including the very strong in vivo upregulation of mrpA. Two genes predicted to be upregulated in vivo by microarray, ipaD and PMI2192, were either downregulated (ipaD) or not differentially regulated (PMI2192) by qRT-PCR. To test the concordance of microarray and qRT-PCR data, the log2-transformed fold changes were plotted against one another (Fig. 3B). Linear regression calculations confirmed the significant agreement between microarray and qRT-PCR data (r2 = 0.5186, P = 0.0025).
Fig. 3.
qRT-PCR validation of in vivo microarrays. (A) Expression of 15 genes in vivo compared to during logarithmic-phase growth in LB culture as measured by qRT-PCR. (B) Concordance of microarray and qRT-PCR data for the 15 genes depicted in panel A. The log2-transformed fold change values are plotted, with linear regression shown. Median fold change at 3 days was used for the microarray data points. The r2 value is 0.5186, and the slope has a significant deviation from zero (P = 0.0025).
Glutamate dehydrogenase is upregulated during urinary tract infection.
Ammonia is the preferred nitrogen source for enteric bacteria. The two primary ammonia incorporation systems, gdhA (PMI3008; glutamate dehydrogenase [GDH]) and glnA (PMI2882; glutamine synthetase [GS]), were inversely regulated in vivo, with gdhA upregulated 10.75-fold and glnA downregulated 2.78-fold (Fig. 1). The opposite result was observed for UPEC in vivo, in which gdhA was slightly downregulated (1.75-fold) and glnA was upregulated 12-fold (74). Glutamine synthetase is the primary ammonia uptake system when nitrogen levels are limited, while glutamate dehydrogenase confers ammonia uptake when ammonia levels are high or, correspondingly, when energy is limited (25).
Nitrogen assimilation appears to be handled differently by P. mirabilis than UPEC during UTI based on microarray and qPCR data assessing transcription. However, because nitrogen assimilation can be regulated posttranslationally as well as transcriptionally, we attempted to correlate the transcript levels of urease, GDH, and GS with the enzyme activities of these gene products (see below).
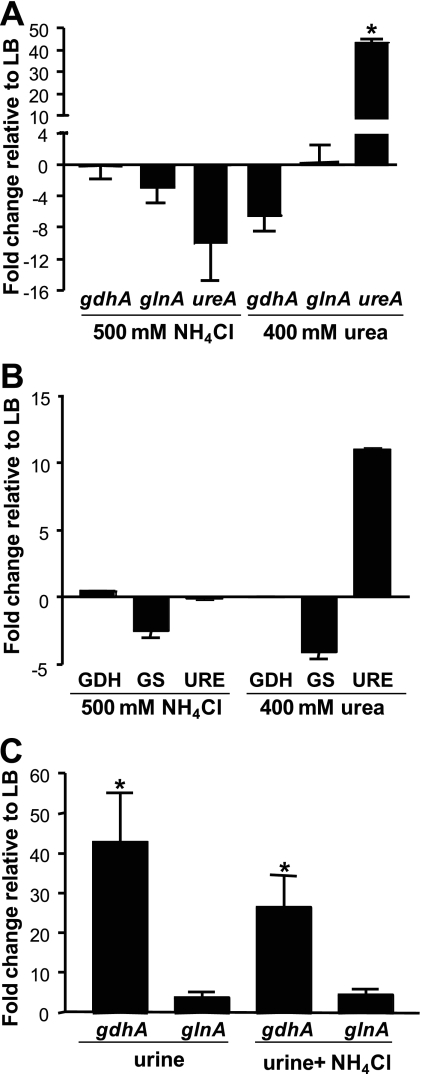
GDH is not upregulated in excess ammonia alone.
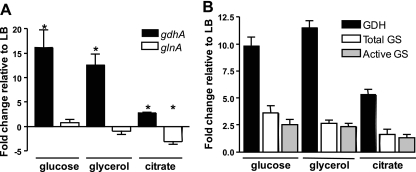
To define in vitro culture conditions that yielded in vivo gene expression patterns, we conducted a series of experiments. Upregulation of gdhA and repression of glnA in vivo by P. mirabilis suggested that ammonia incorporation drives the expression of these genes in P. mirabilis. To test the effect of nitrogen availability on gdhA and glnA gene expression, P. mirabilis HI4320 was cultured in LB broth with or without 500 mM NH4Cl. As assessed by qRT-PCR (Fig. 4A), gdhA was not upregulated; however, as expected, glnA was downregulated. Similar results were obtained by using NH4Cl concentrations ranging from 100 mM to 1 M (data not shown). As assessed by enzyme activity (Fig. 4B), GDH was also not significantly upregulated when P. mirabilis was cultured in 500 mM NH4Cl. GS enzyme activity was downregulated under this condition. Therefore, in this experiment, transcription, as assessed by microarray and qRT-PCR, serves as a proxy for enzyme activity. NH4Cl, however, in this experiment was supplied exogenously, while in vivo, urea in urine is hydrolyzed to ammonia by intracellular urease. Therefore, P. mirabilis was also cultured in LB broth supplemented with 400 mM urea (within the normal human physiological concentration range for urine). Again, gdhA (Fig. 4A) and GDH enzyme activity (Fig. 4B) were not upregulated compared to those in the cells cultured in LB broth, although urease gene ureA expression (mean of 43.5-fold) and urease enzyme activity were highly upregulated during culture in urea (Fig. 4A and B). The pH of the urea culture was approximately 8.5 at the time bacteria were harvested, as a consequence of urease activity.
Fig. 4.
Exogenous nitrogen is not the driving force behind in vivo gdhA and glnA expression. (A) RNA was isolated from P. mirabilis HI4320 cultured in LB supplemented with either 500 mM NH4Cl or 400 mM urea. No significant changes in gdhA or glnA were found by qRT-PCR in comparison to unsupplemented LB. However, addition of urea significantly upregulated expression of ureA. (B) Enzyme activities were determined for whole-cell lysates of P. mirabilis HI4320 cultured under identical conditions to those described for panel A. Glutamate dehydrogenase (GDH), total glutamine synthetase (GS), and urease (URE) enzyme activities (average of five technical replicates) were measured and expressed as fold change relative to preparations from P. mirabilis cultured in LB broth. (C) A ureC mutant was cultured in LB broth or pooled human urine with or without 100 mM NH4Cl, and expression of gdhA and glnA was measured by qRT-PCR. (A and C) Expression was normalized to rpoA. At least three independent experiments were conducted for each condition tested. Error bars represent standard errors of the mean. *, P < 0.05.
To assess whether a component in urine might contribute to the differential expression of gdhA and glnA, we used a ureC mutant that is unable to make functional urease (33) and therefore does not increase the pH of urea-containing cultures. This mutant was cultured to the logarithmic phase in pooled human urine with or without 100 mM NH4Cl supplementation. Compared to ureC cells cultured in LB, gdhA was significantly upregulated in urine with or without NH4Cl (38.6-fold and 24.5-fold, respectively) (Fig. 4C). However, glnA was not differentially regulated. These data suggest that upregulation of gdhA in vivo was not due to ammonia excess per se and that some other undefined factor was regulating gdhA and glnA.
High osmolarity alone does not induce GDH.
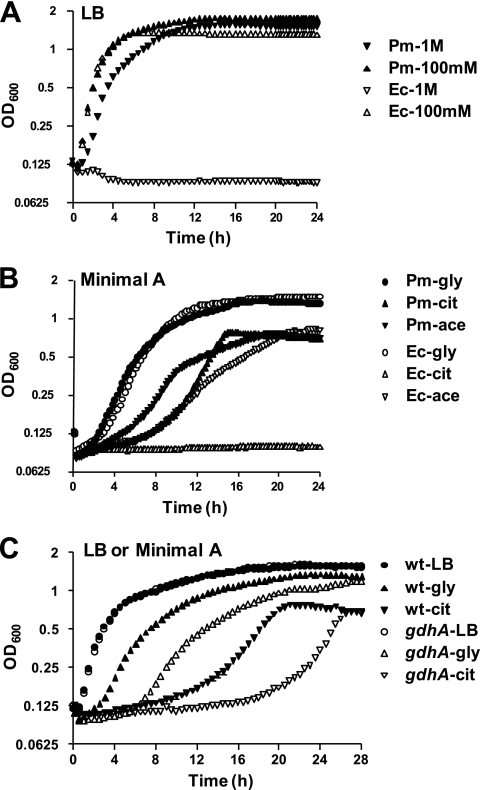
Because gdhA and glnA were expressed differentially between P. mirabilis and UPEC in vivo, we sought to determine additional differences in the physiology of these species that might provide clues regarding this pattern of gene expression. Urine is generally hyperosmotic, averaging 500 to 800 mosmol/kg in humans and 1,040 mosmol/kg in female CBA mice (21), which can interfere with bacterial growth (7). In addition, glutamate, a product of gdhA upregulation, may be used to buffer against osmotic stress (reviewed in reference 45). Thus, P. mirabilis HI4320 and E. coli CFT073 were each cultured in LB supplemented with NH4Cl over a range of 0 to 1 M. While growth was similar for P. mirabilis and E. coli at lower salt concentrations, E. coli was unable to grow at 1 M NH4Cl (Fig. 5A). In contrast, P. mirabilis growth in 1 M NH4Cl was only slightly retarded compared to that at lower salt concentrations (Fig. 5A). However, because gdhA is not upregulated in 500 mM NH4Cl (Fig. 4A) or 1 M NH4Cl (data not shown), osmotic buffering was ruled out as the sole cause for differential gene expression in P. mirabilis and E. coli.
Fig. 5.
Comparison of growth of P. mirabilis HI4320 (Pm) and E. coli CFT073 (Ec) under high-osmolarity conditions or with specific carbon sources. (A) LB broth supplemented with either 100 mM or 1 M NH4Cl; (B) minimal A medium with 1% glycerol, citrate, or acetate as the carbon source; (C) P. mirabilis HI4320 and HI4320 gdhAΩkan cultured in LB broth or minimal A medium with glycerol or citrate as the carbon source. At least three independent experiments were conducted for each condition tested; one representative experiment with three technical replicates is shown.
P. mirabilis uses citrate as a carbon source, while UPEC does not.
Citrate, secreted in human urine at a rate of 3 to 20 mg/kg/day (5), is also a key intermediate of the TCA cycle and was a candidate carbon source that upregulates gdhA. Most P. mirabilis strains can use citrate as the sole carbon source (29), while most E. coli strains cannot. To test whether P. mirabilis HI4320 and E. coli CFT073 differ in their ability to use specific carbon sources, both strains were cultured in minimal medium supplemented with 1% glycerol, citrate, or acetate. P. mirabilis HI4320 was able to use all three carbon sources, while UPEC strain CFT073 was unable to utilize citrate (Fig. 5B).
A gdhA mutant has a growth defect in minimal medium containing citrate as the sole carbon source.
Opposing regulation of gdhA in P. mirabilis and E. coli, coupled with differing abilities to utilize citrate, led us to hypothesize that GDH might play a unique role in central metabolism for P. mirabilis. Therefore, gdhA was inactivated by insertion of a targeted transposon, and the ability of the mutant to grow in rich and minimal media was compared to that of the wild-type isogenic parent strain (Fig. 5C). The gdhA mutant displayed a slight growth defect in minimal medium with glycerol as a carbon source but a more pronounced defect when citrate was the sole carbon source (Fig. 5C). As with E. coli (68), there was no difference in growth rates of wild-type P. mirabilis and the gdhA mutant in LB broth.
Culture in citrate recapitulates in vivo nitrogen gene expression patterns.
Since P. mirabilis gdhA expression is increased in vivo, and a gdhA mutant has a growth defect when cultured in minimal medium with citrate as a carbon source, we postulated that citrate could be directly affecting expression of ammonia incorporation genes gdhA and glnA. Therefore, wild-type P. mirabilis was cultured to the mid-logarithmic phase in LB or minimal medium, and gene expression of gdhA and glnA was measured by qRT-PCR. Expression of gdhA (Fig. 6A) and GDH enzyme activity (Fig. 6B) were significantly increased in all three carbon sources tested (glucose, glycerol, and citrate) compared to the levels in LB broth, but glnA expression was significantly decreased only when citrate was used as the carbon source (Fig. 6A). GS enzyme activity was also lower in citrate than in glucose or glycerol (Fig. 6B). Thus, culture in citrate mimics in vivo conditions with respect to the expression pattern of gdhA and glnA.
Fig. 6.
Citrate as the sole carbon source drives in vivo expression patterns of gdhA and glnA. (A) P. mirabilis HI4320 was cultured in LB or minimal A medium with glucose, glycerol, or citrate as the carbon source. qRT-PCR analysis of RNA revealed upregulation of gdhA in minimal A medium with all three carbon sources, but gdhA was only downregulated when citrate was used as the carbon source. At least three independent experiments were conducted for each condition tested. Expression of rpoA, routinely used for normalization of gene expression by P. mirabilis, was decreased in minimal medium relative to LB; therefore, rpmC was used to normalize expression in these experiments. *, P < 0.05. (B) Enzyme activities were determined for whole-cell lysates of P. mirabilis HI4320 cultured under identical conditions to those described for panel A. Glutamate dehydrogenase (GDH), total glutamine synthetase (GS), and unadenylylated (active) GS enzyme activities (average of five technical replicates from each of two independent preparations) were measured and expressed as fold change relative to preparations from P. mirabilis cultured in LB.
gdhA contributes to in vivo fitness of P. mirabilis.
Based on the experiments described above, we hypothesized that GDH plays a central metabolic role during infection of the urinary tract. To test this, the gdhA mutant was mixed 1:1 with the isogenic parent strain and transurethrally inoculated into the bladders of 10 mice. After 7 days, the bacterial burdens of the gdhA mutant and wild type were enumerated in the bladders, kidneys, and spleens of these mice. As assessed by the competitive indices, the gdhA mutant was defective in colonization of all three sites tested (P < 0.05) (Fig. 7), suggesting that GDH is necessary for P. mirabilis fitness in the urinary tract, perhaps serving as a checkpoint for intracellular carbon and nitrogen levels.
Fig. 7.
HI4320 gdhAΩkan is less fit in vivo than wild-type HI4320. Ten female CBA/J mice were transurethrally infected with a 1:1 mixture of HI4320 and HI4320 gdhAΩkan. After 7 days, bacteria were enumerated in the bladder, kidneys, and spleen. The competitive index (CI) of the gdhA mutant compared to the wild type was plotted; each dot represents the CI from one mouse. The mutant was significantly outcompeted by the wild type in all three organs. Bars indicate median values.
DISCUSSION
This is the first microarray analysis of transcription by an agent of complicated UTI during an infection, as well as the first study examining global temporal control of bacterial gene expression during UTI. Previous studies have examined gene expression by E. coli during UTI in mice (74), in humans with asymptomatic bacteriuria (70), and in patients with UTI (22). There are several similarities in gene expression by P. mirabilis and E. coli during UTI. Both P. mirabilis and E. coli activate systems involved in iron uptake, pyruvate catabolism, peptide uptake, and osmoprotection. Both species have robust metabolism in vivo, as indicated by high expression of ribosomal genes. P. mirabilis and UPEC each upregulate a specific fimbrial operon in vivo (mrp and fim, respectively), although the upregulation of mrp is much stronger than that of fim (13,806-fold versus 7.82-fold for mrpA and fimA, respectively). Likewise, both pathogens downregulate flagellar and chemotaxis genes in vivo.
P. mirabilis in vivo global gene expression not only was verified by qRT-PCR but also agrees with previous reports on P. mirabilis virulence. Genes encoding MR/P fimbriae were the most highly upregulated genes in vivo, with the major structural gene mrpA upregulated 13,800-fold (median). Previous studies have shown that increased expression of MR/P fimbriae correlates with higher levels of colonization in the mouse bladder (36), the majority of bacteria in the bladder and ureters express MR/P fimbriae (31), and an mrp mutant is attenuated in the mouse model of ascending UTI (8). Furthermore, vaccine trials in mice using intact MR/P fimbriae (39) or recombinant components of MR/P fimbriae (38, 39, 73) reduce bacterial burden after challenge. Expression of MR/P fimbriae has not been tested during human infection; this experiment will be critical to future vaccine efforts since UPEC highly expresses type I fimbriae in mice (56, 74), and these fimbriae are required for virulence in mice (14, 46), but type I fimbriae are not well expressed or possibly transiently expressed during human UTI (22). The last gene of the mrp operon, mrpJ, encodes a transcriptional repressor of flagella (40). The high expression of this gene in vivo is consistent with reduced expression of flagella during murine UTI. Interestingly, the mrp operon upregulation is highest at days 1 and 3 postinfection (mrpJ median of 1,189-fold upregulated), and less at day 7 (mrpJ median of 126-fold upregulated); at the same time, the repression of flagellar genes may be easing by day 7. (The flagellar master regulator flhD is downregulated a median of 12.5-fold at days 1 and 3 and upregulated a median of 1.59-fold at day 7.) Additionally, another regulator of flagellum-mediated motility, hexA (64), was upregulated at day 7. These data suggest a potential increased role for flagella and corresponding lesser role for MR/P fimbriae at this later stage in the disease process. During experimental UPEC UTI, flagellar genes are transiently upregulated 4 h postinfection, when the bacteria are ascending the ureters from the bladder to the kidneys (35). It will be useful to examine P. mirabilis fimbrial and flagellar gene expression at early time points (less than 24 h), as well as to study global temporal gene transcription during UPEC UTI.
Other genes found to be upregulated in vivo also agree with single-gene-knockout virulence studies. Iron uptake systems upregulated in vivo are required for colonization (hmuR2) (42) and virulence (pbtA) (27) in mice, and many are immunogenic (PMI0409, PMI0842, hmuR2, ireA, and PMI2596) (58). Zinc uptake systems are also required for virulence (59), are immunogenic in mice (58), and were upregulated in vivo. Likewise, urease, a well-documented virulence factor for P. mirabilis (12, 20, 32, 33, 54), was upregulated. Toxin genes (hpmA, zapA, and pta) were a notable exception; these genes were expressed at or below background levels both in vitro and in vivo. It is possible that expression of these genes is more localized to specific sites in the urinary tract or that subtle changes in transcript level lead to much larger changes in protein activity. Two classes of genes which are involved with virulence were downregulated in vivo: flagella and PMF fimbriae. Flagella are energetically expensive to construct and maintain; they also activate innate immune response via Toll-like receptor 5 (17). Thus, flagellar regulation is likely tightly controlled in vivo, much as it is temporally controlled during experimental UPEC UTI (35). The role of PMF fimbriae is unknown at this time, although knockout studies have shown these fimbriae are required for full virulence (49, 86, 87), and components of PMF fimbriae have been successfully used in experimental vaccines (73). It is possible these fimbriae are required for adherence to specific tissues, which prevents PMF-expressing bacteria from being shed into the urine. Alternatively, like flagella, PMF fimbriae are only required during a limited time during UTI progression and may be tightly regulated in vivo.
There were several differences between P. mirabilis and E. coli transcription in vivo. Although both P. mirabilis and UPEC upregulate pyruvate catabolism pathways in vivo, there are more, and more highly upregulated, genes leading to acetate secretion by P. mirabilis. Increased acetate secretion, which lowers pH, might be a useful counter to urease-induced alkalinization. In addition to peptide uptake, surprisingly, P. mirabilis also upregulates genes involved in glucose uptake (crr and ptsG) and glycolysis (pgi, pfkA, and gpmAB) in vivo. Certainly, both E. coli and P. mirabilis can utilize glucose. With the exception of uncontrolled diabetes, generally glucose is not present in urine. Thus, the apparent ability of P. mirabilis to obtain glucose during UTI warrants further investigation.
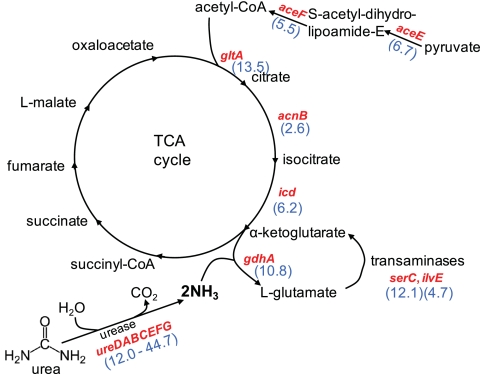
With respect to nitrogen assimilation, there are some intriguing differences between P. mirabilis and UPEC transcription in vivo. The potent urease encoded by P. mirabilis, but absent in UPEC, ensures an abundant source of ammonia in the urinary tract (Fig. 8). Thus, while UPEC is starved for nitrogen in vivo (22, 74), the carbon-nitrogen balance for P. mirabilis may be flipped. Likewise, the genes encoding a portion of the TCA cycle (gltA, acnB, and icd), comprising the steps from acetyl coenzyme A (acetyl-CoA) formation to α-ketoglutarate synthesis, are upregulated by P. mirabilis in vivo (Fig. 8), while the rest of the TCA cycle genes in P. mirabilis were not differentially regulated in vivo. No such upregulation occurs in UPEC during UTI. (Indeed one gene, acnA, is even downregulated in vivo.) At this point in the P. mirabilis cycle, α-ketoglutarate can continue in the TCA cycle to make succinyl-CoA or be converted by incorporation of ammonia to generate l-glutamate via glutamate dehydrogenase (GDH). We initially hypothesized that expression of gdhA and glnA differs in P. mirabilis and UPEC in the urinary tract because of nitrogen availability.
Fig. 8.
In vivo upregulated genes suggest carbon-nitrogen balance drives P. mirabilis central metabolism. The intersection of the TCA cycle with pyruvate catabolism and ammonia assimilation is depicted. Genes upregulated in vivo >2-fold are shown in red; fold increases (blue) are indicated in parentheses.
Indeed, regulation of nitrogen uptake and assimilation is complex, with multiple levels of transcriptional and posttranslational control (reviewed in references 37 and 67). The carbon/nitrogen ratios for P. mirabilis and UPEC in the urinary tract are certainly not the same. Continuous high levels of GDH activity in vivo would lead to diminished α-ketoglutarate pools (30), and therefore, upregulation of upstream TCA cycle enzymes would be required to maintain the full TCA cycle (i.e., to convert α-ketoglutarate to succinyl-CoA) (Fig. 8). Although inverse in vivo regulation of gdhA and glnA by P. mirabilis and UPEC initially was suspected to be solely due to urease activity in P. mirabilis, this was not the case. Addition of exogenous ammonium (NH4Cl) or intracellular ammonia (catalyzed by urease-mediated urea hydrolysis) in culture did not lead to the differential gene expression observed in vivo. However, choice of carbon source, such as citrate, a major component of urine, had a profound effect on gdhA expression. Rich media (19), the presence of specific amino acids in minimal medium (68), or a greater carbon/nitrogen ratio (69) have been reported to repress gdhA. Only when citrate was used as the carbon source, which forces the TCA cycle to operate, did we see a concomitant decrease in glnA expression. However, although P. mirabilis HI4320 can utilize citrate as a carbon source and E. coli cannot, there are more favorable carbon sources available in the urinary tract. Additionally, not all P. mirabilis strains utilize citrate as the sole carbon source (29), and future studies on such strains will be informative.
Glutamate dehydrogenase plays a critical metabolic role during P. mirabilis UTI. gdhA mutants, however, generally have only a subtle phenotype, if any, in other species (68). For example, an E. coli gdhA mutant only showed a defect when placed in direct competition with the wild-type parent strain in batch culture during glucose limitation (25). Neisseria meningitidis has also been shown to require gdhA for full virulence in an infant rat model (76), although this species lacks glutamate synthase (gltBD) (62, 78) and therefore cannot synthesize glutamate via the glutamine synthetase-GOGAT system (67). Furthermore, one of two N. meningitidis gdhA transcripts is regulated by adjoining gene gdhR (61), which is not found in enteric bacteria.
Global analysis of pathogens within the host continues to yield new insights into pathogenesis and central metabolism. This analysis of transcription during UTI will provide a baseline for future studies of early events in transcription, proteomics, tissue-specific expression, and mixed-species infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants AI043363 and AI059722 from the National Institutes of Health. M.M.P. was supported in part by National Research Service Award F32 AI068324.
We gratefully acknowledge critical discussions with Robert Bender, Christopher Alteri, and Patrick Vigil.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 19 April 2011.
REFERENCES
- 1. Alamuri P., et al. 2010. Adhesion, invasion, and agglutination mediated by two trimeric autotransporters in the human uropathogen Proteus mirabilis. Infect. Immun. 78:4882–4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alamuri P., Mobley H. L. 2008. A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol. Microbiol. 68:997–1017 [DOI] [PubMed] [Google Scholar]
- 3. Allison C., Coleman N., Jones P. L., Hughes C. 1992. Ability of Proteus mirabilis to invade human urothelial cells is coupled to motility and swarming differentiation. Infect. Immun. 60:4740–4746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alteri C. J., Smith S. N., Mobley H. L. 2009. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 5:e1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altman P. L. 1961. Physical properties and chemical composition of urine: mammals. Part 1. Man, p. 363–369 In Dittmer D. L. (ed.), Blood and other bodily fluids. Federation of American Societies for Experimental Biology, Washington, DC [Google Scholar]
- 6. Aryee M. J., Gutierrez-Pabello J. A., Kramnik I., Maiti T., Quackenbush J. 2009. An improved empirical Bayes approach to estimating differential gene expression in microarray time-course data: BETR (Bayesian Estimation of Temporal Regulation). BMC Bioinformatics 10:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asscher A. W., Sussman M., Waters W. E., Davis R. H., Chick S. 1966. Urine as a medium for bacterial growth. Lancet ii:1037–1041 [DOI] [PubMed] [Google Scholar]
- 8. Bahrani F. K., et al. 1994. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 62:3363–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bahrani F. K., Mobley H. L. 1993. Proteus mirabilis MR/P fimbriae: molecular cloning, expression, and nucleotide sequence of the major fimbrial subunit gene. J. Bacteriol. 175:457–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belas R., Erskine D., Flaherty D. 1991. Transposon mutagenesis in Proteus mirabilis. J. Bacteriol. 173:6289–6293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bijlsma I. G., van Dijk L., Kusters J. G., Gaastra W. 1995. Nucleotide sequences of two fimbrial major subunit genes, pmpA and ucaA, from canine-uropathogenic Proteus mirabilis strains. Microbiology 141:1349–1357 [DOI] [PubMed] [Google Scholar]
- 12. Burall L. S., et al. 2004. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect. Immun. 72:2922–2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clemmer K. M., Rather P. N. 2008. The Lon protease regulates swarming motility and virulence gene expression in Proteus mirabilis. J. Med. Microbiol. 57:931–937 [DOI] [PubMed] [Google Scholar]
- 14. Connell I., et al. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. U. S. A. 93:9827–9832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cook S. W., Mody N., Valle J., Hull R. 1995. Molecular cloning of Proteus mirabilis uroepithelial cell adherence (uca) genes. Infect. Immun. 63:2082–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dumanski A. J., Hedelin H., Edin-Liljegren A., Beauchemin D., McLean R. J. 1994. Unique ability of the Proteus mirabilis capsule to enhance mineral growth in infectious urinary calculi. Infect. Immun. 62:2998–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feuillet V., et al. 2006. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc. Natl. Acad. Sci. U. S. A. 103:12487–12492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flannery E. L., Mody L., Mobley H. L. 2009. Identification of a modular pathogenicity island that is widespread among urease-producing uropathogens and shares features with a diverse group of mobile elements. Infect. Immun. 77:4887–4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goss T. J., Janes B. K., Bender R. A. 2002. Repression of glutamate dehydrogenase formation in Klebsiella aerogenes requires two binding sites for the nitrogen assimilation control protein, NAC. J. Bacteriol. 184:6966–6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Griffith D. P., Musher D. M., Itin C. 1976. Urease. The primary cause of infection-induced urinary stones. Invest. Urol. 13:346–350 [PubMed] [Google Scholar]
- 21. Hackbarth H., Buttner D., Gartner K. 1982. Intraspecies allometry: correlation between kidney weight and glomerular filtration rate vs. body weight. Am. J. Physiol. 242:R303–R305 [DOI] [PubMed] [Google Scholar]
- 22. Hagan E. C., Lloyd A. L., Rasko D. A., Faerber G. J., Mobley H. L. 2010. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 6:e1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagberg L., et al. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamilton-Miller J. M., Gargan R. A. 1979. Rapid screening for urease inhibitors. Invest. Urol. 16:327–328 [PubMed] [Google Scholar]
- 25. Helling R. B. 1994. Why does Escherichia coli have two primary pathways for synthesis of glutamate? J. Bacteriol. 176:4664–4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Himpsl S. D., Lockatell C. V., Hebel J. R., Johnson D. E., Mobley H. L. 2008. Identification of virulence determinants in uropathogenic Proteus mirabilis using signature-tagged mutagenesis. J. Med. Microbiol. 57:1068–1078 [DOI] [PubMed] [Google Scholar]
- 27. Himpsl S. D., et al. 2010. Proteobactin and a yersiniabactin-related siderophore mediate iron acquisition in Proteus mirabilis. Mol. Microbiol. 78:138–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hooton T. M., et al. 2010. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 50:625–663 [DOI] [PubMed] [Google Scholar]
- 29. Janda J. M., Abbott S. L. 2006. The enterobacteria, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 30. Janes B. K., et al. 2001. Growth inhibition caused by overexpression of the structural gene for glutamate dehydrogenase (gdhA) from Klebsiella aerogenes. J. Bacteriol. 183:2709–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jansen A. M., Lockatell C. V., Johnson D. E., Mobley H. L. 2003. Visualization of Proteus mirabilis morphotypes in the urinary tract: the elongated swarmer cell is rarely observed in ascending urinary tract infection. Infect. Immun. 71:3607–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson D. E., et al. 1993. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect. Immun. 61:2748–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones B. D., Lockatell C. V., Johnson D. E., Warren J. W., Mobley H. L. 1990. Construction of a urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 58:1120–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klevens R. M., et al. 2007. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 122:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lane M. C., Alteri C. J., Smith S. N., Mobley H. L. 2007. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. U. S. A. 104:16669–16674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lane M. C., Li X., Pearson M. M., Simms A. N., Mobley H. L. 2009. Oxygen-limiting conditions enrich for fimbriate cells of uropathogenic Proteus mirabilis and Escherichia coli. J. Bacteriol. 191:1382–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leigh J. A., Dodsworth J. A. 2007. Nitrogen regulation in bacteria and archaea. Annu. Rev. Microbiol. 61:349–377 [DOI] [PubMed] [Google Scholar]
- 38. Li X., et al. 2004. Use of translational fusion of the MrpH fimbrial adhesin-binding domain with the cholera toxin A2 domain, coexpressed with the cholera toxin B subunit, as an intranasal vaccine to prevent experimental urinary tract infection by Proteus mirabilis. Infect. Immun. 72:7306–7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X., et al. 2004. Development of an intranasal vaccine to prevent urinary tract infection by Proteus mirabilis. Infect. Immun. 72:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li X., Rasko D. A., Lockatell C. V., Johnson D. E., Mobley H. L. 2001. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 20:4854–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li X., et al. 2002. Visualization of Proteus mirabilis within the matrix of urease-induced bladder stones during experimental urinary tract infection. Infect. Immun. 70:389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lima A., Zunino P., D'Alessandro B., Piccini C. 2007. An iron-regulated outer-membrane protein of Proteus mirabilis is a haem receptor that plays an important role in urinary tract infection and in in vivo growth. J. Med. Microbiol. 56:1600–1607 [DOI] [PubMed] [Google Scholar]
- 43. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 44. Loomes L. M., Senior B. W., Kerr M. A. 1990. A proteolytic enzyme secreted by Proteus mirabilis degrades immunoglobulins of the immunoglobulin A1 (IgA1), IgA2, and IgG isotypes. Infect. Immun. 58:1979–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lucht J. M., Bremer E. 1994. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system proU. FEMS Microbiol. Rev. 14:3–20 [DOI] [PubMed] [Google Scholar]
- 46. Martinez J. J., Mulvey M. A., Schilling J. D., Pinkner J. S., Hultgren S. J. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Massad G., Bahrani F. K., Mobley H. L. 1994. Proteus mirabilis fimbriae: identification, isolation, and characterization of a new ambient-temperature fimbria. Infect. Immun. 62:1989–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Massad G., Fulkerson J. F., Jr., Watson D. C., Mobley H. L. 1996. Proteus mirabilis ambient-temperature fimbriae: cloning and nucleotide sequence of the atf gene cluster. Infect. Immun. 64:4390–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Massad G., Lockatell C. V., Johnson D. E., Mobley H. L. 1994. Proteus mirabilis fimbriae: construction of an isogenic pmfA mutant and analysis of virulence in a CBA mouse model of ascending urinary tract infection. Infect. Immun. 62:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Massad G., Mobley H. L. 1994. Genetic organization and complete sequence of the Proteus mirabilis pmf fimbrial operon. Gene 150:101–104 [DOI] [PubMed] [Google Scholar]
- 51. Mobley H. L., et al. 1996. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 64:5332–5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mobley H. L., et al. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mobley H. L., Hausinger R. P. 1989. Microbial ureases: significance, regulation, and molecular characterization. Microbiol. Rev. 53:85–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mobley H. L., Warren J. W. 1987. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J. Clin. Microbiol. 25:2216–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morgenstein R. M., Szostek B., Rather P. N. 2010. Regulation of gene expression during swarmer cell differentiation in Proteus mirabilis. FEMS Microbiol. Rev. 34:753–763 [DOI] [PubMed] [Google Scholar]
- 56. Mulvey M. A., et al. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497 [DOI] [PubMed] [Google Scholar]
- 57. Nielubowicz G. R., Mobley H. L. 2010. Host-pathogen interactions in urinary tract infection. Nat. Rev. Urol. 7:430–441 [DOI] [PubMed] [Google Scholar]
- 58. Nielubowicz G. R., Smith S. N., Mobley H. L. 2008. Outer membrane antigens of the uropathogen Proteus mirabilis recognized by the humoral response during experimental murine urinary tract infection. Infect. Immun. 76:4222–4231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nielubowicz G. R., Smith S. N., Mobley H. L. 2010. Zinc uptake contributes to motility and provides a competitive advantage to Proteus mirabilis during experimental urinary tract infection. Infect. Immun. 78:2823–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Old D. C., Adegbola R. A. 1982. Haemagglutinins and fimbriae of Morganella, Proteus and Providencia. J. Med. Microbiol. 15:551. [DOI] [PubMed] [Google Scholar]
- 61. Pagliarulo C., et al. 2004. Regulation and differential expression of gdhA encoding NADP-specific glutamate dehydrogenase in Neisseria meningitidis clinical isolates. Mol. Microbiol. 51:1757–1772 [DOI] [PubMed] [Google Scholar]
- 62. Parkhill J., et al. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502–506 [DOI] [PubMed] [Google Scholar]
- 63. Pearson M. M., Mobley H. L. 2007. The type III secretion system of Proteus mirabilis HI4320 does not contribute to virulence in the mouse model of ascending urinary tract infection. J. Med. Microbiol. 56:1277–1283 [DOI] [PubMed] [Google Scholar]
- 64. Pearson M. M., Rasko D. A., Smith S. N., Mobley H. L. 2010. Transcriptome of swarming Proteus mirabilis. Infect. Immun. 78:2834–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pearson M. M., et al. 2008. Complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility. J. Bacteriol. 190:4027–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pellegrino R., Galvalisi U., Scavone P., Sosa V., Zunino P. 2003. Evaluation of Proteus mirabilis structural fimbrial proteins as antigens against urinary tract infections. FEMS Immunol. Med. Microbiol. 36:103–110 [DOI] [PubMed] [Google Scholar]
- 67. Reitzer L. 2003. Nitrogen assimilation and global regulation in Escherichia coli. Annu. Rev. Microbiol. 57:155–176 [DOI] [PubMed] [Google Scholar]
- 68. Reitzer L. J. 1996. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, L-alanine, and D-alanine, p. 391–407 In Neidhardt F. C., et al. (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. ASM Press, Washington, DC [Google Scholar]
- 69. Riba L., Becerril B., Servin-Gonzalez L., Valle F., Bolivar F. 1988. Identification of a functional promoter for the Escherichia coli gdhA gene and its regulation. Gene 71:233–246 [DOI] [PubMed] [Google Scholar]
- 70. Roos V., Klemm P. 2006. Global gene expression profiling of the asymptomatic bacteriuria Escherichia coli strain 83972 in the human urinary tract. Infect. Immun. 74:3565–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Saeed A. I., et al. 2003. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378 [DOI] [PubMed] [Google Scholar]
- 72. Sarada K. V., Rao N. A., Venkitasubramanian T. A. 1980. Isolation and characterisation of glutamate dehydrogenase from Mycobacterium smegmatis CDC 46. Biochim. Biophys. Acta 615:299–308 [DOI] [PubMed] [Google Scholar]
- 73. Scavone P., Sosa V., Pellegrino R., Galvalisi U., Zunino P. 2004. Mucosal vaccination of mice with recombinant Proteus mirabilis structural fimbrial proteins. Microbes Infect. 6:853–860 [DOI] [PubMed] [Google Scholar]
- 74. Snyder J. A., et al. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72:6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stadtman E. R., Smyrniotis P. Z., Davis J. N., Wittenberger M. E. 1979. Enzymic procedures for determining the average state of adenylylation of Escherichia coli glutamine synthetase. Anal. Biochem. 95:275–285 [DOI] [PubMed] [Google Scholar]
- 76. Sun Y. H., Bakshi S., Chalmers R., Tang C. M. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269–1273 [DOI] [PubMed] [Google Scholar]
- 77. Swihart K. G., Welch R. A. 1990. Cytotoxic activity of the Proteus hemolysin HpmA. Infect. Immun. 58:1861–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tettelin H., et al. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809. [DOI] [PubMed] [Google Scholar]
- 79. Tusher V. G., Tibshirani R., Chu G. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci.U. S. A. 98:5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Uphoff T. S., Welch R. A. 1990. Nucleotide sequencing of the Proteus mirabilis calcium-independent hemolysin genes (hpmA and hpmB) reveals sequence similarity with the Serratia marcescens hemolysin genes (shlA and shlB). J. Bacteriol. 172:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Warren J. W., et al. 1987. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J. Infect. Dis. 155:1151–1158 [DOI] [PubMed] [Google Scholar]
- 82. Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. 1982. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J. Infect. Dis. 146:719–723 [DOI] [PubMed] [Google Scholar]
- 83. Wassif C., Cheek D., Belas R. 1995. Molecular analysis of a metalloprotease from Proteus mirabilis. J. Bacteriol. 177:5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Welch R. A., et al. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:17020–17024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wray S. K., Hull S. I., Cook R. G., Barrish J., Hull R. A. 1986. Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect. Immun. 54:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zunino P., et al. 2003. Proteus mirabilis fimbriae (PMF) are important for both bladder and kidney colonization in mice. Microbiology 149:3231–3237 [DOI] [PubMed] [Google Scholar]
- 87. Zunino P., et al. 2007. Mannose-resistant Proteus-like and P. mirabilis fimbriae have specific and additive roles in P. mirabilis urinary tract infections. FEMS Immunol. Med. Microbiol. 51:125–133 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.