Abstract
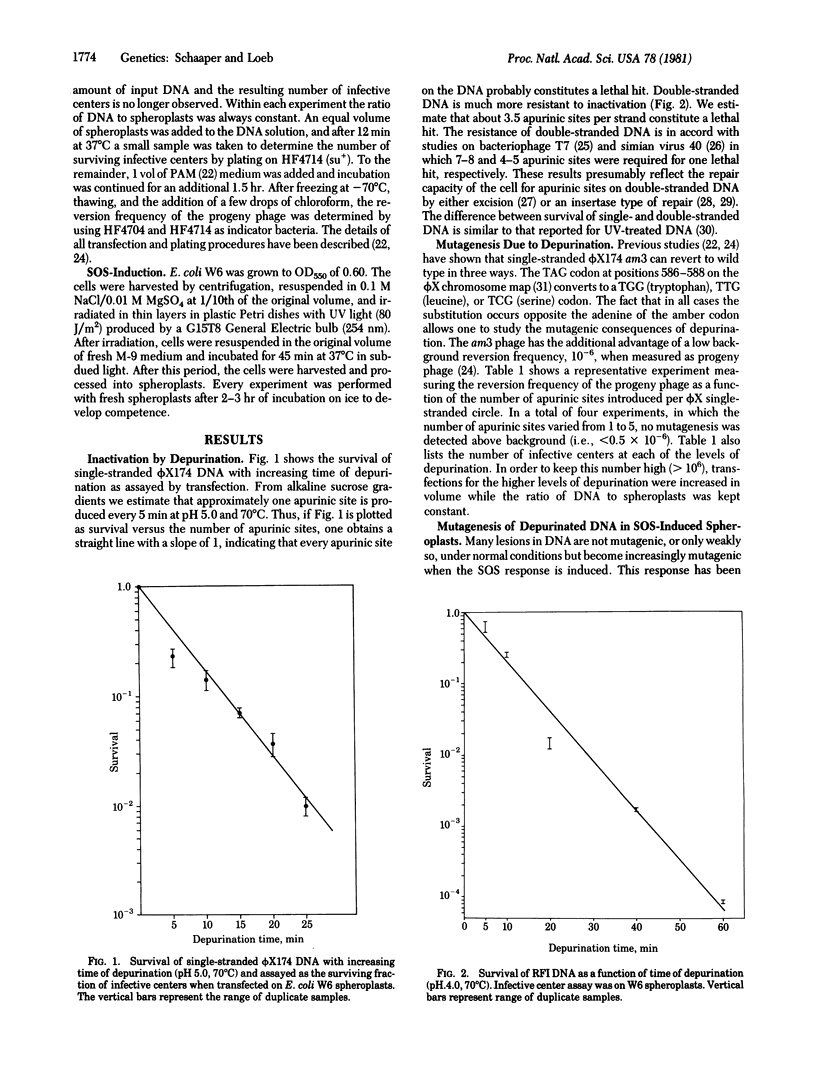
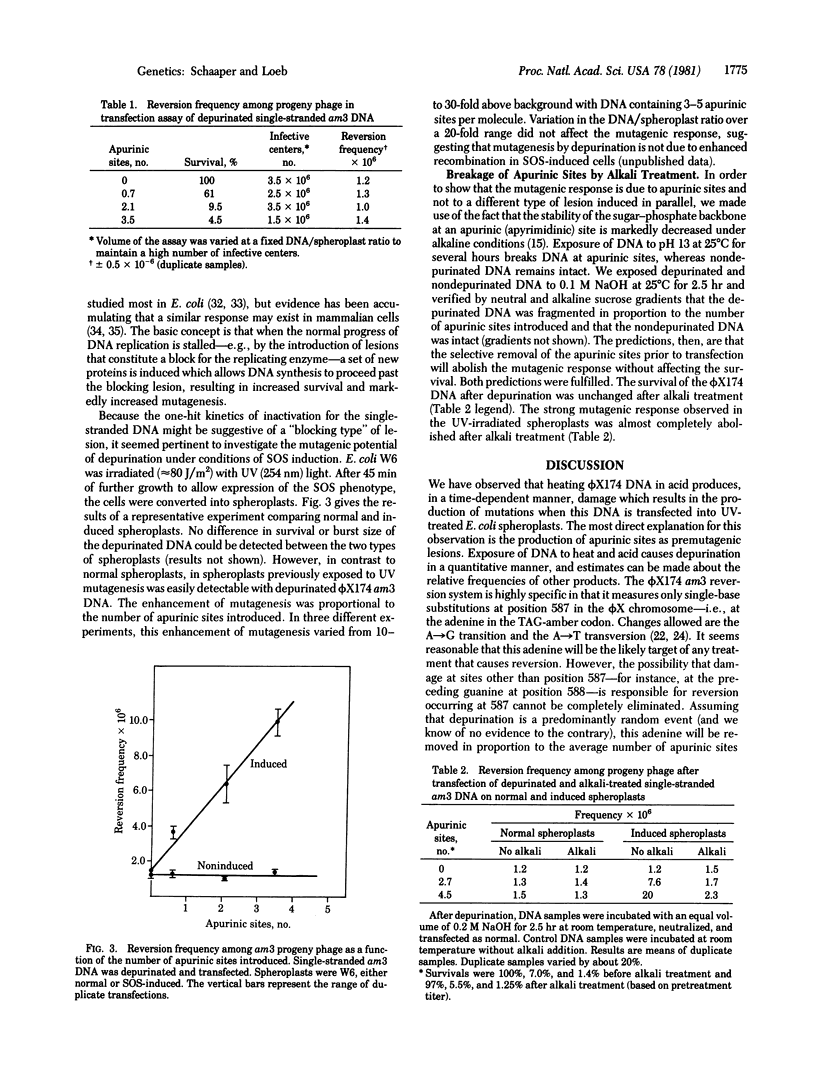
Introduction of apurinic sites into phi X174 am3 DNA leads to loss of biological activity when measured in a transfection assay. For single-stranded DNA, approximately one apurinic site constitutes a lethal hit; for double-stranded (RFI) DNA, approximately 3.5 hits per strand are lethal. When the reversion frequency of am3 DNA is measured, no increase due to depurination is observed above the background level. However, a large increase in reversion frequency is observed when the same DNA is assayed by using spheroplasts derived from bacteria previously exposed to UV light. The results suggest that apurinic sites are impediments to a replicating DNA polymerase; however, nucleotides can be incorporated opposite these sites under SOS-induced conditions. We estimate the frequency of mutagenesis per apurinic site to be less than 1 in 1400 in normal spheroplasts and 1 in 100 in SOS-induced spheroplasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott P. J., Saffhill R. DNA synthesis with methylated poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O(6)-methylguanine. Biochim Biophys Acta. 1979 Mar 28;562(1):51–61. doi: 10.1016/0005-2787(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz R. H., Bingham P. M., Drake J. W. Heat mutagenesis in bacteriophage T4: the transition pathway. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1269–1273. doi: 10.1073/pnas.73.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Baltz R. H., Ripley L. S., Drake J. W. Heat mutagenesis in bacteriophage T4: the transversion pathway. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4159–4163. doi: 10.1073/pnas.73.11.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W., Baltz R. H. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11–37. doi: 10.1146/annurev.bi.45.070176.000303. [DOI] [PubMed] [Google Scholar]
- Essigmann J. M., Croy R. G., Nadzan A. M., Busby W. F., Jr, Reinhold V. N., Büchi G., Wogan G. N. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci U S A. 1977 May;74(5):1870–1874. doi: 10.1073/pnas.74.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett E. R., Tsau J. Solvolyses of cytosine and cytidine. J Pharm Sci. 1972 Jul;61(7):1052–1061. doi: 10.1002/jps.2600610703. [DOI] [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Grunberger D., Weinstein I. B. Biochemical effects of the modification of nucleic acids by certain polycyclic aromatic carcinogens. Prog Nucleic Acid Res Mol Biol. 1979;23:105–149. doi: 10.1016/s0079-6603(08)60132-4. [DOI] [PubMed] [Google Scholar]
- Hsu W. T., Lin E. J., Harvey R. G., Weiss S. B. Mechanism of phage phiX174 DNA inactivation by benzo(a)pyrene-7,8-dihydrodiol-9,10-epoxide. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3335–3339. doi: 10.1073/pnas.74.8.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrna R. D., Smith J., Linn S., Penhoet E. E. Survival of apurinic SV40 DNA in the d-complementation group of xeroderma pigmentosum. Mutat Res. 1979 Aug;62(1):173–181. doi: 10.1016/0027-5107(79)90230-6. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. Effect of divalent metal ion activators and deoxyrionucleoside triphosphate pools on in vitro mutagenesis. J Biol Chem. 1979 Jul 10;254(13):5718–5725. [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. The accuracy of Escherichia coli DNA polymerase I in copying natural DNA in vitro. J Biol Chem. 1980 Oct 25;255(20):9961–9966. [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval F. Effect of uncouplers on radiosensitivity and mutagenicity in x-irradiated mammalian cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2702–2705. doi: 10.1073/pnas.77.5.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R., Ormerod M. G. The replication of DNA in murine lymphoma cells (L5178Y). I. Rate of replication. Biochim Biophys Acta. 1970 Mar 19;204(1):128–143. doi: 10.1016/0005-2787(70)90496-x. [DOI] [PubMed] [Google Scholar]
- Lin J. K., Miller J. A., Miller E. C. 2,3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1, a major acid hydrolysis product of aflatoxin B1-DNA or -ribosomal RNA adducts formed in hepatic microsome-mediated reactions and in rat liver in vivo. Cancer Res. 1977 Dec;37(12):4430–4438. [PubMed] [Google Scholar]
- Lindahl T., Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry. 1974 Jul 30;13(16):3405–3410. doi: 10.1021/bi00713a035. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Livneh Z., Elad D., Sperling J. Enzymatic insertion of purine bases into depurinated DNA in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1089–1093. doi: 10.1073/pnas.76.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Z., Elad D., Sperling J. Enzymatic insertion of purine bases into depurinated DNA in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1089–1093. doi: 10.1073/pnas.76.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Springgate C. F., Battula N. Errors in DNA replication as a basis of malignant changes. Cancer Res. 1974 Sep;34(9):2311–2321. [PubMed] [Google Scholar]
- Margison G. P., O'Connor P. J. Biological implications of the instability of the N-glycosidic bone of 3-methyldeoxyadenosine in DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):349–356. doi: 10.1016/0005-2787(73)90021-x. [DOI] [PubMed] [Google Scholar]
- Masamune Y. Effect of ultraviolet irradiation of bacteriophage f1 DNA on its conversion to replicative form by extracts of Escherichia coli. Mol Gen Genet. 1976 Dec 22;149(3):335–345. doi: 10.1007/BF00268536. [DOI] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Carcinogens enhance survival of UV-irradiated simian virus 40 in treated monkey kidney cells: induction of a recovery pathway? Proc Natl Acad Sci U S A. 1978 Jan;75(1):346–350. doi: 10.1073/pnas.75.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman C. W., Loeb L. A. Depurination decreases fidelity of DNA synthesis in vitro. Nature. 1977 Dec 8;270(5637):537–538. doi: 10.1038/270537a0. [DOI] [PubMed] [Google Scholar]
- Shearman C. W., Loeb L. A. Effects of depurination on the fidelity of DNA synthesis. J Mol Biol. 1979 Feb 25;128(2):197–218. doi: 10.1016/0022-2836(79)90126-8. [DOI] [PubMed] [Google Scholar]
- Singer B., Kröger M. Participation of modified nucleosides in translation and transcription. Prog Nucleic Acid Res Mol Biol. 1979;23:151–194. doi: 10.1016/s0079-6603(08)60133-6. [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Restriction of carcinogen-induced error incorporation during in vitro DNA synthesis. Cancer Res. 1976 Feb;36(2 Pt 1):516–523. [PubMed] [Google Scholar]
- Stark A. A., Essigmann J. M., Demain A. L., Skopek T. R., Wogan G. N. Aflatoxin B1 mutagenesis, DNA binding, and adduct formation in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1343–1347. doi: 10.1073/pnas.76.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly W. G. Commentary. Monofunctional alkylating agents and apurinic sites in DNA. Biochem Pharmacol. 1974 Jan 1;23(1):3–8. doi: 10.1016/0006-2952(74)90307-4. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


