1. Introduction
Free radicals derived primarily from molecular oxygen have been implicated in a variety of human disorders including atherosclerosis, cancer, neurodegenerative diseases, and aging.1 Damage to tissue biomolecules, including lipids, proteins, and DNA, by free radicals is postulated to contribute importantly to the pathophysiology of oxidative stress. Lipids are readily attacked by free radicals resulting in the formation of a number of peroxidation products.2 The isoprostanes (IsoPs) are a unique series of prostaglandin-like compounds formed in vivo via the non-enzymatic free radical-initiated peroxidation of arachidonic acid, a ubiquitous polyunsaturated fatty acid (PUFA). Since discovery of these molecules over twenty years ago by Morrow and Roberts, one class of IsoPs, the F2-IsoPs, have become the biomarker of choice for assessing endogenous oxidative stress because these molecules are chemically stability and have been detected in all biological fluids and tissues analyzed.3-5 In addition to F2-IsoPs, a variety of IsoPs with different ring structures have been identified. Several of these compounds possess potent biological activities that could account for some of the pathophysiological effects of oxidative injury. Further, IsoP-like molecules are also generated from a number of different PUFAs including α-linolenic acid, eicosapentaenoic acid (EPA), adrenic acid, and docosahexaenoic acid (DHA) (Figure 1). There are many excellent reviews in the literature describing not only the quantification of F2-IsoPs in human health and disease but also the biological activities of these molecules.6-9 Thus, this review seeks to give readers a comprehensive, up-to-date overview of our current knowledge regarding IsoPs including the chemistry and biochemistry of their formation and metabolism, the utility of measuring these compounds as markers of in vivo oxidant stress, and their biological properties.
Figure 1.
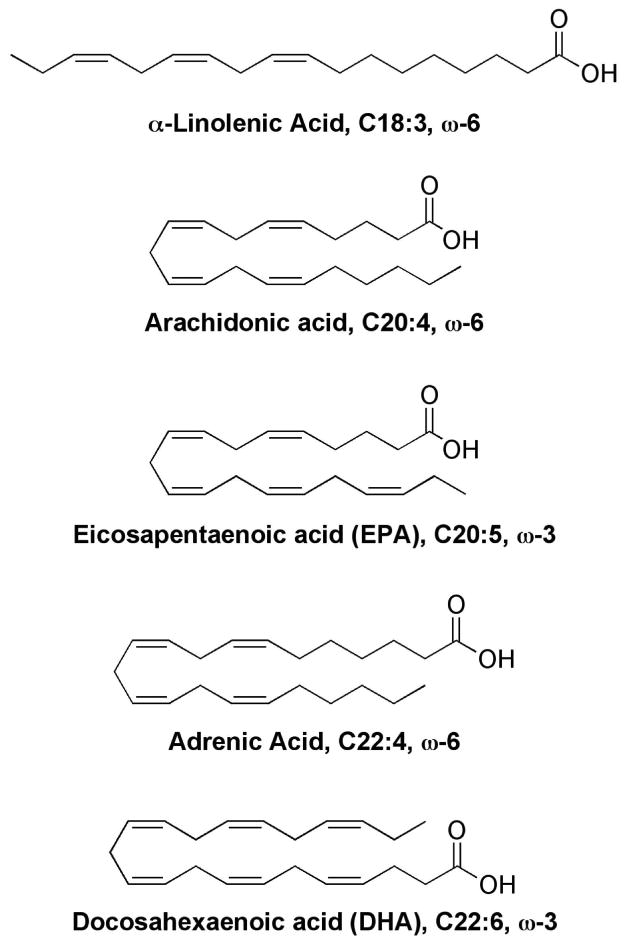
Structures of polyunsaturated fatty acids that have been shown to generate isoprostane-like compounds.
1.1 Reactive oxygen species and oxidative stress
Free radicals are reactive chemical species containing one or more unpaired electrons in their outer orbitals. Reactive oxygen species (ROS) is a term used to describe chemically reactive, oxygen-containing molecules including oxygen-centered free radicals as well as oxygen ions and peroxides.10-13 The most common ROS generated in living systems are superoxide anion (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), alkoxyl radicals (RO•), peroxyl radicals (ROO•), singlet oxygen (1O2),14 and ozone.15 Other related reactive species formed in cells include peroxynitrite (ONOO−), nitric oxide (•NO), hypochlorus acid (HOCl), and other carbon centered radicals. ROS generated in vivo are derived from both endogenous and exogenous sources.
ROS are continuously generated during aerobic metabolism through reduction-oxidation (redox) reactions in the cell. The mitochondria within a cell are a major source of endogenous ROS. The mitochondrial electron-transport chain (ETC) is the main source of energy, in the form of adenosine triphosphate (ATP), in mammalian cells and is essential for life. Movement of electrons from oxidizable organic molecules to molecular oxygen (O2) is responsible for ATP generation by the ETC, and O2 is the final electron acceptor in this processes yielding H2O.10 The mitochondrial ETC produces small amounts of O2•− during normal function due to the leaking of electrons directly to oxygen. During energy transduction, an estimated 1-4% of oxygen reacting with the electron transport chain is incompletely reduced to superoxide anion by leaking electrons. Complexes I and III of the ETC have been shown to be responsible for generation of O2•−.16 Under conditions of cellular stress and ATP depletion, an excess of the superoxide radical causes release of free iron (FeII) from iron-containing molecules, such as iron-sulfur proteins within the inner mitochondrial membrane, promoting the formation of the reactive hydroxyl radical. 17,18
Peroxisomes are also a significant source of cellular ROS. Peroxisomes are specialized cytoplasmic organelles present in most plant and animal cells, and they are major sites of oxygen consumption. These organelles carry out important metabolic functions, including β-oxidation of long-chain and very long-chain fatty acids, degradation of uric acid, and synthesis of ether-linked lipids. Several peroxisomal oxidases produce H2O2 through the oxidation of various metabolites.19,20 Peroxisomal oxidases have been to be responsible for approximately 35% of all H2O2 formed in rat liver.21 Acyl-CoA oxidase, which is involved in the β-oxidation of fatty acids, is one such enzyme.20 During this reaction, O2 is reduced to H2O2, which can be further reduced to H2O by the enzyme catalase (CAT). The reaction of xanthine and xanthine oxidase yields both superoxide anion and hydrogen peroxide through the one-electron and two-electron reduction of O2, respectively, to form uric acid.20
ROS production by phagocytic cells during oxidative burst is important in host immune defense against microbial pathogens. Oxidative or respiratory burst is a process in which activated phagocytes, such as neutrophils and macrophages, produce large, toxic quantities of ROS to kill ingested bacteria.22,23 Phagocytes are activated in response to microorganisms and inflammatory mediators. Upon activation, nicotine adenine dinucleotide phosphate (NADPH) oxidase (NOX) produces superoxide anion within the phagosomal membrane. This superoxide-generating oxidase catalyzes the transfer of electrons from NADPH to molecular oxygen (O2) to form O2•−.24 In addition, the heme-containing phagocytic enzyme myeloperoxidase (MPO) produces hypochlorus acid (HOCl) in neutrophils by the reaction of hydrogen peroxide and chloride at sites of inflammation.25-28 Production of ROS at sites of inflammation can cause injury to surrounding tissues.
ROS are also generated from exogenous genotoxic sources, including UV radiation, environmental toxicants, cigarette smoke, and other chemical carcinogens.29
ROS have important biological functions. Low to moderate levels of ROS have been shown to play a key role in mediating signal transduction and normal physiological processes. H2O2 can freely diffuse across cell membranes to participate in intra- and intercellular signaling.20 H2O2 is a potent oxidant capable of oxidizing methionine and reactive cysteine residues of proteins. Reversible protein oxidation by H2O2 is thought to be an important signaling mechanism to modulate the activity of various kinases and phosphatases involved in the regulation of cell growth, proliferation, and apoptosis.30 In fact, recent reports suggest that H2O2 may be involved in post-translational modification of proteins in a manner that is analogous to regulatory protein phosphorylation.31 Serine/threonine kinases of the mitogen activated protein kinase (MAPK) family, including extracellular-regulated kinases (ERKs), c-Jun-NH2-terminal kinase (JNK), and p38 MAPK are among the signaling molecules whose activity may be regulated by ROS-mediated post-translational modification.30 ROS are also thought to be involved in regulation of the phosphoinositol 3 kinase (PI3K)-Akt-p53 signaling pathway. Protein tyrosine phosphatases, such as protein tyrosine phosphatase 1B (PTP1B) 31 and the tumor suppressor PTEN,32 have been shown to be reversibly oxidized and inactivated by H2O2. Further, the activity of transcription factors, such as activator protein (AP)-1 and nuclear factor κB (NF-κB), has been shown to be modulated by ROS.33
A series of antioxidant defense mechanisms have been developed to maintain redox homeostasis and protect cells against free radical-induced oxidative damage. The redox (reduction-oxidation) environment within a cell is characterized by the concentration of electrons stored in many cellular constituents, and redox homeostasis is achieved when ROS levels and antioxidant defenses are in proper balance. The primary antioxidant defenses include antioxidant enzymes and low molecular weight antioxidant molecules. Antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase play a key role in the degradation of ROS. Superoxide dismutases (SODs), such as manganese SOD (MnSOD) in the mitochondrial matrix and copper-zinc SOD (CuZnSOD) catalyze the dismutation of O2•− to H2O2 and O2. Glutathione peroxidase (GPx) scavenges hydrogen peroxide with reduced glutathione (GSH) as the electron donor.34 Catalase decomposes H2O2 to oxygen and water. Small molecule antioxidants, such as glutathione (GSH), ascorbic acid (Vitamin C), a-tocopherol (Vitamin E), carotenoids, and coenzyme Q, serve as free radical scavengers and/or reducing agents. In addition, the thioredoxin (Trx) system, including Trx, Trx reductase, and NADPH, is important in facilitating the enzymatic reduction of protein disulfides to regulate cellular redox status.35 Because the biological status of a cell is closely related to its redox environment, the balance between both the activities and intracellular levels of each of these antioxidants is critical for the health and survival of organisms.36
Oxidant stress is characterized by an imbalance between cellular antioxidant defenses and overproduction of free radicals, particularly ROS. Oxidative stress has become increasingly implicated in the etiology of many human diseases, including cancer, cardiovascular disease, ischemia/reperfusion injury, neurodegenerative diseases, lung disease and even the normal aging process.34,37-40 For example, Harman characterized the aging process as a decline in normal cellular function due to the accumulation of biomolecules damaged by free radicals.41 Under conditions of oxidative stress, high levels of ROS can react non-specifically and rapidly with cellular biomolecules, including DNA, proteins, and lipids.42 This leads to molecular damage such as DNA mutations, protein oxidation, and lipid peroxidation. Direct evidence, however, for the association between oxidative stress and disease has often been lacking. A number of methods exist to quantify free radicals and their oxidation products, although many of these techniques suffer from lack of sensitivity and specificity, especially when used to assess oxidant stress status in vivo.7,43
1.2 Lipid peroxidation
As mentioned, lipids are major targets for free radical attack under settings of oxidative stress.44,45 Lipid peroxidation is a hallmark of oxidative stress, and excessive production of lipid peroxidation products has been implicated in the pathogenesis of a number of human diseases. Lipid peroxidation can cause damage to cellular membranes through disturbance of membrane organization and alteration of membrane integrity, fluidity, and permeability. Free radical-mediated lipid peroxidation proceeds by a chain mechanism and involves three events: initiation, propagation, and termination. One initiating free radical can oxidize many lipid molecules through sequential, self-propagating chain reactions. Radical species that can initiate the chain reaction include hydroxyl radicals, alkoxyl radicals, peroxyl radicals, and peroxynitrite; the hydroxyl radical is the most active. Catalytic metal ions such as copper (CuI) or iron (FeII) can also contribute to chain initiation. The mechanism of free radical-initiated lipid peroxidation begins with abstraction of a bisallylic hydrogen from a polyunsaturated lipid to form a carbon-centered radical which rearranges to a more stable pentadienyl radical. The pentadienyl radical then combines with molecular oxygen (O2) to generate a peroxyl radical, which facilitates chain propagation through abstraction of a bisallylic hydrogen from a second lipid to give a conjugated diene lipid hydroperoxide and new lipid pentadienyl radical. This permits the continuation of another chain reaction. The distribution of lipid peroxidation products is determined by the relative contribution of competing reactions and the free radical-initiated chain reactions are terminated by antioxidants. 11,12,45,46
Biologically important polyunsaturated fatty acids (PUFAs), such as linoleic acid (LA) (18:2), arachidonic acid (AA) (20:4), eicosapentaenoic acid (EPA) (20:5), and docosahexaenoic acid (DHA) (22:6) are all subject to free radical-initiated lipid peroxidation, yielding a diverse array of products. The rates of oxidation of these molecules have been systematically studied and depend upon the number of -CH2- centers in the molecule that are flanked by two double bonds (bisallylic methylenes). Thus, the oxidizability of common fatty acids is as follows: linoleic acid < arachidonic acid < eicosapentaenoic acid (EPA) < docosahexaenoic acid (DHA).47 Likewise, oxidation of linoleic acid and their esters proceeds by a simpler mechanism than that of more highly unsaturated fatty acids such as AA, EPA, and DHA.11,12,46 Lipid hydroperoxides are primary products of free radical-initiated peroxidation of PUFAs. Oxidation of linoleates forms hydro(pero)xyoctadienoates (H(P)ODEs). Aldehydes such as acrolein, malondialdehyde (MDA), and 4-hydroxy-2-nonenal (HNE) are formed from lipid hydroperoxides as decomposition products.48,49 HNE, the most extensively studied of these aldehyde products, is a highly reactive α,β-unsaturated aldehyde that can readily react with proteins, DNA, and phospholipids to cause deleterious effects.50,51 Further, more complex oxidation products, generally termed secondary oxidation products, are generated from the non-enzymatic free radical-catalyzed peroxidation of arachidonic acid and other highly unsaturated PUFAs. Secondary oxidation products include a series of prostaglandin (PG)-like products termed isoprostanes (IsoPs) as well as monocylic and serial cyclic peroxides.46 Importantly, IsoPs are considered to be the “gold standard” biomarker of endogenous lipid peroxidation and oxidative stress.7
2. Historical perspectives on isoprostanes
In the mid-1970's, it was shown that PG-like compounds could be formed in vitro by the non-enzyamtic peroxidation of purified polyunsaturated fatty acids. Seminal in vitro mechanistic studies by Porter, Pryor, and others led to a proposed mechanism by which these compounds are generated via bicycloendoperoxide intermediates.52,53 However, this work was never carried beyond in vitro studies. Further, it was not determined whether PG-like compounds could be formed in biological fluids containing unsaturated fatty acids.
In the 1980's, a study showed that PGD2 derived from the cyclooxygenase (COX) is primarily metabolized in vivo in humans to form 9α,11β-PGF2α by the enzyme 11-ketoreductase.54 In aqueous solutions, however, PGD2 is an unstable compound that undergoes isomerization of the lower side chain and these isomers can likewise be reduced by 11-ketoreductase to yield isomers of 9α,11β-PGF2α. In studies undertaken to further characterize these compounds utilizing gas chromatography (GC)/mass spectrometry (MS), it was found that when plasma samples from normal volunteers were processed and analyzed immediately, a series of peaks were detected possessing characteristics of F-ring PGs. Interestingly, however, when plasma samples that had been stored at -20°C for several months were reanalyzed, identical chromatographic peaks were detected but levels of putative PGF2-like compounds were up to 100-fold higher. In addition, base-catalyzed hydrolysis of plasma lipids also yielded significant amounts of the PGF2-like compounds. Antioxidants and reducing agents suppressed the formation of these compounds.55 Further, Morrow et al. demonstrated that treatment of rats with the hepatotoxicant carbon tetrachloride (CCl4) resulted in a 50 to 55-fold increase of these compounds in plasma and over a 100-fold increase of these compounds in liver compared to untreated rats (Morrow, Awad, Boss et al. 1992; Morrow, Awad, Kato et al. 1992).3,56 CCl4 is a potent inducer of lipid peroxidation due to its cytochrome P450-catalylzed conversion to the trichloromethyl radical (CCl3•).57 These experiments confirmed that PGF2-like compounds were generated in vivo, not by a COX-derived mechanism, but rather non-enzymatically by auto-oxidation of arachidonic acid. Because these compounds contain F-type prostane rings and were comprised of many different isomers, they are termed F2-IsoPs.
Since the initial discovery of the F2-IsoPs, various classes of IsoPs that differ in regards to the functional groups on the prostane ring have been discovered; the structures of these different compounds are summarized in Figure 2. The compounds are named based upon the structure of the functional groups on the cyclopentane ring in a manner analogous to the prostaglandins. In 1997, Taber, Morrow, and Roberts proposed a nomenclature system for the IsoPs that has been approved by the Eicosanoid Nomenclature Committee, which is sanctioned by JCBN of IUPAC, and is used throughout this manuscript. An alternative nomenclature system for the IsoPs has been proposed by FitzGerald and colleagues in which the abbreviation iP is used for isoprostane, and the regioisomers are denoted as III-VI based upon the number of carbons between the omega carbon and the first double bond. 58
Figure 2.
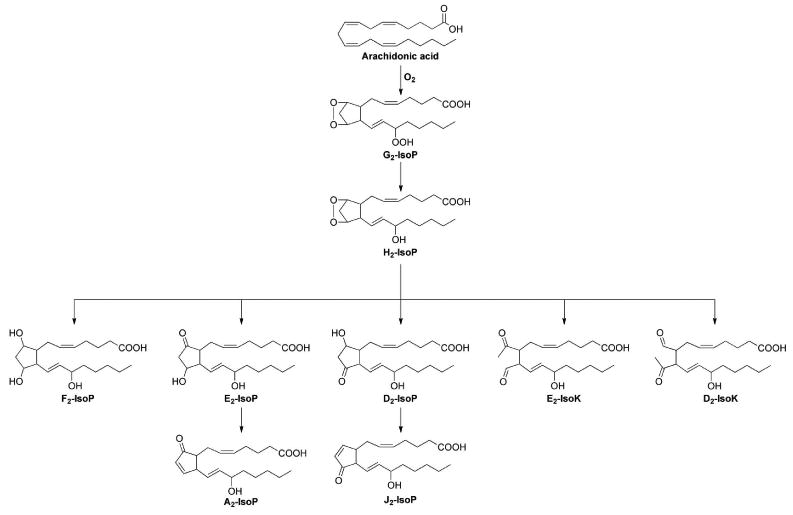
Oxidation of arachidonic acid via free radical-catalyzed peroxidation can yield a number of isoprostanes with varying ring structures.
3. Formation of the F2-isoprostanes
A mechanism to explain the formation of the F2-IsoPs from arachidonic acid is outlined in Figure 3 and is based on that proposed by Pryor for the generation of bicycloendoperoxide intermediates. Following abstraction of a bisallylic hydrogen atom and the addition of a molecule of oxygen to arachidonic acid to form a peroxyl radical, the peroxyl radical undergoes 5-exo cyclization and a second molecule of oxygen adds to the backbone of the compound to form PGG2-like compounds. These unstable bicycloendoperoxide intermediates are then reduced to the F2-IsoPs. Based on this mechanism of formation, four F2-IsoP regioisomers, each of which is comprised of 8 racemic diastereomers for a total of 64 compounds, are generated. Based upon the Taber, Morrow, and Roberts nomenclature system, the four regioisomer classes are named according to the carbon number on which the side chain hydroxyl group is attached with the carboxyl carbon being 1, as indicated in Figure 3.59
Figure 3.
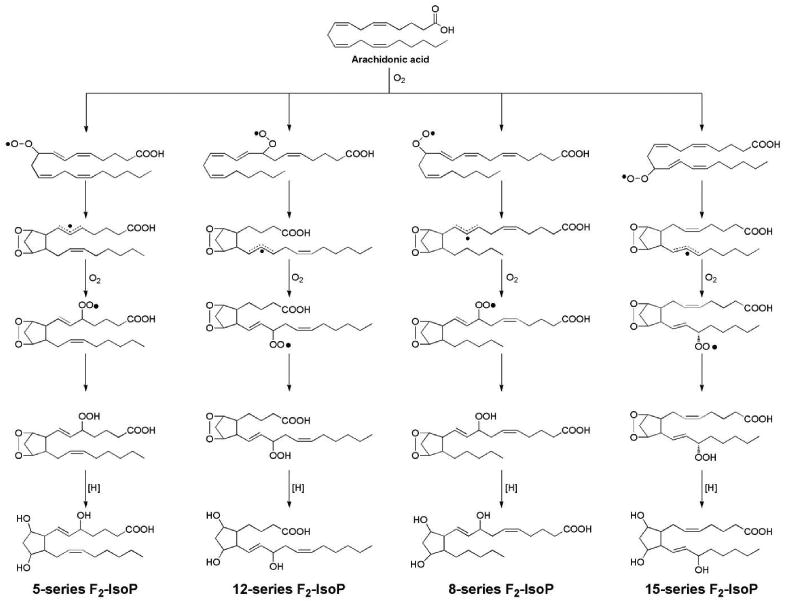
Mechanism of formation of F2-isoprostanes from the free radical-initiated peroxidation of arachidonic acid.
A central structural distinction between IsoPs and cyclooxygenase-derived PGs is that the former contain side chains that are predominantly oriented cis to the prostane ring while the latter possess exclusively trans side chains. Importantly, while PGs are generated from free arachidonic acid, IsoPs are formed predominantly from oxidation of arachidonic acid esterified in membrane phospholipids. Stafforini and colleagues have shown that F2-IsoP hydrolysis from phospholipids is catalyzed, at least in part, by the platelet-activating factor (PAF) acetylhydrolases I and II. 60
It is important to note that during the formation of F2-IsoPs the initial abstraction of any bisallylic hydrogen atom from arachidonic acid is equally likely. However, quantification of the different IsoP regioisomers finds that these compounds are not formed in equal amounts. When arachidonic acid is oxidized either in vitro or in vivo, the 5- and 15-series regioisomers are formed in significantly greater abundance than the 8- and 12-series regioisomers (Figure 4). One explanation for this difference is that that the arachidonyl hydroperoxides that give rise to the 8- and 12-series regioisomers readily undergo further oxidation to yield a novel class of compounds that contains both bicycloendoperoxide and cyclic peroxide moieties; these compounds are termed dioxolane-IsoPs and have been reported to form in vivo.61 The regioisomers of 5- and 15-series cannot undergo this further oxidation and can, therefore, accumulate at higher concentrations in tissues and fluids as they represent terminal oxidation products of arachidonic acid. The dioxolane-IsoPs have been extensively characterized using mass spectrometry.61-63 The biological activity and the utility of the dioxolane-IsoPs as biomarkers of oxidative stress have not been explored.
Figure 4.
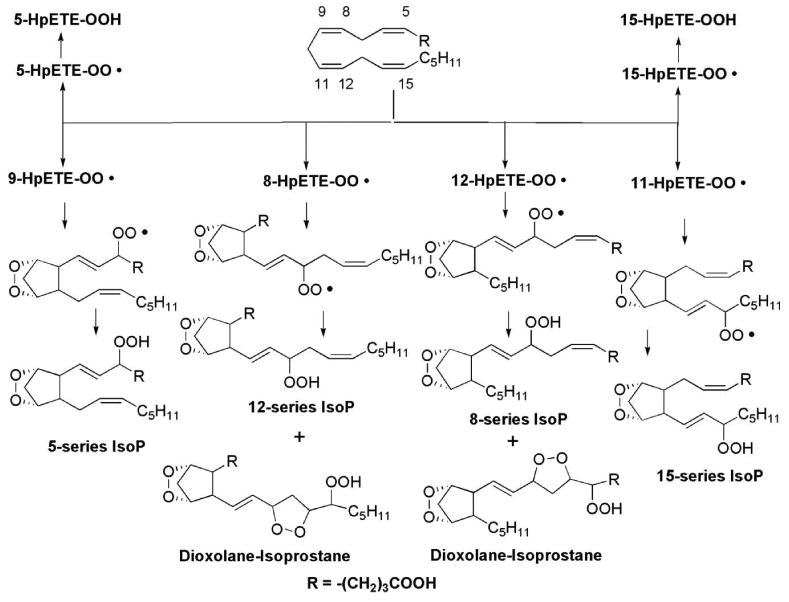
Dioxolane-isoprostanes are generated from 8-series and 12-series isoprostanes but not from 5-series and 15-series isoprostanes.
4. Quantification of F2-isoprostanes as an index of oxidant stress in vivo
Several methods have been developed to quantify the F2-IsoPs including gas chromatography-mass spectrometry (GC/MS), GC-tandem mass spectrometry (GC/MS/MS) liquid chromatography-tandem mass spectrometry (LC/MS/MS), and immunoassays. Each of these assays varies in the particular mixture of F2-IsoP regioisomers that are measured. Typically, 15-F2t-IsoP (also referred to as 8-iso-PGF2α), which is one of the most abundant IsoP isomers formed in vivo, is measured along with other isomers. Several internal standards are available from commercial sources to quantify the IsoPs. [2H4]-15-F2t-IsoP ([2H4]-8-iso-PGF2α) and [2H4]-PGF2α are the two most commonly used isotopically-labeled internal standards and their structures are shown in Figure 5. (Nomenclature note: 15-F2t-IsoP is designated as such because the hydroxyl groups on the molecule are oriented trans to side chain stereochemistry.) Recently, Mas and colleagues have reported a GC/MS method for quantification of F2-IsoPs in urine that utilizes 4(RS)-F4t-neuroprostane (derived from DHA) as the internal standard in order to eliminate compounds that possibly interfere with the ionization of [2H4]-15-F2t-IsoP and thus confound the analysis.64 However, a potential downside of this approach is that 4(RS)-F4t-neuroprostane contains conjugated double bonds that are susceptible to undergoing oxidation during sample processing, which cannot occur using [2H4]-15-F2t-IsoP as the internal standard.
Figure 5.
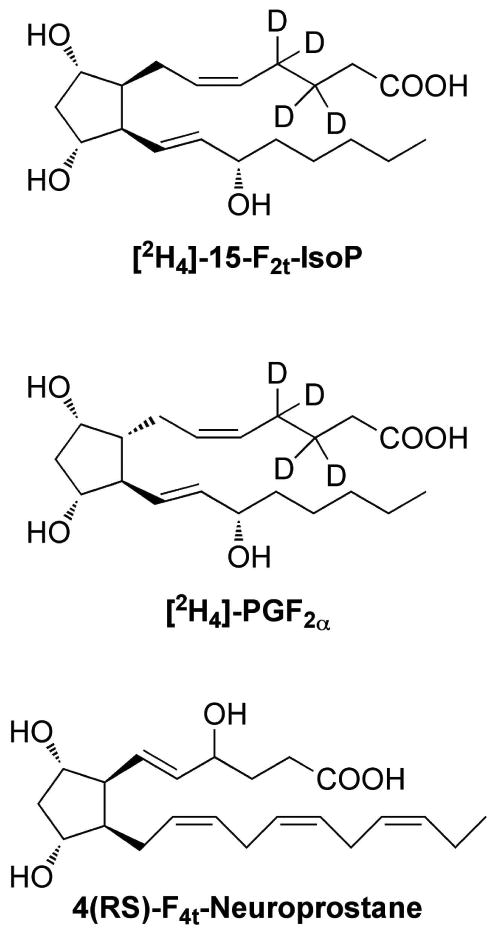
Structures of isotopically-labeled internal standards typically used to quantify F2-isoprostanes.
Quantification of F2-IsoPs by MS has distinct advantages compared to analysis by immunoassay methodologies such as ELISA. Although ELISA measurement offers high throughput analysis and does not require costly instrumentation, the polyclonal antibodies used to bind F2-IsoPs exhibit cross-reactivity other many other molecules similar in structure, including COX-derived PGF2α.65 This cross-reactivity results in the quantification of inflated concentrations of F2-IsoPs. Additionally, biological impurities can interfere with antibody binding.65 MS offers high sensitivity and specificity yielding quantitative results in the low picogram per mL range. Esterified F2-IsoPs can also be quantified by measurement of free compounds after base hydrolysis. GC/MS offers the greatest sensitivity, which is particularly beneficial for quantification of low-level F2-IsoPs in plasma, but requires time-consuming sample preparation and molecule derivatization prior to analysis. Using LC/MS, the single isomer 15-F2t-IsoP can be quantified and sample throughput is greater than GC/MS because chemical derivatization is not required.66 Smith and colleagues recently reported an elegant comparison study in which they examined correlations between a newly developed LC/MS/MS methodology and two commercially available ELISA kits (Cayman Chemicals and Oxford Biomedical Research) to quantify 15-F2t-IsoP in 156 urine samples. These authors found that there was significant correlation between the three methods but the ELISA from Oxford Biomedical Research had a positive bias compared with the other methods while the ELISA from Cayman Chemicals revealed a large spread of results when analyzed using a Bland-Altman plot. These discrepancies are most likely the result of the polyclonal antibody cross-reacting with other compounds.
The true utility of the F2-IsoPs is in the quantification of lipid peroxidation and thus oxidant stress status in vivo. F2-IsoPs are stable, robust molecules and are detectable in all human tissues and biological fluids analyzed, including plasma, urine, bronchoalveolar lavage fluid, cerebrospinal fluid, bile, and amniotic fluid.67 The quantification of F2-IsoPs in urine and plasma, however, is most convenient and least invasive. Notably, the Biomarkers of Oxidative Stress (BOSS) Study, an independent, multi-investigator study sponsored by the National Institute of Environmental Health Sciences, compared more than 30 endogenous biomarkers of oxidative stress and determined that the most accurate method to assess in vivo oxidant stress status is the quantification of plasma or urinary F2-IsoPs.68
Normal levels of F2-IsoPs in healthy humans have been defined.67,69,70 Defining these levels is particularly important in that it allows for an assessment of the effects of diseases on endogenous oxidant tone and allows for the determination of the extent to which various therapeutic interventions affect levels of oxidant stress. Elevations of IsoPs in human body fluids and tissues have been found increased in a diverse array of human disorders and the findings have been extensively reviewed. Some of the diseases associated with increased levels of F2-IsoPs include atherosclerosis and its risk factors such as obesity and cigarette smoking,71-75 hypercholesterolemia,76 diabetes,77 HIV,78-81 asthma,82-85 neurodegenerative diseases,86,87 rheumatoid arthritis,88-90 certain types of cancer,91-94 and many others.71-94
Given that the IsoPs are not only biomarkers but also mediators of oxidative-stress related diseases, multiple investigators have focused on possible therapeutic interventions that can decrease endogenous production of IsoPs. In fact, medical treatments of the diseases associated with oxidative stress have had some success in inhibiting IsoP formation. Antioxidant supplementation, anti-diabetic treatments, cessation of smoking, weight loss, and even a decrease in daily caloric intake have been shown to decrease endogenous IsoP formation.72,95,96 Antioxidant therapy in particular has been studied in detail in humans, with Vitamin E (α-tocopherol) as one of the most widespread antioxidants studied. Studies have demonstrated, however, that that daily doses of 1600 IU or greater for at least 8 weeks were required to statistically reduce plasma levels of F2-IsoPs.95,97 Interestingly, Block and colleagues have shown that 1000 mg/day vitamin C or 800 IU/day vitamin E for two months can lower levels of plasma F2-IsoPs by 22% (P=0.01) or 9.8% (P=0.46), respectively, when baseline levels of F2-IsoPs are high (> 50μg/mL), such as in obese populations.98 Neither treatment decreased levels of F2-IsoPs in individuals with normal baseline levels. In other studies, pre-treatment of skin with topical Vitamin E has also been shown to reduce levels of F2-IsoPs present post-UV irradiation.93 Finally, consumption of tart cherry juice, which is high in polyphenols, particularly anthocyanins, increases the capacity of elderly humans to resist oxidative damage as measured by changes in plasma F2-IsoP levels in response to forearm ischemia-reperfusion (I/R) before and after treatment. The tart cherry juice intervention reduced the I/R-induced F2-isoprostane response (P < 0.05), whereas placebo had no significant effect. Tart cherry juice has no effect on basal F2-IsoP levels.
Despite the prevalence of reports in the literature on F2-IsoPs in human disease, care must be taken when examining the data and comparing multiple studies. In an excellent commentary published recently, Halliwell and Lee broached several controversial topics regarding sampling, method of quantification, and data interpretation.99,100 One primary discussion point in the article centered on the choice of biological matrix and the particular F2-IsoP species measured. Most studies in which F2-IsoPs were quantified have focused on measuring these molecules in either plasma or urine and have assumed that these measures were equivalent. However, in the few reported studies in which F2-IsoPs were measured in both urine and plasma, discrepancies between the two measurements were often found. Halliwell and Lee suggest that these differences could relate to the in vivo generation of free radicals and subsequent F2-IsoP formation as well as to the hydrolysis, metabolism, and excretion of these compounds. For completeness, these authors suggest measuring total F2-IsoPs (both molecules esterified in phospholipids and those which are unesterified) in plasma along with free F2-IsoPs in urine. They also propose that measurement of F2-IsoP urinary metabolites should be considered.
5. Metabolism of F2-isoprostanes
In contrast to cyclooxygenase-derived PGs that are generated from free arachidonic acid, IsoPs are initially formed from arachidonic acid esterified in tissues phospholipids. F2-IsoPs can then be released from the phospholipid backbone as free fatty acids by phospholipase action. Free F2-IsoPs are found circulating in plasma; circulating F2-IsoPs are filtered in the kidney and appear in the urine. F2-IsoPs can also undergo metabolism in the liver. Two major urinary metabolites of 15-F2t-IsoP, one of the most abundant endogenous F2-IsoPs, have been identified as 2,3-dinor-15-F2t-IsoP (2,3-dinor-8-IsoP-F2a) and 2,3-dinor-5,6-dihydro-15-F2t-IsoP (2,3-dinor-5,6-dihydro-8-IsoP-F2a) (Figure 6). 101,102 Multiple mass spectrometric methodologies have been developed to quantify each of these metabolites yet most clinical reports in the literature have focused on the measurement of unmetabolized F2-IsoPs in urine. Interestingly, Dai et al. reported complex relationships between the excretion of F2-IsoPs and 2,3-dinor-5,6-dihydro-8-IsoP-F2a, oxidative stress, obesity, and breast cancer risk in a nested-case control study within the Shanghai Women's Health Study.92 These findings support Halliwell's and Lee's contention that measurement of F2-IsoPs and its metabolites should be carefully considered in clinical studies.
Figure 6.
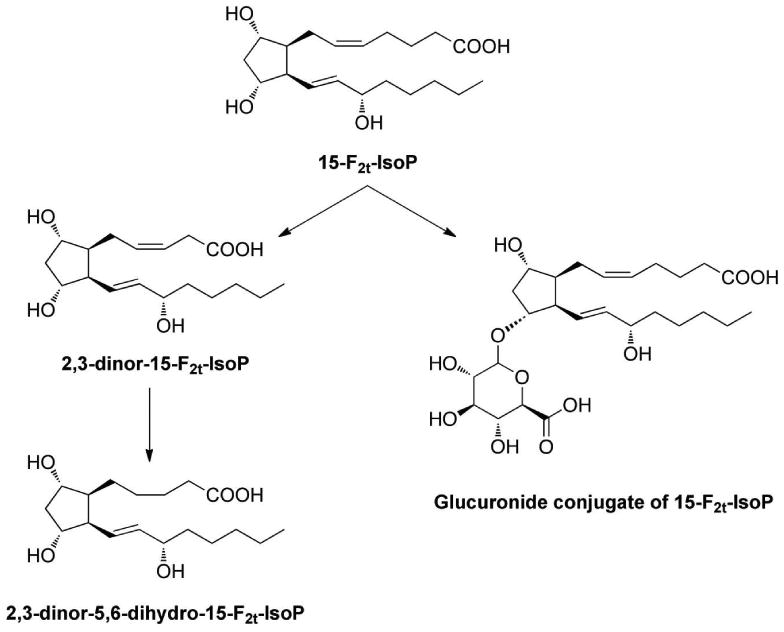
Urinary metabolites of 15-F2t-IsoP. Note: Glucuronide could conjugate with any of the three hydroxyl groups on 15-F2t-IsoP. Additionally, it is possible that multiple glucuronides could conjugate with 15-F2t-IsoP. As the exact glucuronide species generated have not been characterized, the current structure is shown for simplicity.
It is worth noting here that the majority of PGF2α observed in human urine is from the free radical catalyzed peroxidation of arachidonic acid and not from enzymatically derived from COX. Yin et al. have shown that PGF2α and its enantiomer (ent-PGF2α) are present the urine of healthy humans at levels near 1 ng/mg Cr. PGF2α itself, however, is readily metabolized by 15-prostaglandin dehydrogenase and enzymatic reductases to yield 13,14-dihydro-15-keto-PGF2α whereas its enantiomer is present unmetabolized in the urine. Thus, ent-PGF2α represents a major F2-IsoP present in the urine.
In addition to the metabolites discussed above, Yan and colleagues recently established that F2-IsoPs are excreted in the urine as glucuronide conjugates.103 These authors noted that levels of urinary F2-IsoPs were significantly increased after treatment of the urine with b-glucuronidase (0.43 ± 0.02 vs. 0.61 ± 0.03 nmol/mmol Cr) and that this increase was, in fact, dependent upon the activity of the enzyme. Further study is needed to determine how biological factors affect the extent of glucuronidation of F2-IsoPs.
6. Biological activities of the F2-isoprostanes
In addition to being robust markers of in vivo oxidant stress, F2-IsoPs can exert potent biological effects and potentially mediate some of the adverse effects of oxidant injury. As mentioned previously, IsoPs are initially formed in vivo esterified in glycerophospholipids. Molecular modeling of IsoP-containing phospholipids reveals them to be remarkably distorted molecules.55 Thus, the formation of these distorted phospholipids would be expected to exert profound effects on membrane fluidity and integrity, a well-know sequelae of oxidant injury. The majority of the studies exploring their bioactivity, however, have been performed using unesterified IsoPs.
One particular F2-IsoP that is produced abundantly in vivo and that has been extensively tested for biological activity is 15-F2t-IsoP (8-iso-PGF2α), which differs from cyclooxygenase-derived PGF2α only in the inversion of the upper side chain stereochemistry. In initial studies, 15-F2t-IsoP was found to be a potent renal vasoconstrictor with biological activity in the low nanomolar range.104 Subsequent studies demonstrated that it has similar effect in most species and vascular systems.105 F2-IsoPs are thought to exert their biological activity through thromboxane receptor (TPR) activation as TPR antagonists block the ability of 15-F2t-IsoP (8-iso-PGF2α) to active platelets and induce vasoconstriction of vascular smooth muscle cells, carotid arteries, and renal glomeruli. More recently, however, other mechanisms by which F2-IsoPs exert biological activity have been proposed. In 2008, Khasawneh and colleagues described a systematic study in which they explored the signaling and molecular interactions of 15-F2t-IsoP (8-iso-PGF2α) with TPRs as well as signaling through TPR-independent mechanisms in the platelet.106 Several landmark findings were reported therein: (1) 15-F2t-IsoP (8-iso-PGF2α) coordinated with Phe184, Asp193, and Phe196 in the active site of platelet TPRs, (2) Phe196 is a unique TPR binding site for 15-F2t-IsoP (8-iso-PGF2α) and not other TPR agonists, (3) 15-F2t-IsoP (8-iso-PGF2α) both stimulates and inhibits human platelet activation, and (4) inhibition of human platelet activation by 15-F2t-IsoP (8-iso-PGF2α) is TPR independent and is regulated by a yet to be determined cAMP-coupled receptor. In a recent comprehensive review of platelet function and isoprostane biology, Ting and Khasawneh suggested that F2-IsoPs should be listed as orphan ligands until further receptors regulating their biological activity are identified.107
Other regio- and diastereomers of F2-IsoPs have also been studied, although studies have been limited. 15-F2c-IsoP (12-iso-PGF2α) activates the PGF2α receptor and induces hypertrophy in cardiac smooth muscle cells. Interestingly, 5-F2t-IsoP, a major product of F2-IsoP formation like 15-F2t-IsoP, and its 5-epimer do not induce vasomotor effects in the rat thoracic aorta, the human internal mammary artery, nor the saphenous vein.108 Other series of F2-IsoPs have also been studied on pig retinal and brain vasculature and majority of them showed potent vasoconstriction.109 Notably, Ting and Khasawneh have recently published an elegant review of current knowledge surrounding the complex F2-IsoP bioactivity in the vasculature. 110
7. D2/E2-isoprostanes
In addition to undergoing reduction to yield F2-IsoPs, the arachidonyl endoperoxide intermediate can undergo isomerization to yield E- and D-ring IsoPs (Figure 2), which are isomeric to PGE2 and PGD2, respectively.111 E2/D2-IsoPs are formed competitively with F2-IsoPs, and recent studies have demonstrated that the depletion of cellular reducing agents, such as glutathione (GSH) or α-tocopherol, favors the formation of E2/D2-IsoPs over that of reduced F2-IsoPs. Depletion of GSH and a-tocopherol occurs in various human tissues under conditions of oxidant injury, including the brains of patients with Alzheimer's disease (AD). Reich et al. thus examined the ratios of F-ring to E/D-ring IsoPs in post-mortem brain tissues from patients with AD; not only were levels of both E2/D2- and F2-IsoPs significantly elevated, but E2/D2-IsoPs were the favored products of the IsoP pathway in affected brain regions. This increased ratio of E2/D2-IsoPs to F2-IsoPs provides information not only about lipid peroxidation in a given organ, but also about the redox environment in that tissue.
As a result of the above findings in the brain, formation of D2/E2-IsoPs has been explored in cerebral ischemia. A critical reduction in cerebral blood flow and increased pro-inflammatory mediators in the affected region characterize this condition. In 2008, Farias et al. reported that levels of E2/D2-IsoPs are increased in the brain of rats subjected to head-focused microwave irradiation for five minutes following decapitation, a model of complete ischemia.112 This finding was recently confirmed by Brose et al. using a newly developed LC/MS methodology to quantify these molecules.113 Most recently, E2/D2-IsoPs have been tentatively identified in cerebral spinal fluid of traumatic brain injured patients.114 Formation of E2/D2-IsoPs in these and other disease settings has important implications as 15-E2t-IsoP, one abundantly generated isomer, has been found to be a more potent vasoconstrictor than 15-F2t-IsoP or PGF2α and is an inhibitor of TP-mediated platelet aggregation.115,116
In addition to D2/E2-IsoPs, PGD2 and PGE2 can also be generated from oxidative stress, not simply from the COX. Gao et al. reported the formation of these molecules and their respective enantiomers in vivo via the spontaneous epimerization of 15-E2t-IsoP and 15-D2c-IsoP, respectively.117
The metabolism of E2/D2-IsoPs has not been extensively studied but Jahn and Dinca have synthesized a potential metabolite of 15-E2-IsoP although the formation of this metabolite in vivo has not been confirmed.118
8. A2/J2-isoprostanes
E2/D2-IsoPs, however, are not terminal products of the IsoP pathway. These compounds readily dehydrate in vivo to yield A2/J2-IsoPs (Figure 2), which are also known as cyclopentenone IsoPs because they contain an α,β-unsaturated cyclopentenone ring structure.119,120 A2/J2-IsoPs are highly reactive electrophiles that readily form Michael adducts with cellular thiols, including those found on cysteine residues in proteins and glutathione. In fact, structural studies suggest that distinct cyclopentenone IsoPs can selectively adduct specific residues on proteins. Stamatakis and Perez-Sala have written a comprehensive review on the interactions between electrophilic lipids, including cyclopentenone IsoPs, and proteins.121 The metabolism of cyclopentenone IsoPs has been studied in HepG2 cells, a cell line derived from human hepatocytes, as well as in the rat. These molecules are rapidly metabolized by glutathione transferase enzymes yielding water-soluble modified glutathione conjugates. The major urinary cyclopentenone IsoP metabolite in rats is a 15-A2t-IsoP mercapturic acid sulfoxide conjugate (Figure 7). 122
Figure 7.
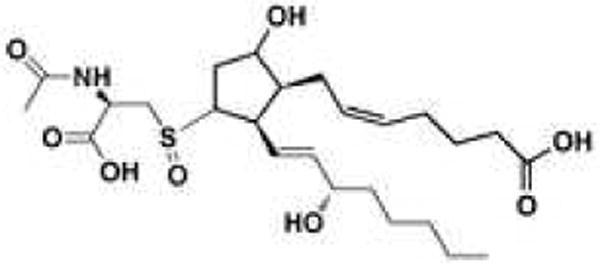
The major urinary metabolite of 15-A2t-IsoP identified in the rat.
The chemical reactivity of cyclopentenone IsoPs suggested that these compounds might be biologically active. The synthesis of two groups of cyclopentenone IsoPs regioisomers, 15-A2-IsoPs and 15-J2-IsoPs, has allowed the examination of their bioactivity. Studies employing primary cortical neuronal cultures demonstrated that both 15-A2-IsoPs and 15-J2-IsoPs potently induce neuronal apoptosis and exacerbate neurodegeneration caused by other insults at concentrations as low as 100nM.123 Musiek et al. propose that these molecules initially induce mitochondrial dysfunction and GSH depletion, causing marked increases in intracellular ROS production and further lipid peroxidation.123-125 This interruption of redox homeostasis leads to subsequent phosphorylation of ERK1/2 and serine 36 of p66shc as well as translocation and activation of 12-lipoxygenase (12-LOX). All of these factors contribute to cell death. It is important to note that in this system 15-A2t-IsoP is a both a product and inducer of oxidative stress; the production of 15-A2t-IsoPs in response to an initial oxidant injury sets in motion a feed-forward cycle that leads to a loss of intracellular redox homeostasis and consequent apoptosis. Interruption of this cycle with antioxidants can prevent cell death. Importantly, 15-A2/J2-IsoPs have more recently been shown to be produced abundantly in stroke-infarcted human cortical tissue and could be thus be partially responsible for neurological damage following stroke events in vivo.126 Taken together, these data suggest that A2/J2-IsoPs, or the downstream signaling pathways activated by these compounds, might represent novel therapeutic targets.
Cyclopentenone IsoPs also exert biological effects in non-neural tissue. Musiek et al. reported that 15-A2-IsoPs potently suppress lipopolysacharide (LPS)-induced inflammatory signaling via inhibition of the NF-κB pathway in macrophages.127 15-A2-IsoPs inhibit the induction of COX-2 and the inducible nitric oxide synthase as well as the elaboration of several pro-inflammatory cytokines in LPS-stimulated RAW264.7 cells and primary murine macrophages. Similar anti-inflammatory effects were seen with 15-J2-IsoPs. 15-J2-IsoPs also activate the peroxisome proliferator activated receptor-gamma (PPARγ) with an EC50 of approximately 3μM. This receptor modulates a wide variety of biological processes, including inflammatory signaling and fatty acid metabolism. 15-J2-IsoPs also induce macrophage apoptosis at low micromolar concentrations in a PPARγ-independent manner. Thus, there is a diversity of actions among cyclopentenone IsoP isomers. It appears that these compounds could act as negative-feedback regulators of the inflammatory response, since oxidative stress and lipid peroxidation often occur under conditions of chronic inflammation.
15-A2-IsoPs have also been shown to inhibit NF-κB activation, through impairment of IkB-α degradation, in LPS-stimulated human placenta and fetal membranes.128 Like in macrophages, this inhibition was associated with a reduction in the release of pro-inflammatory cytokines including IL-1β, IL-6, IL-8, and TNF-α as well as PGE2 and PGF2α. These cytokines and PGs are involved in both term and preterm labor and delivery and these authors suggest that 15-A2-IsoPs or other cyclopentenone IsoPs could abrogate early labor. The potential role that cyclopentenone IsoPs could play in labor and delivery is of particular interest in light of a recent study by Menon and colleagues which demonstrated that F2-IsoPs are increased in normal term human fetal membrane explant cultures exposed to cigarette smoke extract.129 In that publication, the authors suggest that generation of F2-IsoPs may be an alternate pathway, in regards to traditional thinking about the bioactivities of the COX-generated PGE2 and PGF2α that mediates preterm pre-labor rupture of membranes. The data presented in these two studies suggests that the redox environment and consequent generation of either F2-IsoPs or E2/D2- and A2/J2-IsoPs in the human placenta and fetal membranes may be an important and previously understudied mechanism regulating onset of labor and delivery. Further studies are needed in this area to determine what role, if any, IsoPs play in the pathophysiology of childbirth.
9. Deoxy-J2-isoprostanes
15-deoxy-Δ12,14-PGJ2 (15-d-PGJ2) is a cyclopentenone PG that formed following the dehydration and isomerization of PGJ2, a metabolite of PGD2. Like PGA2/PGJ2 and A2/J2-IsoPs, 15-d-PGJ2 contains αβ-unsaturated carbonyl functionalities that contribute to a wide array of biological activities exerted by this molecule. This compound actually exhibits greater reactivity and biological potency in many cellular systems compared to other PGA2/PGJ2 and A2/J2-IsoPs as 15-d-PGJ2 contains two electrophilic carbon centers at both C-9 and C-13, which can undergo Michael addition with free sulfhydryls of cysteine residues on proteins.130 Some of the biological activities of 15-d-PGJ2 are related to the observation that this molecule activates the PPARγ nuclear receptor.131 Previous studies indicate that 15-d-PGJ2 mediates PPARγ-dependent repression of NF-κB and AP-1, inhibiting the expression of pro-inflammatory proteins. 132 15-d-PGJ2 has also been shown to possess PPARγ-independent activity through direct adduction to intracellular proteins targets. In this context, 15-d-PGJ2, like 15-A2/J2-IsoPs, has been shown to exert anti-inflammatory effects by inhibiting NF-κB-dependent gene transcription via covalent modification of cysteine residues on IκB kinase and in the DNA binding domains of NF-κB subunits.133,134 In addition to its proposed role inflammation, 15-d-PGJ2 exhibits potent antiproliferative and pro-apoptotic effects in various cancer cell lines by modulating MAPK135 and p53 activity.136,137 15-d-PGJ2 also stimulates antioxidant responses through activation of Nrf2-mediated signaling via adduction to critical cysteine residues on the electrophile sensor Keap1.138-141
Despite significant interest in the biological activity of 15-d-PGJ2, evidence for its formation in vivo is controversial. Production of 15-d-PGJ2 has been reported in selenium-supplemented macrophages following LPS stimulation142 and in rodent models of inflammation, including carrageenan-induced pleurisy in rats133 and zymosan-induced resolving peritonitis in mice.143 15-d-PGJ2 was also detected in macrophages in human atherosclerotic lesions using immunohistochemical approaches.144 In addition, Hirata et al. reported the detection of Δ12-PGJ2, the precursor to 15-d-PGJ2, in human urine, and its formation was suppressed to some extent by COX inhibitors.145 On the other hand, Bell-Parikh et al. reported very low levels of 15-d-PGJ2 (∼5 pM) in human urine, and these levels are likely insufficient to exert bioactivity.146 Additionally, levels of 15-d-PGJ2 were unaltered in the pathophysiological settings in which 15-d-PGJ2-mediated activation of PPARγ has been implicated, namely inflammation and diabetes. Based on these and other findings, the physiological relevance of 15-d-PGJ2 has been questioned.146,147 Further, the extent to which 15-d-PGJ2 is formed in vivo in humans and the mechanisms that regulate its formation remain unclear.
Recently, Hardy et al. described for the first time that 15-d-PGJ2 is generated from the free radical catalyzed peroxidation of arachidonic acid (Figure 8). Further, a series of 15-d-PGJ2-like compounds, termed deoxy-J2-IsoPs, are formed in vivo from the dehydration of J2-IsoP regioisomers (Figure 9). 15-d-PGJ2 and deoxy-J2-IsoPs were significantly generated in vivo esterified in membrane phospholipids in rat liver after induction of lipid peroxidation by CCl4.148 The finding that 15-d-PGJ2 is produced in vivo via free radical-catalyzed lipid peroxidation, independent of the COX pathway, offers new insights into the possible mechanisms that contribute to 15-d-PGJ2 formation and provides the impetus for examination of the systemic production of 15-d-PGJ2 in humans.
Figure 8.
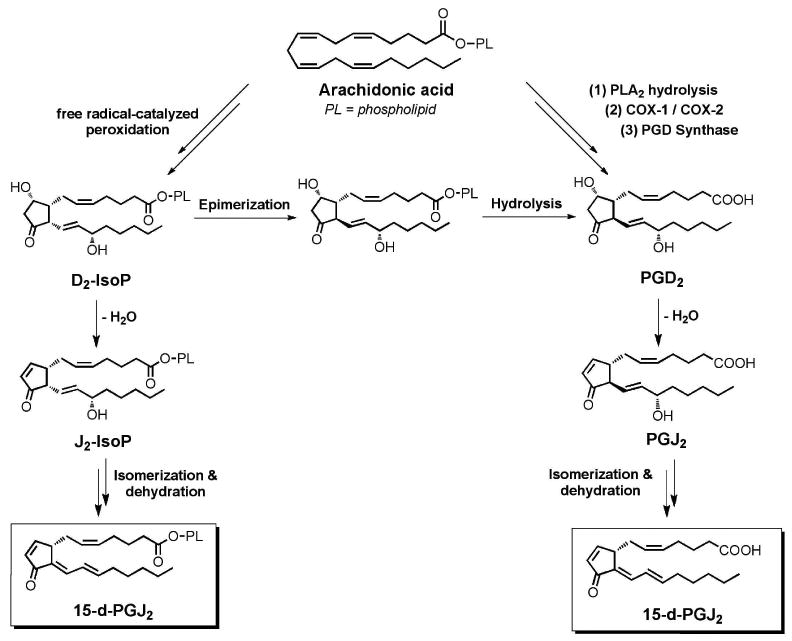
15-deoxy-Δ12,14-PGJ2 can be formed from cyclooxygenase-derived PGD2 as well as from the non-enzymatic free radical-catalyzed peroxidation of arachidonic acid.
Figure 9.
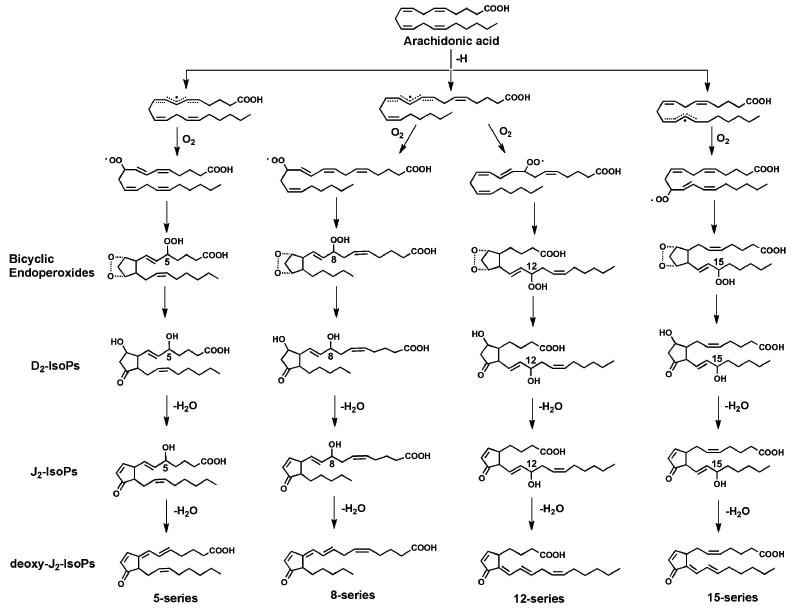
Multiple 15-d-PGJ2 regioisomers can be generated during free radical-catalyzed peroxidation of arachidonic acid.
10. Isoketals/isolevuglandins
In addition to rearranging to generate E2/D2-IsoPs, H2-IsoPs rearrange to form highly reactive γ-ketoaldehydes (γKAs, also called isoketals or isolevuglandins) in the same manner that PGH2 derived from cyclooxygenase non-enzymatically rearranges to form levuglandins. Oxidation of AA generates eight E2- or D2- γKA regioisomers and 64 individual stereoisomers (Figure 10).149 E2-isomers have the ketone group adjacent to the carboxylate side chain and the aldehyde adjacent to the second (or lower) side chain; D2-isomers have the aldehyde group adjacent to the carboxylate side chain and the ketone group adjacent to the second side chain.
Figure 10.
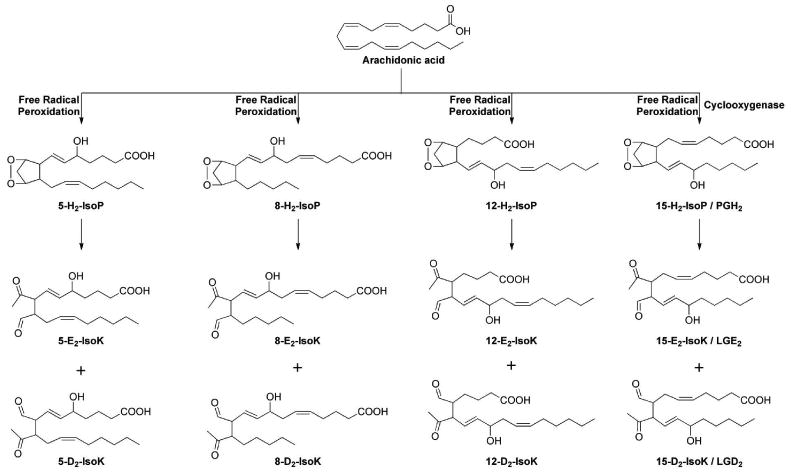
Four different regioisomers of E2/D2-isoketals are be generated from isoprostane bicycloendoperoxides.
Interest in these molecules as potential mediators of cellular dysfunction induced by oxidative stress stems from three cardinal features of their biochemistry: 1) their rapid adduction to lysine residues of proteins, 150 2) their proclivity to crosslink proteins, 151-154 and 3) their adduction to aminophospholipids. 155 It is important to note that most studies examining the biological activities of these molecules have used a single γKA regioisomer, referred to as levuglandin E2 or 15-E2-isoketal, which is produced by either pathway. For simplicity, we will refer to this compound as γKA throughout.
10.1. The γKAs react with the lysyl residues of proteins
The reaction of γKAs with primary amines including lysine and PE is shown in Figure 11.150 Formation of the essentially non-reversible pyrrole adduct underlies the far greater reactivity of γKAs compared to other commonly studied lipid aldehydes such as HNE which typically form Michael adducts. The dramatic differences in reactivity of γKA and HNE was demonstrated in experiments where each of the lipid aldehydes were incubated with albumin in vitro. Greater than half of all γKA adducted to the protein in the first 20 seconds, while less than half of the HNE was adducted after 60 min incubation time.150 Formation of γKAs in vivo can be quantified by measuring the highly stable γKA-lysyl-lactam adducts using mass spectrometry.156 Because these adducts are not rapidly cleared, their tissue level serves as a dosimeter of oxidative stress over time.157 Increased γKA-protein adducts have been reported in a number of disease conditions including atherosclerosis, myocardial infarction, end-stage renal disease, experimental sepsis, hyperoxia, and carbon tetrachloride poisoning.158-161 Formation of protein adducts may underlie some of the reported effects of these compounds including alterations in ion channels.162
Figure 11.

Reaction of γ-ketoaldehydes with primary amines yields formation of stable adducts.
10.2. The γKAs crosslink proteins
The initial reaction of γKA with lysine forms pyrrole adducts which then mature under oxidizing conditions into lactam, hydroxylactam, or cross-linked adducts (Figure 11).151-154 Oxidation of protein pyrrole adducts in the immediate vicinity of other nucleophiles such as cysteines form crosslinks between the amino acid residues,163 and alter the conformation and function of proteins.164 γKAs crosslink proteins more potently and rapidly than HNE,154 and this protein-protein crosslinking can be prevented experimentally by adding excess of glycine.154 If and to what extent cross-linking contributes to γKA action needs to be further explored.
10.3. The γKAs react rapidly with phosphatidylethanolamine
In addition to lysine residues, γKA can react with other primary amines present in cells including phosphatidylethanolamine (PE) and DNA.165-167 One of the most important of these cellular amines is the aminophospholipid phosphatidylethanolamine (PE). Because non-enzymatic formation of γKA occurs on membrane phospholipids, they are well positioned to react with PE in the membrane. Exogenous addition of γKA to human umbilical vein endothelial cells (HUVEC) leads to approximately five-fold more abundant γKA-PE adduct formation than protein adduct formation.155 Although little is known about the levels of γKA-PE in vivo, Li et al reported significant increases in mice chronically treated with ethanol and in human subjects with age-related macular degeneration.168 Likewise, there is a paucity of data relating to the bioactivity of γKA-PE, but recent studies suggest the possibility that they might mediate some of the pro-inflammatory effects of lipid peroxidation. For instance, in studies with cultured HUVEC, γKA-PE induces expression of inflammatory cytokines and adhesion molecules that results in the adhesion of THP-1 monocytes.161 This effect appears to be mediated by induction of endoplasmic recticulum (ER) stress, as exposure of endothelial cells lead to increased expression of ER stress signaling molecules such as BiP and CHOP. Furthermore, chemical inhibitors of ER stress signaling blocked monocyte adhesion to HUVEC induced by γKA-PE. Although the exact mechanism whereby γKA-PE induces ER stress is unknown, γKA-PE significantly alters membrane curvature and the N-pyrrole moiety of γKA-PE appears to mediate this effect.
10.4. γKA Scavengers
To better define the biological role of γKAs in oxidative injury and potentially prevent their detrimental effects, studies were performed to identify selective scavengers of γKAs. Initial screens identified pyridoxamine (PM) as a compound that effectively intercepts γKAs from adducting to cellular amines.169 To understand the basis of PM's reactivity with gKAs, Amarnath and colleagues determined second order reaction rates for a series of primary amines relative to N-α-acetyl-lysine. These structure-activity relationship studies identified the critical moiety to be a phenolic amine with the hydroxyl group adjacent to the methyl amine (Figure 12).169 Therefore, other phenolic amines such as salicylamine (SA) and pentyl-pyridoxamine (PPM) are similarly potent and are as selective as PM for scavenging γKA. None of these three scavengers significantly reduced the levels of F2-IsoPs during in vitro oxidation of AA, indicating that reduction in the level of γKA adducts can be directly attributed to γKA scavenging and not inhibition of lipid peroxidation.170 Further, these compounds did not significantly scavenge other lipid aldehydes such as HNE169,170 and they reacted with α-ketoaldehydes such as methylglyoxal at a rate approximately 187 times slower than γKAs.169 Thus, PM and related phenolic amines preferentially scavenge γKAs compared to other lipid aldehydes. The mechanism for this selectivity of these phenolic amines appears to be the ability of the hydroxyl group adjacent to the methylamine to catalyze pyrrole formation.169 In aqueous solutions, all three scavengers markedly reduced the levels of Lys-lactam adduct, but in platelets, SA and PPM reduced levels of Lys-lactam adducts to a greater extent than PM.170 Additionally, in intact cells exposed hydrogen peroxide, both SA and PPM protected cellular viability, while PM did not.170 Similarly, SA and PPM protected cells against peroxide induced inhibition of sodium currents, while PM failed to do so.162 SA is orally bioavailable171 and administering SA in the drinking water of ApoE4 mice prevents the age-related loss of working memory in these mice.172 γKA scavengers could thus be valuable tools for future in vivo studies exploring the contribution of γKA to disease processes.
Figure 12.
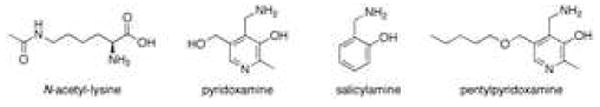
Structures of γ-ketoaldehyde scavengers.
11. Isofurans
As discussed, the value of F2-IsoPs as a biomarker of endogenous oxidative stress is well appreciated. Nonetheless, a shortcoming of this approach exists during situations involving oxidant injury in settings of elevated oxygen tension. When IsoPs are formed there is a carbon-centered radical that undergoes 5-exo-cyclization to form the cyclopentane ring. Alternatively, this carbon-centered could react with a molecule of oxygen to generate oxidized products of a different structure as shown in Figure 13. These products have a substituted tetrahydrofuran ring and thus have been named isofurans (IsoFs).173,174 Two mechanisms have been proposed for the formation of these molecules – the cyclic peroxide cleavage pathway and the epoxide hydrolysis pathway.175 A total of 8 regioisomers are formed that are comprised of 16 racemic diastereomers for a total of 256 compounds. IsoFs are routinely quantified using the same GC/MS assay utilized to measure F2-IsoPs; the primary m/z noted for the pentafluorobenzyl ester, trimethylsilyl ether derivatives of the IsoFs.
Figure 13.
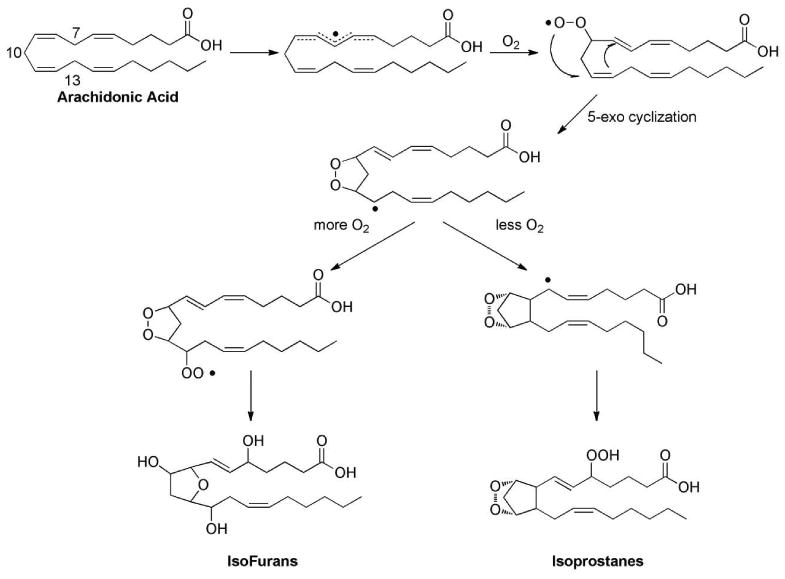
Mechanism of formation of isofurans compared to the mechanism of formation of F2-isoprostanes.
As hypothesized by Fessel et al., it was found that whereas levels of F2-IsoPs from arachidonic acid remain virtually unchanged when oxygen tension is increased from 21% to 100%, levels of IsoFs are increased significantly.174 Further, it has been shown that levels of IsoFs esterified in lungs of mice exposed to 100% O2 were significantly increased compared to mice breathing 21% O2. In these animals, level of F2-IsoPs remained unchanged.
More current studies have demonstrated the utility of quantifying IsoFs as biomarkers of oxidative stress in clinical settings in which high concentrations of oxygen are used during treatments or procedures. For example, in 2009, Vento et al. examined the effect of using either 30% or 90% O2 for the resuscitation of preterm neonates (24-28 weeks gestational age).176 In the study it was found that urinary IsoFs, but not F2-IsoPs, were significantly increased one week after birth in the high-oxygen group compared to the low-oxygen group. Additionally, increased levels of urinary IsoFs as well as increased levels of GSSG and 8-hydroxy-2′-deoxyguanosine, other biomarkers of oxidative stress, correlated with development of chronic lung disease. The authors concluded that resuscitation of preterm neonates with 30% oxygen caused less oxidative stress, inflammation, need for oxygen, and risk of bronchopulmonary dysplasia.
Additionally, two recently published studies have examined the utility of IsoFs as biomarkers of oxidative injury during surgical procedures. Mas et al. compared levels of F2-IsoPs and IsoFs and the effects of general or spinal anesthesia during ischemia/reperfusion of the leg in patients undergoing knee replacement surgery.177 Interestingly, F2-IsoPs were significantly lower in the general anesthesia patient group compared to the spinal anesthesia group whereas levels of IsoFs were significantly increased when general anesthesia compared to spinal anesthesia. The authors proposed that the increase in IsoFs was related to increased oxygen concentrations administered during general anesthesia. The pathophysiological consequences of this increase oxidative stress need to be explored.
Notably, Billings and colleagues examined the relationship between development of postoperative acute kidney injury (AKI) following cardiopulmonary bypass (CPB) and oxidative stress, as measured by both plasma and urine F2-IsoPs and IsoFs.178 AKI occurs in up to 30% of cardiac surgery patients and independently predicts in-hospital mortality, morbidity, and midterm as well as long-term survival; thus, understanding the mechanism(s) leading to AKI and development of a biomarker to predict AKI is of central importance. Plasma and urine F2-IsoPs and IsoFs were increased significantly during CPB with the magnitude of increase of IsoFs being much greater. Further, concentrations of both biomarkers in plasma and urine increased more significantly in subjects who subsequently went on to develop AKI. The magnitude of increase in urinary IsoFs was considerably greater compared to plasma IsoFs and was sustained over a period of two days following CPB. F2-IsoPs and IsoFs generated in the kidney are directly excreted into the urine. Therefore, the authors suggest the large increase in urinary IsoFs could reflect increased renal oxygen tension and oxidative stress due to dysfunctional mitochondria, which results in diminished mitochondrial oxygen consumption and thus higher cellular oxygen tension. In a separate study, also recently published, Snoeijs and colleagues examined the role of F2-IsoP signaling via the thromboxane receptor in renal ischemia reperfusion injury in wild type and thromboxane (TP) receptor knockout mice.179 F2-IsoPs as well as thromboxane B2 were readily detected in the urine of both groups following surgery; and kidney dysfunction, histological injury, and the number of infiltrated neutrophils was similar between the two groups. The authors thus concluded that F2-IsoPs might not play a central role in oxidative stress signaling in this setting. Taken together, these studies emphasize the need to carefully consider the mechanism of formation of oxidized lipid biomarkers as well as the clinical setting and disease state when quantifying endogenous oxidative injury.
12. Formation of isoprostanes in situ on phospholipids
As noted, IsoPs are primarily formed from the free radical attack on the phospholipids of cellular membranes and are subsequently released by phospholipases such as PAF-acetyl hydrolases. Most of the biological activities of isoprostanes, however, have been studied using the free form of the molecule as esterified isoprostanes are perceived as reservoir of the free isoprostanes. Recently, a novel class of esterified isoprostanes containing an epoxide moiety have been characterized in mildly oxidized LDL (MM-LDL). These phospholipid isoprostanes are called epoxyisoprostanes.180 Extensive studies have been carried out on these esterified isoprostanes and roles of these isoprostanes have been uncovered in the initiation and progression of atherosclerosis. There are many excellent reviews in the literature summarizing the biological activity of oxidized phospholipids including these phopholipid-bound isoprostanes.13,181,182 This review will focus on the chemical mechanisms of formation of esterified isoprostanes, detection by mass spectrometry, and comparison of the biology of the esterified isoprostanes to free analogues.
12.1. Mechanism of formation of epoxyisoprostanes esterified on phospholipids
The general free radical mechanism on the formation of different classes of isoprostanes has already been summarized herein. The G2- and H2-IsoPs, or bicyclic endoperoxides, are the critical intermediates that can be converted to the other IsoPs, such as F2-, E2/D2-IsoPs. Epoxy-F2-IsoPs have never been identified in vivo, thus these epoxy-IsoPs are most likely derived from the rearrangement from the G2/H2-IsoPs to E2/D2-analogues.183 The chemical mechanism of formation is summarized in Figure 14. Briefly, the rearrangement of the bicyclic endoperoxides in G2-IsoPs gives rise to intermediate E2/D2-IsoPs in which the hydroperoxides are not reduced. The acidity of the α-hydrogen atom of the carbonyl group on the cyclopentane ring renders a unique 1,5-dehydration reaction with the concomitant formation of an epoxy ring to give epoxy-E2/D2-IsoPs, in this case esterified in phosphatidylcholine (PC). It should be noted that the hydroperoxide functionality on the side chains is a prerequisite for the subsequent dehydration to occur. If the hydroperoxide is reduced while the rearrangement occurs, the E2/D2-IsoPs will be generated instead. Thus the redox enivronment in a biological system is an important determining factor for the formation of these epoxy-IsoPs. It is plausible that the product distribution of these IsoPs is strongly dependent on the reducing equivalents that they are exposed to. As discussed, a strong reducing environment favors the formation of F2-IsoPs because the bicyclic endoperoxides and the hydroperoxides are readily reduced. The bicyclic endoperoxide functionality has limited stability and it readily rearranges to D2/E2-IsoPs upon exposure to aqueous media. Thus, the formation of these epoxy-IsoPs most likely occurs after the oxidized sn-2 side chains on the phospholipid flip outside of the hydrophobic environment of the membrane.
Figure 14.
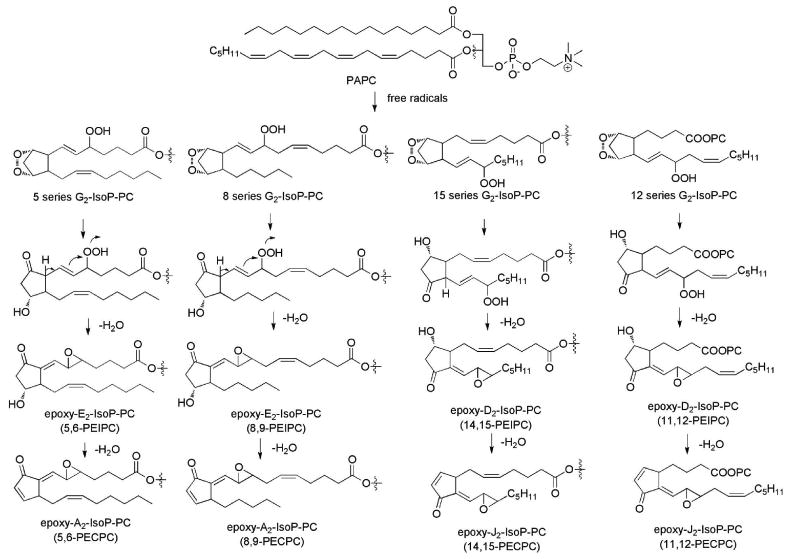
Formation of epoxy-isoprostanes esterified in phosphatidylcholine.
The regioselectivity of epoxy-IsoP-PCs is quite interesting. The two regioisomers of 5- and 8-series of G2-IsoPs gives rise to E2/A2 types of epoxy-IsoPs while 12- and 15-series G2-IsoPs generates D2/J2 epoxy-IsoPs, respectively. It should be pointed out that D2/E2-IsoPs without epoxide function groups can be produced from each of the four G2-IsoPs. The regioselectivity of these novel epoxy-IsoP-PCs has not been studied. Based on the regioselectivity of F2-IsoPs and the formation of dioxolane-IsoPs from 8- and 12-G2-IsoPs, one can predict that 5- and 15-series of G2-IsoPs generate more abundant epoxy-IsoPs than those from 8- and 12-series G2-IsoPs. It is important to note that the epoxy-D2/J2-IsoP-PCs should be more reactive than epoxy-E2/A2-IsoP-PCs.
12.2. Biological activity of isoprostanes esterifed on phospholipids
As noted, our current knowledge on the biological activities of isoprostanes primarily focuses on the free acid analogues of F2-IsoPs, specifically 15-F2t-IsoP; E2-IsoPs, specifically 15-E2t-IsoP; and 15-A2/J2-IsoPs. In contrast to the free acid analogues of IsoPs, the biological activity of phospholipid IsoPs have not been well studied.184-186 The epoxide containing IsoP-phospholipids, epoxy-E2/D2-isoprostane phosphatidycholine (PEIPC) and their dehydration products epoxy-cyclopentenone isoprostane PC (PECPC), were initially identified in MM-LDL. In LDL, oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-PC (PAPC) activates endothelial cells to produce monocyte chemotactic protein (MCP-1) and interleukin-8 (IL-8), two chemokines that play an important role in atherosclerosis. PEIPC and PECPC are the major components in oxidized PAPC that mediates this effect.
PEIPC and PECPC can also modulate a variety of intracellular signal transduction pathways: (1) PEIPC has been shown to activate the receptors of PGE2 and PGD2, EP2 and DP receptors respectively.187 This effect is implicated in atherosclerosis because EP2 receptors are expressed in all cell types relevant to atherosclerosis including endothelial cells (ECs), monocytes, macrophages and vascular smooth muscle cells. Activation of EP2 receptors on ECs by PEIPC activates integrins and increases binding of monocytes while EP2 receptor antagonists abolish this effect. The epoxide moiety in the molecules seemed to be required for the activation of EP2 receptor.
Additionally, phospholipid-bound IsoPs activate PPARs, intracellular ligand-activated transcription factors. Specifically, PEIPC and PECPC activate PPARα in transfected HeLa cells. PPARα is involved in MCP-1 synthesis; MCP-1 mediates monocyte recruitment during atherogenesis. Accumulation of PEIPC and PECPC in cells exposed to cytokines and in atherosclerotic lesions suggests that these lipids may play a role in the chronic disease processes.183
(2) It has long been demonstrated that MM-LDL caused a saturable dose-dependent increase in cAMP levels in aortic ECs that may result from stimulation of Gs and inhibition of Gi heterotrimeric G-protein complexes. More recently, it has been found that PEIPC in oxPAPC increase intracellular cAMP levels.188 Later studies have identified Gs-coupled prostaglandin E2 and D receptors (EP2 and DP) as mediators of this cAMP elevation induced by OxPAPC and PEIPC.189 Elevation of cAMP induced by PEIPC causes cellular effects relevant to inflammation including activation of the small GTPase R-RAS, which stimulates apical expression of connecting segment-1-containing fibronectin and thus promotes the entry of monocytes into sites of chronic inflammation. On the other hand, cAMP-dependent PKA plays a role in OxPAPC-induced phosphorylation of transcription factor CREB leading to enhanced expression of heme oxygenase-1, an enzyme with prominent antioxidant and anti-inflammatory properties.190 Furthermore, cAMP and PKA play mechanistic roles in barrier-protective effects of OxPLs in pulmonary vascular endothelium.
3) OxPAPC regulates genes related to inflammation, lipid metabolism, cellular stress, proliferation, and differentiation. In human aortic ECs, oxidation mixture of PAPC modulates expression of ∼1,000 genes.191 Transcription factors characterized as mediators of the effects of PEIPC are described in Table 1.
Table 1. Transcription Factors Activated By Epoxy-IsoP-PC.
| Transcription Factors | Cell Type | Target | Cofactors | References |
|---|---|---|---|---|
| STAT3 | HAEC | IL-8 | c-SRC JAKs | Gharavi, 2007, J. Biol. Chem. |
| PPARs | HAEC | IL-8 MCP-1 | Subga, 2002, J. Biol. Chem. | |
| NRF2 | HUVEC | NQO1 HO-1 | NADPH oxidase | Jyrkkanen, 2008, Circ. Res. Li, 2007, J. Lipid Res. |
12.3. Detection of intact isoprostanes esterified on phospholipids by mass spectrometry
Historically, quantification of F2-IsoPs has been accomplished by GC-MS techniques after steps of purification and derivatization. However, more recently, direct characterization and quantification of the PL esterified isoprostanes using LC/MS has been performed. F2-IsoP-PAPC was first identified by fast atom bombardment mass spectrometry in the negative ion mode.3 Recently, we employed iontrap MS to characterize the isoprostanes esterified on different phospholipid head groups after reverse phase separation. The different intact regioisomers have been identified using MSn in the negative ion mode.192 The F2- and D2/E2-IsoP esterified to 1-stearoyl-2-arachidonyl-PE have been detected in vitro and in vivo. The levels of intact E2- and F2-IsoPs on 1-palmitoyl-2-arachidonyl-PC and 1-stearoyl-2-arachidonyl-PC atherosclerotic plaques at different stages were analyzed by ESI-MS in the positive ion mode.193 There is, however, no clear trend of the levels of these IsoP-PCs at different stages of atheroma development.
PEIPC and PECPC were structurally characterized by MS, UV and NMR (Figure 15). Chemical derivatization was also used to assist with identification and characterization of functional groups in these molecules. Derivatization with methoxyamine HCl helped to identify the carbonyl group and epoxide in the structure. Treatment with sodium borohydride reduced the carbonyl group as well as the epoxide ring. Catalytic hydrogenation on the free acid analogue indicated the presence of two double bonds and two reducible oxygens in the molecules. After hydrolysis using the enzyme PLA2, the free acid of the epoxy-E2-IsoP was characterized by 1H-NMR. Thus the structure of epoxy-E2-IsoP-PAPC was unambiguously identified. Independent chemically synthesis also confirmed the structures.
Figure 15.
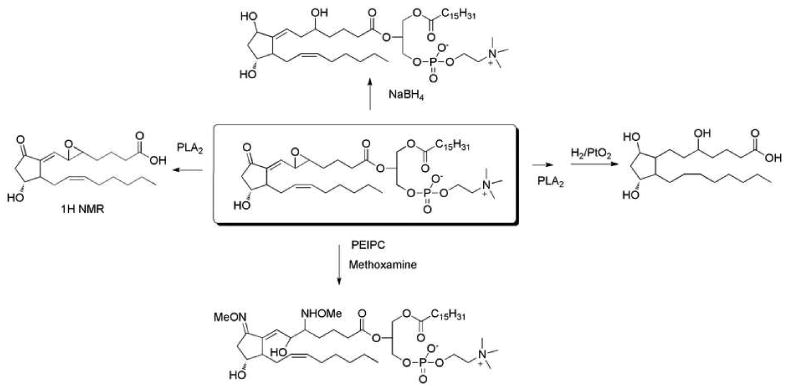
Chemical strategies to characterize epoxy-isoprostane-PC.
Levels of intact PEIPC were determined in rabbit aortas using LC/MS. In healthy controls 29.2 ± 3.1 ng/ mg of wet tissue was detected while the levels increased to 141.5 ± 45.2 ng/mg in New Zealand white rabbit aortas.194 PEIPCs were also detected in cultured HAECs. The levels of these compounds increased from 13 ng/mg in the control to 22.5 ng/mg after treatment with IL-1β. 183
13. Formation of isoprostanes from other polyunsaturated fatty acids
Arachidonic acid is not the only polyunsaturated fatty acid that can be oxidized to generate IsoPs. The basic requirement for cyclization to occur is the presence of at least three double bonds. F-ring IsoPs have are formed from the peroxidation of linolenic acid [C18:3, F1-IsoPs or phytoprostanes]195, EPA [C20:5, ω-3, F3-IsoPs]196, DHA [C22:6, ω-3, F4-IsoPs or F4-neuroprostanes (NPs)], and, most recently, adrenic acid [C22:4, ω-6, F2-dihomo-IsoPs] (Figure 16).197 Several excellent reviews of the phytoprostanes including recent perspectives on phytoprostane and IsoP nomenclature systems have been recently reported in the literature; thus, this review will focus on IsoPs generated from DHA, adrenic acid, and EPA.198,199
Figure 16.
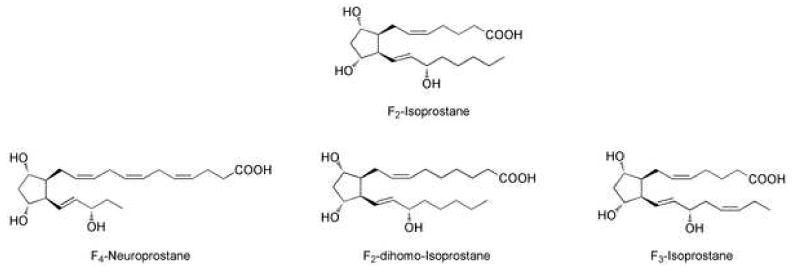
F-ring isoprostanes from arachidonic acid, docosahexaenoic acid, adrenic acid, and eicosapentaenoic acid.
13.1. Isoprostanes/Neuroprostanes generated from docosahexaenoic acid
Docosahexaenoic Acid (DHA) is highly enriched in human brain and neurons comprising ∼25% to 35% of the total fatty acids in aminophospholipids.200 DHA is more susceptible than AA to oxidation owing to the fact that it contains a greater number of double bonds.201 Initial studies of DHA oxidation were undertaken due to the abundance of this PUFA in the brain with the idea that measurement of IsoP-like compounds formed from oxidation of DHA, termed neuroprostanes (NPs), might provide a more sensitive biomarker of neuronal oxidant injury than IsoPs.197 DHA, however, is also abundant in the eye, heart, liver, and testes. Additionally, it is a major component of marine fish oil as well as many food supplements including infant formula. Studies initiated to understand the mechanism(s) underlying the oxidation of this PUFA are thus of great importance.46
Analogous to the pathway of IsoP formation from arachidonic acid, F-ring NPs are formed by reduction of H-ring intermediates. Because of the larger number of double bonds in DHA, 8 rather than 4 regioisomers are formed as a result of abstraction of a bisallylic hydrogen atom from C6, C9, C12, C15, and C18. This gives rise to 8 NP regioisomers (4-, 7-, 10-, 11-, 13-, 14-, 17-, and 20-series NPs).202 4-series and 20-series NPs are formed in greatest abundance as the other series of compounds can be further oxidized to generate dioxolane NPs in the same manner that 8- and 12-series IsoPs can generate dioxolane ring-containing IsoPs. The ring structures of the NPs are also similar to the spectrum of ring structures noted for IsoPs. F4-NPs are formed both during oxidation of DHA in vitro and in normal brain tissue.203 Moreover, F4-NPs are present in CSF from normal individuals and levels are higher in patients with Alzheimer disease (AD).204 Recently, Brown and colleagues have shown that F2-IsoPs and to a greater extent, F4-NPs, are significantly increased in neurons exposed to ischemic preconditioning.205 In addition to F4-NPs, E4-/D4-NPs, A4/J4-NPs, and γ-ketoaldehyde products similar to the isoketals (neuroketals, NK) are formed during oxidation of DHA in vitro and levels of these NPs are present in detectable quantities in brain tissue.206 Interestingly, NK protein adducts in normal human brain reach levels of ∼10 ng/g brain. Further, levels of NK adducts measured by LC/MS/MS in hippocampus from brains of patients with AD were significantly increased compared to hippocampus from age-matched controls; intense immunostaining, using the single chain antibody which recognizes both isoketal and NK adducts, was noted in virtually every neuron in the hippocampus from patients with AD while staining was absent in aged-matched controls.207,208
The biological activity of these molecules remains largely unexplored due to the complex strategies required to chemically synthesize these molecules. In recent years, however, three primary research groups including Vidari and colleagues, Taber and colleagues, and Durand and colleagues have successfully synthesized neuroprostane molecules of varying ring structures.209-211 Hopefully the synthesis of these molecules will lead to a greater understanding of the biological activities of NPs in tissues enriched with DHA. Musiek et al. have shown that 14-A4-NPs, synthesized by Vidari and colleagues, are potent inhibitors of NF-kappaB signaling similar to 15-A2-IsoPs.212
13.2. Isoprostanes from adrenic acid
VanRollins and colleages have examined the formation of F-ring IsoPs, termed F2-dihomo-IsoPs, generated from adrenic acid (C22:4, w-6).213 Adrenic acid, like DHA, is highly enriched in the brain but is primarily found in white matter and is associated with myelin. White matter is commonly damaged by ischemic stroke and is uniformly damaged in multiple sclerosis. These authors found that F2-dihomo-IsoPs are formed in significant amounts from adrenic acid and levels are markedly increased in settings of oxidant stress occurring specifically in the white matter in human brain. Proportionally, levels of dihomo-IsoPs in white matter undergoing oxidative injury increase to a greater extent than IsoPs and NPs derived from arachidonic acid and DHA, respectively. These studies suggest that quantification of dihomo-IsoPs may be a selective marker of white matter injury in vivo. Studying the formation of D2/E2-dihomo-IsoPs in white matter may be of particular importance due to the reducing environment in the brain and the identification of D2/E2-IsoPs from arachidonic acid molecules in ischemic stroke and traumatic brain injury.
13.3. Isoprostanes from eicosapentaenoic acid
Eicosapentaenoic Acid (EPA) is a polyunsaturated ω-3 fatty acid that is a major component of marine fish oil. Evidence from both epidemiological studies and clinical trials has shown that increased intake of fish oil, and, specifically, the ω-3 fatty acids, have beneficial effects on diseases associated with oxidative stress, particularly cardiovascular diseases. As early as 1980, studies suggested that the low mortality rates from coronary heart diseases among Greenland Eskimos compared with Danes may be due to the Eskimos' high consumption of seafood. 214 Since that time a number of cohort studies have been published that demonstrate a cardioprotective effect from fish consumption.215-222 These epidemiological studies have provided evidence that fish consumption favorably affects coronary heart disease mortality. In support of these studies, the hypothesis that consumption of ω-3 polyunsaturated fatty acids found in marine fish oils act to protect individuals from cardiovascular disease has been tested in a number of randomized control interventional trials. One of the larger prospective studies to date was the Gruppo Italian per lo Studio della Sopravvivenzia nell-Infarto (GISSI)-Prevention study. In this study, 11,324 patients with pre-existing heart disease were randomized to 300 mg/d vitamin E, 850 mg/d EPA + DHA, both, or neither. After 3.5 years of follow-up, the group given the ω-3 PUFAs alone demonstrated a 15% reduction in the primary endpoints of death, non-fatal myocardial infarction, and non-fatal stroke. This study also found that there was a 20% reduction in all causes of mortality as well as a 45% reduction in sudden cardiac death compared to the control diet group. Interestingly, in these studies vitamin E supplementation was found to provide no additional benefit. 223 This result, however, is not unexpected as the dose of vitamin E tested is known not to effectively reduce endogenous levels of lipid peroxidation.95
EPA alone is also helpful in treating cardiovascular disease patients. A large-scale study to determine the effects of the EPA, entitled the Japan EPA Lipid Intervention Study (JELIS), sought to determine the effect(s) dietary EPA supplementation would have on hypercholesterolemic patients suffering from coronary artery disease. 18,645 patients with a total cholesterol of 6.5 mmol/L or greater were recruited throughout Japan, and patients were randomly assigned to receive either 1800 mg of EPA daily with a statin treatment or statins alone. After a five-year follow-up, major coronary events were decreased 19% in the EPA-fed group versus the control statin group, indicating the promise of fish oil supplementation in the prevention of cardiac events in patients with high cholesterol. 224-226
Multiple other smaller trials have also shown additional cardioprotective effects associated with EPA. 227,228 Importantly, Gao et al. quantified the effect of EPA supplementation on arachidonate content and F2-IsoP levels in the heart tissue and found that levels of F2-IsoPs decreased dramatically, up to 64%, suggesting that EPA effectively decreases levels of pro-inflammatory F2-IsoPs formed from arachidonate.196 Recent, related work has suggested that metabolism of EPA yields oxidized bioactive compounds that mediate its effects. Serhan and co-workers have described novel anti-inflammatory hydroxylated EPA metabolites (termed E-series resolvins) that are derived from the enzymatic-mediated oxidation of EPA.229,230 These findings have led to considerable interest in determining other oxidation products of EPA that may mediate the anti-inflammatory effects of this fatty acid. Thus, Gao et al. went on to describe the formation of F3-IsoPs from the oxidation of EPA and showed that levels of these compounds dramatically increased in concordance with the noted decrease in F2-IsoPs.231
The significant generation of F3-IsoPs in cardiac tissue brings into question the biological activity of these molecules and the similarity to F2-IsoPs. One limited report, which provided no data, stated that the EPA-derived IsoP, 15-F3t-IsoP, possessed activity that is significantly different from the 15-F2t-IsoP acid in that the EPA-derived compound does not affect human platelet shape change.232 The lack of activity of 15-F3t-IsoP is consistent with observations regarding EPA-derived 3-series prostaglandins in that these latter compounds exert weaker agonist or no effects in comparison to arachidonic acid-derived 2-series prostaglandin.233,234 Further study is needed to determine the biological consequences of IsoP generation from EPA and whether EPA supplementation might be beneficial to populations with increased oxidative stress.
Analogous to arachidonic acid oxidation, multiple classes of IsoPs can also form from EPA oxidation. A3/J3-IsoPs, also known as the EPA-derived cyclopentenone IsoPs, have been definitively described to form in abundance in vivo in settings of oxidative stress as well. They have been found to exert potent anti-inflammatory activity much like their arachidonic acid and DHA counterparts through inhibition of NF-κB. 235 Unlike A2-IsoPs and A4-NPs, 15-A3t-IsoP did not promote lipid peroxidation in RAW cells. In addition to A3-IsoPs, a J3-IsoP isomer formed from EPA peroxidation has been shown to induce the NF-E2 related factor 2 (Nrf2)-based antioxidant response through inhibition of Keap1, a negative regulator of Nrf2. 236
14. Summary
The discovery of the IsoPs as products of non-enzymatic lipid peroxidation has been a major breakthrough in the field of free radical research. The quantification of these molecules has opened up new areas of investigation regarding the role of free radicals in human physiology and pathophysiology, and appears to be the most useful tool currently available to explore the role of endogenous lipid peroxidation in the pathogenesis of human disease. Our understanding of the IsoP pathway continues to expand, providing new insights into the nature of lipid peroxidation in vivo, and revealing new molecules that exert potent biological actions and might serve as unique indices of disease. Basic research into the biochemistry and pharmacology of the IsoPs, coupled with clinical studies employing these molecules as biomarkers, should continue to provide important insights into the role of oxidant stress in human disease.
Acknowledgments
The authors would like to acknowledge NIH grants GM15431, ES13125, ES000267, and DK20593.
Abbreviations
- AA
arachidonic acid
- AD
Alzheimer's Disease
- AKI
acute kidney injury
- AP
activator protein
- ATP
adenosine triphosphate
- CAT
catalase
- COX
cyclooxygenase
- CPB
cardiopulmonary bypass
- DHA
docosahexaenoic acid
- EC
endothelial cell
- EPA
eicosapentaenoic acid
- ER
endoplasmic reticulum
- ERK
extracellular-regulated kinase
- ESI
electrospray ionization
- ETC
electron transport chain
- GC
gas chromatography
- GPx
glutathione peroxidase
- GSH
glutathione
- HNE
4-hydroxynonenal
- HUVEC
human umbilical vein endothelial cell
- I/R
ischemic-reperfusion
- IL
interleukin
- IsoF
isofuran
- IsoPs
isoprostane
- JNK
Jun-NH2-terminal kinase
- LA
linoleic acid
- LC
liquid chromatography
- LOX
lipoxygenase
- LPS
lipopolysaccaride
- MAPK
mitogen activated protein kinase
- MCP-1
monocyte chemotactic protein 1
- MDA
malondialdehyde
- MM-LDL
minimally modified low density lipoprotein
- MPO
myleoperoxidase
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- NADPH
nicotine adenine dinucleotide phosphate
- NFkB
nuclear factor kappa B
- NK
neuroketal
- NP
neuroprostane
- Nrf2
NF-E2 related factor 2
- PAF
platelet activating factor
- PAPC
1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphatidylcholine
- PC
phosphatidylchoine
- PE
phosphatidylethanolamine
- PECPC
expoxy cyclopentenone isoprostane phosphatidylcholine
- PEIPC
epoxy E2/D2-isoprostane phosphatidylcholine
- PG
prostaglandin
- PI3K
phosphoinositol 3 kinase
- PLA2
phospholipase A2
- PM
pyridoxamine
- PPM
pentyl-pyridoxamine
- PTPIB
protein tyrosine phosphatase 1B
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SA
salicylamine
- SAPC
1-stearoyl-2-arachidonyl-sn-glycero-3-phosphatidylcholine
- SOD
superoxide dismutase
- TNFα
tumor necrosis factor α
- TP
thromboxane receptor
- Trx
thioredoxin
- UV
ultraviolet
Biographies

Dr. Ginger L. Milne received her B.S. in Chemistry with Honors in 1997 from Wake Forest University, graduating Magna Cum Laude. She received her Ph.D. in Chemistry in 2002 from Vanderbilt University under the direction of Dr. Ned A. Porter. Dr. Milne then completed a post-doctoral fellowship with Dr. Jason D. Morrow in the Division of Clinical Pharmacology at Vanderbilt University and subsequently joined the faculty there. She is currently Research Assistant Professor of Medicine, Research Assistant Professor of Pharmacology, and the Director of the Eicosanoid Core Laboratory, a facility developed at Vanderbilt University more than 20 years ago for the quantitative analysis of prostaglandins, leukotrienes, isoprostanes, and their metabolites in humans.

Dr. Huiyong Yin received his B.S. in Chemistry from Tongji University in Shanghai, China. He subsequently received his M.S. in Chemistry from the Shanghai Institute of Organic Chemistry at the Chinese Academy of Sciences in Shanghai in 1995. He received his Ph.D. in Chemistry in 2002 from Vanderbilt University under the direction of Dr. Ned A. Porter. He joined the faculty there in 2003 and is currently Research Associate Professor of Pharmacology, Chemistry, and Medicine.

Dr. Klarissa D. Hardy received her B.S. in Chemistry from Jackson State University in 2006. She received her Ph.D. in Pharmacology in 2011 under the direction of Dr. L. Jackson Roberts, II. During her pre-doctoral work, she was awarded a Ruth L. Kirschstein National Research Service Award Individual Fellowship. Dr. Hardy is currently pursuing a post-doctoral fellowship with Dr. Sidney Nelson at the University of Washington. She is a 2011 United Negro College Fund/Merck Postdoctoral Science Research Fellowship awardee.

Dr. Sean S. Davies received his B.S. in chemistry in 1993 and his Ph.D. in Experimental Pathology in 1999, both from the University of Utah. He was a post-doctoral fellow with Dr. Jack Roberts at Vanderbilt University, before being appointed as an assistant professor in the Department of Pharmacology there in 2008. He received one of the first NIH New Innovator Awards in 2007. His research focuses on the role of lipid mediators in chronic disease and on therapeutic strategies to treat these diseases.

Dr. L. Jackson Roberts, II received his M.D. degree from the University of Iowa and then did a residency in internal medicine at Washington University. He then did a postdoctoral fellowship in Clinical Pharmacology at Vanderbilt University and subsequently joined the faculty there. He is currently the William Stokes Professor of Experimental Therapeutics, Professor of Pharmacology, and Professor of Medicine. He has received numerous awards including a MERIT Award from the National Institutes of Health and a Discovery Award from the Society for Free Radical Biology & Medicine. He is an Associate Editor of the journal Free Radical Biology & Medicine. He is best known for his discovery in 1990 of isoprostanes, which has greatly advanced the ability to reliably assess oxidative stress status in vivo. He has over 250 peer reviewed original publications and over 120 book chapters.
References
- 1.Halliwell BG. Free Radicals in Biology and Medicine. 3rd. Oxford University Press; New York: 1999. [Google Scholar]
- 2.Yin H, Porter NA. Antioxid Redox Signal. 2005;7:170. doi: 10.1089/ars.2005.7.170. [DOI] [PubMed] [Google Scholar]
- 3.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ. Proc Natl Acad Sci USA. 1992;89:10721. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrow JD, Hill E, Burk RF, Nammour TM, Badr KF, Roberts LJ., Jr Proc Natl Acad Sci USA. 1990;87:9383. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, FitzGerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, R LJ, II, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Thiel DHV, Wellner D, Walter PB, Tome KB, Mason RP, Barrett JC. Free Radic Biol Med. 2005;38:698. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Musiek ES, Yin H, Milne GL, Morrow JD. Lipids. 2005;40:987. doi: 10.1007/s11745-005-1460-7. [DOI] [PubMed] [Google Scholar]
- 7.Milne GL, Yin H, Morrow JD. J Biol Chem. 2008;283:15533. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts LJ, II, Milne GL. J Lipid Res. 2009;50:S219. doi: 10.1194/jlr.R800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahn U, Galano JM, Durand T. Angewandte Chemie Int Ed. 2008;47:5894. doi: 10.1002/anie.200705122. [DOI] [PubMed] [Google Scholar]
- 10.Davies KJA. IUBMB Life. 2000;50:279. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 11.Porter NA. Acc Chem Res. 1986;19:262. [Google Scholar]
- 12.Porter NA, Caldwell SE, Mills KA. Lipids. 1995;30:277. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 13.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Antiox Redox Signal. 2010;12:1009(51). doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girotti AW, Kriska T. Antioxid Redox Signal. 2004;6:301. doi: 10.1089/152308604322899369. [DOI] [PubMed] [Google Scholar]
- 15.Cooper PR, Mesaros AC, Zhang J, Christmas P, Stark CM, Douaidy K, Mittelman MA, Soberman RJ, Blair IA, Panettieri RA., Jr PLoS One. 2010;5:e10235. doi: 10.1371/journal.pone.0010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller FL, Liu Y, Van Remmen H. J Biol Chem. 2004;279:49064. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 17.Liochev SI, Fridovich I. Free Radic Biol Med. 1994;16:29. doi: 10.1016/0891-5849(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 18.Lenaz G, Bovina C, Formiggini G, Parenti Castelli G. Acta Biochim Pol. 1999;46:1. [PubMed] [Google Scholar]
- 19.De Duve C, Baudhuin P. Physiol Rev. 1966;46:323. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- 20.Schrader M, Fahimi HD. Biochim Biophys Acta. 2006;1763:1755. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Boveris A, Oshino N, Chance B. Biochem J. 1972;128:617. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babior BM, Lambeth JD, Nauseef W. Arch Biochem Biophys. 2002;397:342. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 23.Dang PM, Cross AR, Quinn MT, Babior BM. Proc Natl Acad Sci USA. 2002;99:4262. doi: 10.1073/pnas.072345299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambeth JD. Nat Rev Immunol. 2004;4:181. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 25.Klebanoff SJ. Ann Intern Med. 1980;93:480. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 26.Clark RA, Klebanoff SJ. J Immunol. 1980;124:399. [PubMed] [Google Scholar]
- 27.Nicholls SJ, Hazen SL. J Lipid Res. 2009;50:S346. doi: 10.1194/jlr.R800086-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podrez EA, Abu-Soud HM, Hazen SL. Free Radic Biol Med. 2000;28:1717. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 29.Burke KE, Wei H. Toxicol Ind Health. 2009;25:219. doi: 10.1177/0748233709106067. [DOI] [PubMed] [Google Scholar]
- 30.Adler V, Yin Z, Tew KD, Ronai Z. Oncogene. 1999;18:6104. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 31.Lee SA, Dritschilo A, Jung M. J Biol Chem. 1998;273:32889. doi: 10.1074/jbc.273.49.32889. [DOI] [PubMed] [Google Scholar]
- 32.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. J Biol Chem. 2002;277:20336. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 33.Loukili N, Rosenblatt-Velin N, Rolli J, Levrand S, Feihl F, Waeber B, Pacher P, Liaudet L. J Biol Chem. 2010;285:15746. doi: 10.1074/jbc.M110.103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Mol Cell Biochem. 2004;266:37. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Holmgren A. J Biol Chem. 2009;284:723. doi: 10.1074/jbc.R800045200. [DOI] [PubMed] [Google Scholar]
- 36.Schafer FQ, Buettner GR. Free Radic Biol Med. 2001;30:1191. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 37.Berliner JA, Watson ADN. Engl J Med. 2005;353:9. doi: 10.1056/NEJMp058118. [DOI] [PubMed] [Google Scholar]
- 38.Hazen SL. Circulation. 2010;122:1786. doi: 10.1161/CIRCULATIONAHA.110.984062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montine TJ, Morrow JD. Am J Pathol. 2005;166:1283. doi: 10.1016/S0002-9440(10)62347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halliwell B. Cardiovasc Res. 2000;47:410. doi: 10.1016/s0008-6363(00)00097-3. [DOI] [PubMed] [Google Scholar]
- 41.Harman D. Proc Natl Acad Sci USA. 1981;78:7124. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balaban RS, Nemoto S, Finkel T. Cell. 2005;120:483. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Morrow JD, Zackert WE, VanderEnde DS, Reich EE, Terry ES, Cox B, Sanchez SC, Montine TJ, Roberts LJ., II . Handbook of Antioxidants. Vol. 2. Lester Packer and Enrique Cadenas/Marcel Dekker; New York: 2002. pp. 57–74. [Google Scholar]
- 44.Gardner HW. Free Radic Biol Med. 1989;7:65. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 45.Niki E. Free Radic Biol Med. 2009;47:469. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 46.Yin H, Porter NA. Antiox Redox Signal. 2005;7:170. doi: 10.1089/ars.2005.7.170. [DOI] [PubMed] [Google Scholar]
- 47.Pratt DA, Tallman KA, Porter NA. Acc Chem Res. 2011;44:458. doi: 10.1021/ar200024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benedetti A, Comporti M, Esterbauer H. Biochim Biophys Acta. 1980;620:281. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 49.Esterbauer H, Schaur RJ, Zollner H. Free Radic Biol Med. 1991;11:81. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 50.Schneider C, Porter NA, Brash AR. J Biol Chem. 2008;283:15539. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu W, Porter NA, Schneider C, Brash AR, Yin H. Free Radic Biol Med. 2011;50:166. doi: 10.1016/j.freeradbiomed.2010.10.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porter NA, Funk MO. J Org Chem. 1975;40:3614. doi: 10.1021/jo00912a037. [DOI] [PubMed] [Google Scholar]
- 53.Pryor WA, Stanley JP. J Org Chem. 1975;40:3615. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- 54.Liston TE, Roberts LJ. Proc Natl Acad Sci USA. 1985;82:6030. doi: 10.1073/pnas.82.18.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrow JD, Harris TM, Roberts LJ., 2nd Anal Biochem. 1990;184:1. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 56.Morrow JD, Awad JA, Kato T, Takahashi K, Badr KF, Roberts LJ, 2nd, Burk RF. J Clin Invest. 1992;90:2502. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Recknagel RO. Pharmacol Rev. 1967;19:145. [PubMed] [Google Scholar]
- 58.Rokach J, Khanapure SP, Hwang SW, Adiyaman M, Lawson JA, FitzGerald GA. Prostaglandins. 1997;54:853. doi: 10.1016/s0090-6980(97)00184-6. [DOI] [PubMed] [Google Scholar]
- 59.Taber DF, Morrow JD, Roberts LJ., 2nd Prostaglandins. 1997;53:63. doi: 10.1016/s0090-6980(97)00005-1. [DOI] [PubMed] [Google Scholar]
- 60.Stafforini DM, Sheller JR, Blackwell TS, Sapirstein A, Yull FE, McIntyre TM, Bonventre JV, Prescott SM, Roberts LJ., 2nd J Biol Chem. 2006;281:4616. doi: 10.1074/jbc.M507340200. [DOI] [PubMed] [Google Scholar]
- 61.Yin H, Morrow JD, Porter NA. J Biol Chem. 2004;279:3766. doi: 10.1074/jbc.M307137200. [DOI] [PubMed] [Google Scholar]
- 62.Yin H, Porter NA. Antioxid Redox Signal. 2005;7:170. doi: 10.1089/ars.2005.7.170. [DOI] [PubMed] [Google Scholar]
- 63.Yin H, Porter NA. Methods Enzymol. 2007;433:193. doi: 10.1016/S0076-6879(07)33011-5. [DOI] [PubMed] [Google Scholar]
- 64.Mas E, Michel F, Guy A, Bultel V, Falquet Y, Chardon P, Rossi JC, Cristol JP, Durand TJ. Chromatog B. 2008;872:133. doi: 10.1016/j.jchromb.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 65.Il'yasova D, Morrow JD, Ivanova A, Wagenknecht LE. Ann Epidemiol. 2004;14:793. doi: 10.1016/j.annepidem.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Yin H, Porter NA, Morrow JD. J Chromatogr B. 2005;827:157. doi: 10.1016/j.jchromb.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 67.Morrow JD, Zackert WE, Yang JP, Kurhts EH, Callewaert D, Dworski R, Kanai K, Taber D, Moore K, Oates JA, Roberts LJ. Anal Biochem. 1999;269:326. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- 68.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Free Radic Biol Med. 2005;38:698. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 69.Morrow JD, Roberts LJ. Meth Enzymol. 1999;300:3. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 70.Liang Y, Wei P, Duke RW, Reaven PD, Harman SM, Cutler RG, Heward CB. Free Radic Biol Med. 2003;34:409. doi: 10.1016/s0891-5849(02)01018-3. [DOI] [PubMed] [Google Scholar]
- 71.Morrow JD. Arterioscler Thromb Vasc Biol. 2003;23:368. doi: 10.1161/01.ATV.0000063107.86298.FD. [DOI] [PubMed] [Google Scholar]
- 72.Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. Hypertension. 2003;41:422. doi: 10.1161/01.HYP.0000053450.19998.11. [DOI] [PubMed] [Google Scholar]
- 73.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd N Engl J Med. 1995;332:1198. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 74.Taylor AW, Bruno RS, Traber MG. Lipids. 2008;43:925. doi: 10.1007/s11745-008-3222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seet RC, Lee CY, Loke WM, Huang SH, Huang H, Looi WF, Chew ES, Quek AM, Lim EC, Halliwell B. Free Radic Biol Med. 2011;12:1787. doi: 10.1016/j.freeradbiomed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 76.Davi G, Alessandrini P, Mezzetti A, Minotti G, Bucciarelli T, Costantini F, Cipollone F, Bon GB, Ciabattoni G, Patrono C. Arterioscler Thromb Vasc Biol. 1997;17:3230. doi: 10.1161/01.atv.17.11.3230. [DOI] [PubMed] [Google Scholar]
- 77.Mezzetti A, Cipollone F, Cuccurullo F. Cardiovasc Res. 2000;47:475. doi: 10.1016/s0008-6363(00)00118-8. [DOI] [PubMed] [Google Scholar]
- 78.Redhage LA, Shintani A, Haas DW, Emeagwali N, Markovic M, Oboho I, Mwenya C, Erdem H, Acosta EP, Morrow JD, Hulgan T. HIV Clin Trials. 2009;10:181. doi: 10.1310/hct1003-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hulgan T, Morrow J, D'Aquila RT, Raffanti S, Morgan M, Rebeiro P, Haas DW. Clin Infect Dis. 2003;37:1711. doi: 10.1086/379776. [DOI] [PubMed] [Google Scholar]
- 80.McComsey GA, Morrow JD. J Acquir Immune Defic Syndr. 2003;34:45. doi: 10.1097/00126334-200309010-00006. [DOI] [PubMed] [Google Scholar]
- 81.Glesby MJ, Hoover DR, Raiszadeh F, Lee I, Shi Q, Milne G, Sanchez SC, Gao W, Kaplan RC, Morrow JD, Anastos K. Antivir Ther. 2009;14:763. doi: 10.3851/1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brussino L, Badiu I, Sciascia S, Bugiani M, Heffler E, Guida G, Malinovschi A, Bucca C, Rolla G. Clin Exp Allergy. 40:1642. doi: 10.1111/j.1365-2222.2010.03604.x. [DOI] [PubMed] [Google Scholar]
- 83.Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Am J Respir Crit Care Med. 1999;160:216. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- 84.Kawikova I, Barnes PJ, Takahashi T, Tadjkarimi S, Yacoub MH, Belvisi MG. Am J Respir Crit Care Med. 1996;153:590. doi: 10.1164/ajrccm.153.2.8564103. [DOI] [PubMed] [Google Scholar]
- 85.Dworski R, Roberts LJ, 2nd, Murray JJ, Morrow JD, Hartert TV, Sheller JR. Clin Exp Allergy. 2001;31:387. doi: 10.1046/j.1365-2222.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- 86.Montine TJ, Markesbery WR, Morrow JD, Roberts LJ., 2nd Ann Neurol. 1998;44:410. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- 87.Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Ann Neurol. 2005;58:730. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- 88.Basu S, Whiteman M, Mattey DL, Halliwell B. Ann Rheum Dis. 2001;60:627. doi: 10.1136/ard.60.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferrante E, Vazzana N, Santilli F, Di Cicco M, Lauriti C, Di Battista L, Ciabattoni G, Di Matteo L, Davi G. Free Radic Biol Med. 49:857. doi: 10.1016/j.freeradbiomed.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 90.Rho YH, Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Raggi P, Milne GL, Stein CM. Arthritis Care Res. 2010;62:1473. doi: 10.1002/acr.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barocas DA, Motley S, Cookson MS, Chang SS, Penson DF, Dai Q, Milne G, Roberts LJ, 2nd, Morrow J, Concepcion RS, Smith JA, Jr, Fowke JH. J Urol. 2011;6:2102. doi: 10.1016/j.juro.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dai Q, Gao YT, Shu XO, Yang G, Milne G, Cai Q, Wen W, Rothman N, Cai H, Li H, Xiang Y, Chow WH, Zheng W. J Clin Oncol. 2009;27:2482. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Belli R, Amerio P, Brunetti L, Orlando G, Toto P, Proietto G, Vacca M, Tulli A. Int J Immunopathol Pharmacol. 2005;18:497. doi: 10.1177/039463200501800309. [DOI] [PubMed] [Google Scholar]
- 94.Owen RW. IARC Sci Publ. 2001;154:101. [PubMed] [Google Scholar]
- 95.Roberts LJ, 2nd, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. Free Radic Biol Med. 2007;43:1388. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, Capani F, Patrono C. Circulation. 1999;99:224. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 97.Levine M, Wang Y, Padayatty SJ, Morrow J. Proc Natl Acad Sci USA. 2001;98:9842. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Block G, Jensen CD, Morrow JD, Holland N, Norkus EP, Milne GL, Hudes M, Dalvi TB, Crawford PB, Fung EB, Schumacher L, Harmatz P. Free Radic Biol Med. 2008;45:377. doi: 10.1016/j.freeradbiomed.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Halliwell B, Lee CY. Antioxid Redox Signal. 2010;13:145. doi: 10.1089/ars.2009.2934. [DOI] [PubMed] [Google Scholar]
- 100.Lee CY, Seet RC, Huang SH, Long LH, Halliwell B. Antioxid Redox Signal. 2009;11:407. doi: 10.1089/ars.2008.2179. [DOI] [PubMed] [Google Scholar]
- 101.Roberts LJ, II, Moore KP, Zackert WE, Oates JA, Morrow JD. J Biol Chem. 1996;271:20617. doi: 10.1074/jbc.271.34.20617. [DOI] [PubMed] [Google Scholar]
- 102.Morales CR, Terry ES, Zackert WE, Montine TJ, Morrow JD. Clin Chim Acta. 2001;314:93. doi: 10.1016/s0009-8981(01)00637-4. [DOI] [PubMed] [Google Scholar]
- 103.Yan Z, Mas E, Mori TA, Croft KD, Barden AE. Anal Biochem. 2010;403:126. doi: 10.1016/j.ab.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 104.Takahashi K, Nammour TM, Fukunaga M, Ebert J, Morrow JD, Roberts LJ, Hoover RL, Badr KF. J Clin Invest. 1992;90:136. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kinsella BT, Mahony DJ, Fitzgerald GA. J Pharm Exp Ther. 1997;281:957. [PubMed] [Google Scholar]
- 106.Khasawneh FT, Huang JS, Mir F, Srinivasan S, Tiruppathi C, Le Breton GC. Biochem Pharmacol. 2008;75:2301. doi: 10.1016/j.bcp.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ting HJ, Khasawneh FT. J Biomed Sci. 2010;17:24. doi: 10.1186/1423-0127-17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marliere S, Cracowski JL, Durand T, Chavanon O, Bessard J, Guy A, Stanke-Labesque F, Rossi JC, Bessard G. Br J Pharmacol. 2002;135:1276. doi: 10.1038/sj.bjp.0704558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hou X, Roberts LJ, 2nd, Gobeil F, Jr, Taber D, Kanai K, Abran D, Brault S, Checchin D, Sennlaub F, Lachapelle P, Varma D, Chemtob S. Free Radic Biol Med. 2004;36:163. doi: 10.1016/j.freeradbiomed.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 110.Ting HJ, Khasawneh FT. J Biomed Sci. 2010;17:24. doi: 10.1186/1423-0127-17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reich EE, Markesbery WR, Roberts LJ, 2nd, Swift LL, Morrow JD, Montine TJ. Am J Pathol. 2001;158:293. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farias SE, Basselin M, Chang L, Heidenreich KA, Rapoport SI, Murphy RC. J Lipid Res. 2008;49:1990. doi: 10.1194/jlr.M800200-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brose SA, Thuen BT, Golovko MY. J Lipid Res. 2011;4:850. doi: 10.1194/jlr.D013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Varma S, Janesko KL, Wisniewski SR, Bayir H, Adelson PD, Thomas NJ, Kochanek PM. J Neurotrauma. 2003;20:781. doi: 10.1089/089771503767870005. [DOI] [PubMed] [Google Scholar]
- 115.Longmire AW, Roberts LJ, Morrow JD. Prostaglandins. 1994;48:247. doi: 10.1016/0090-6980(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 116.Fukunaga M, Takahashi K, Badr KF. Biochem Biophys Res Commun. 1993;195:507. doi: 10.1006/bbrc.1993.2075. [DOI] [PubMed] [Google Scholar]
- 117.Gao L, Zackert WE, Hasford JJ, Danekis ME, Milne GL, Remmert C, Reese J, Yin H, Tai HH, Dey SK, Porter NA, Morrow JD. J Biol Chem. 2003;278:28479. doi: 10.1074/jbc.M303984200. [DOI] [PubMed] [Google Scholar]
- 118.Jahn U, Dinca E. J Org Chem. 2010;75:4480. doi: 10.1021/jo1006569. [DOI] [PubMed] [Google Scholar]
- 119.Chen Y, Morrow JD, Roberts LJ., 2nd J Biol Chem. 1999;274:10863. doi: 10.1074/jbc.274.16.10863. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y, Zackert WE, Roberts LJ, 2nd, Morrow JD. Biochim Biophys Acta. 1999;1436:550. doi: 10.1016/s0005-2760(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 121.Stamatakis K, Perez-Sala D. Ann New York Acad Sci. 2006;1091:548. doi: 10.1196/annals.1378.096. [DOI] [PubMed] [Google Scholar]
- 122.Milne GL, Gao L, Porta A, Zanoni G, Vidari G, Morrow JD. J Biol Chem. 2005;280:25178. doi: 10.1074/jbc.M502891200. [DOI] [PubMed] [Google Scholar]
- 123.Musiek ES, Breeding RS, Milne GL, Zanoni G, Morrow JD, McLaughlin B. J Neurochem. 2006;97:1301. doi: 10.1111/j.1471-4159.2006.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Musiek ES, McLaughlin B, Morrow JD. J Mol Neurosci. 2007;33:80. doi: 10.1007/s12031-007-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Musiek ES, Milne GL, McLaughlin B, Morrow JD. Brain Pathol. 2005;15:149. doi: 10.1111/j.1750-3639.2005.tb00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zeiger SL, Musiek ES, Zanoni G, Vidari G, Morrow JD, Milne GJ, McLaughlin B. Free Radic Biol Med. 2009;47:1422. doi: 10.1016/j.freeradbiomed.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Musiek ES, Gao L, Milne GL, Han W, Everhart MB, Wang D, Backlund MG, DuBois RN, Zanoni G, Vidari G, Blackwell TS, Morrow JD. J Biol Chem. 2005;280:35562. doi: 10.1074/jbc.M504785200. [DOI] [PubMed] [Google Scholar]
- 128.Lappas M, Permezel M, Holdsworth SJ, Zanoni G, Porta A, Rice GE. Free Radic Biol Med. 2007;42:1791. doi: 10.1016/j.freeradbiomed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 129.Menon R, Fortunato SJ, Yu J, Milne GL, Sanchez S, Drobek CO, Lappas M, Taylor RN. Placenta. 2011;32:317. doi: 10.1016/j.placenta.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 130.Fitzpatrick FA, Wynalda MA. J Biol Chem. 1983;258:11713. [PubMed] [Google Scholar]
- 131.Straus DS, Glass CK. Med Res Rev. 2001;21:185. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 132.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. Cell. 1995;83:813. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 133.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Nat Med. 1999;5:698. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 134.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Nature. 2000;403:103. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 135.Perez-Sala D, Cernuda-Morollon E, Canada FJ. J Biol Chem. 2003;278:51251. doi: 10.1074/jbc.M309409200. [DOI] [PubMed] [Google Scholar]
- 136.Kondo M, Shibata T, Kumagai T, Osawa T, Shibata N, Kobayashi M, Sasaki S, Iwata M, Noguchi N, Uchida K. Proc Natl Acad Sci USA. 2002;99:7367. doi: 10.1073/pnas.112212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shibata T, Yamada T, Kondo M, Tanahashi N, Tanaka K, Nakamura H, Masutani H, Yodoi J, Uchida K. Biochemistry. 2003;42:13960. doi: 10.1021/bi035215a. [DOI] [PubMed] [Google Scholar]
- 138.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. Chem Res Toxicol. 2005;18:1779. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 139.Itoh K, Tong KI, Yamamoto M. Free Radic Biol Med. 2004;36:1208. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 140.Chen ZH, Yoshida Y, Saito Y, Sekine A, Noguchi N, Niki E. J Biol Chem. 2006;281:14440. doi: 10.1074/jbc.M600260200. [DOI] [PubMed] [Google Scholar]
- 141.Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. Biochem J. 2004;378:373. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vunta H, Davis F, Palempalli UD, Bhat D, Arner RJ, Thompson JT, Peterson DG, Reddy CC, Prabhu KS. J Biol Chem. 2007;282:17964. doi: 10.1074/jbc.M703075200. [DOI] [PubMed] [Google Scholar]
- 143.Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Proc Natl Acad Sci USA. 2007;104:20979. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shibata T, Kondo M, Osawa T, Shibata N, Kobayashi M, Uchida K. J Biol Chem. 2002;277:10459. doi: 10.1074/jbc.M110314200. [DOI] [PubMed] [Google Scholar]
- 145.Hirata Y, Hayashi H, Ito S, Kikawa Y, Ishibashi M, Sudo M, Miyazaki H, Fukushima M, Narumiya S, Hayaishi O. J Biol Chem. 1988;263:16619. [PubMed] [Google Scholar]
- 146.Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. J Clin Invest. 2003;112:945. doi: 10.1172/JCI18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Powell WS. J Clin Invest. 2003;112:828. doi: 10.1172/JCI19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hardy KD, Cox BE, Milne GL, Yin H, Roberts LJ. J Lipid Res. 2011;52:113. doi: 10.1194/jlr.M010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Davies SS, Amarnath V, Roberts LJ. Chem Phys Lipids. 2004;128:85. doi: 10.1016/j.chemphyslip.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 150.Brame CJ, Salomon RG, Morrow JD, Roberts LJ., 2nd J Biol Chem. 1999;274:13139. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- 151.Boutaud O, Li J, Chaurand P, Brame CJ, Marnett LJ, Roberts LJ, Oates JA. Adv Exp Med Biol. 2001;500:133. doi: 10.1007/978-1-4615-0667-6_16. [DOI] [PubMed] [Google Scholar]
- 152.Boutaud O, Ou JJ, Chaurand P, Caprioli RM, Montine TJ, Oates JA. J Neurochem. 2002;82:1003. doi: 10.1046/j.1471-4159.2002.01064.x. [DOI] [PubMed] [Google Scholar]
- 153.Davies SS, Amarnath V, Montine KS, Bernoud-Hubac N, Boutaud O, Montine TJ, Roberts LJ., 2nd FASEB J. 2002;16:715. doi: 10.1096/fj.01-0696fje. [DOI] [PubMed] [Google Scholar]
- 154.Iyer RS, Ghosh S, Salomon RG. Prostaglandins. 1989;37:471. doi: 10.1016/0090-6980(89)90096-8. [DOI] [PubMed] [Google Scholar]
- 155.Sullivan CB, Matafonova E, Roberts LJ, 2nd, Amarnath V, Davies SS. J Lipid Res. 2009;5:999. doi: 10.1194/jlr.M001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Davies SS, Amarnath V, Brame CJ, Boutaud O, Roberts LJ., 2nd Nat Protoc. 2007;2:2079. doi: 10.1038/nprot.2007.298. [DOI] [PubMed] [Google Scholar]
- 157.Poliakov E, Brennan ML, Macpherson J, Zhang R, Sha W, Narine L, Salomon RG, Hazen SL. FASEB J. 2003;17:2209. doi: 10.1096/fj.03-0086com. [DOI] [PubMed] [Google Scholar]
- 158.Fukuda K, Davies SS, Nakajima T, Ong BH, Kupershmidt S, Fessel J, Amarnath V, Anderson ME, Boyden PA, Viswanathan PC, Roberts LJ, 2nd, Balser JR. Circ Res. 2005;97:1262. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- 159.Davies SS, Brantley EJ, Voziyan PA, Amarnath V, Zagol-Ikapitte I, Boutaud O, Hudson BG, Oates JA, Roberts LJ., 2nd Biochemistry. 2006;45:15756. doi: 10.1021/bi061860g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brame CJ, Boutaud O, Davies SS, Yang T, Oates JA, Roden D, Roberts LJ., 2nd J Biol Chem. 2004;279:13447. doi: 10.1074/jbc.M313349200. [DOI] [PubMed] [Google Scholar]
- 161.Guo L, Chen Z, Cox BE, Amarnath V, Epand RF, Epand RM, Davies SS. J Biol Chem. 2011;20:18170. doi: 10.1074/jbc.M110.213470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nakajima T, Davies SS, Matafonova E, Potet F, Amarnath V, Tallman KA, Serwa RA, Porter NA, Balser JR, Kupershmidt S, Roberts LJ., 3rd J Mol Cell Cardiol. 2010;48:352. doi: 10.1016/j.yjmcc.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Amarnath V, Valentine WM, Amarnath K, Eng MA, Graham DG. Chem Res Toxicol. 1994;7:56. doi: 10.1021/tx00037a008. [DOI] [PubMed] [Google Scholar]
- 164.Boutaud O, Montine TJ, Chang L, Klein WL, Oates JA. J Neurochem. 2006;96:917. doi: 10.1111/j.1471-4159.2005.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sullivan CB, Matafonova E, Roberts LJ, 2nd, Amarnath V, Davies SS. J Lipid Res. 51:999. doi: 10.1194/jlr.M001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bernoud-Hubac N, Fay LB, Armarnath V, Guichardant M, Bacot S, Davies SS, Roberts LJ, II, Lagarde M. Free Radic Biol Med. 2004;37:1604. doi: 10.1016/j.freeradbiomed.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 167.Carrier EJ, Amarnath; Oates JA, Boutaud O. Biochemistry. 2009;48:10775. doi: 10.1021/bi9015132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roychowdhury S, McMullen MR, Pritchard MT, Li W, Salomon RG, Nagy LE. Free Radic Biol Med. 2009;47:1526. doi: 10.1016/j.freeradbiomed.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Amarnath V, Amarnath K, Amarnath K, Davies S, Roberts LJ., II Chem Res Tox. 2004;17:410. doi: 10.1021/tx0300535. [DOI] [PubMed] [Google Scholar]
- 170.Davies SS, Brantley EJ, Voziyan PA, Amarnath V, Zagol-Ikapitte I, Boutaud O, Hudson BG, Oates JA, Ii L. J Biochemistry. 2006;45:15756. doi: 10.1021/bi061860g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zagol-Ikapitte I, Matafonova E, Amarnath V, Bodine CL, Boutaud O, Tirona RG, Oates JA, Roberts LJ, II, Davies SS. Pharmaceutics. 2010;2:18. doi: 10.3390/pharmaceutics2010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Davies SS, Bodine C, Matafonova E, Pantazides BG, Bernoud-Hubac N, Harrison FE, Olson SJ, Montine TJ, Amarnath V, Roberts LJ., Ii J Alzheimers Dis. 2011 June 27; doi: 10.3233/JAD-2011-102118. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Davies SS, Talati M, Wang X, Mernaugh RL, Amarnath V, Fessel J, Meyrick BO, Sheller J, Roberts I. L Jackson Free Radic Biol Med. 2004;36:1163. doi: 10.1016/j.freeradbiomed.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 174.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ., Jr Proc Natl Acad Sci USA. 2002;99:16713. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jahn U, Galano JM, Durand T. Angew Chem Int Ed. 2008;47:5894. doi: 10.1002/anie.200705122. [DOI] [PubMed] [Google Scholar]
- 176.Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, Roberts LJ, 2nd, Arduini A, Escobar JJ, Sastre J, Asensi MA. Pediatrics. 2009;124:e439. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 177.Mas E, Barden AE, Corcoran TB, Phillips M, Roberts LJ, Ii, Mori TA. Free Radic Biol Med. 2011;50:1171. doi: 10.1016/j.freeradbiomed.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 178.Billings FTt, Ball SK, Roberts LJ, 2nd, Pretorius M. Free Radic Biol Med. 2011;11:1480. doi: 10.1016/j.freeradbiomed.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Snoeijs MG, Hoogland PR, Boonen B, Coffman TM, Peutz-Kootstra CJ, Buurman WA, van Heurn LW. Free Radic Res. 2011;6:699. doi: 10.3109/10715762.2011.571686. [DOI] [PubMed] [Google Scholar]
- 180.Watson AD, Subbanagounder G, Welsbie DS, Faull KF, Navab M, Jung ME, Fogelman AM, Berliner JA. J Biol Chem. 1999;274:24787. doi: 10.1074/jbc.274.35.24787. [DOI] [PubMed] [Google Scholar]
- 181.Bochkov VN. Thromb Haemost. 2007;97:348. [PubMed] [Google Scholar]
- 182.Berliner JA, Leitinger N, Tsimikas S. J Lipid Res. 2009 50:S207. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Subbanagounder G, Wong JW, Lee H, Faull KF, Miller E, Witztum JL, Berliner JA. J Biol Chem. 2002;277:7271. doi: 10.1074/jbc.M107602200. [DOI] [PubMed] [Google Scholar]
- 184.Berliner JA, Gharavi NM. Free Radic Biol Med. 2008;45:119. doi: 10.1016/j.freeradbiomed.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Berliner JA, Leitinger N, Tsimikas S. JLipid Res. 2009;50:S207. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gargalovic PS, Imura M, Zhang B, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Patel S, Nelson SF, Horvath S, Berliner JA, Kirchgessner TG, Lusis AJ. Proc Natl Acad Sci USA. 2006;103:12741. doi: 10.1073/pnas.0605457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li R, Mouillesseaux KP, Montoya D, Cruz D, Gharavi N, Dun M, Koroniak L, Berliner JA. Circ Res. 2006;98:642. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 188.Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Arterioscler Thromb Vasc Biol. 2003;23:1384. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- 189.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, Vora DK. Proc Nat Acad Sci USA. 1999;96:12010. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, Kadl A, Binder BR, Leitinger N. J Biol Chem. 2003;278:51006. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 191.Gargalovic PS, Imura M, Zhang B, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Patel S, Nelson SF, Horvath S, Berliner JA, Kirchgessner TG, Lusis AJ. Proc Natl Acad Sci USA. 2006;103:12741. doi: 10.1073/pnas.0605457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yin H, Cox BE, Liu W, Porter NA, Morrow JD, Milne GL. J Mass Spectrom. 2009;44:672. doi: 10.1002/jms.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ravandi A, Babaei S, Leung R, Monge JC, Hoppe G, Hoff H, Kamido H, Kuksis A. Lipids. 2004;39:97. doi: 10.1007/s11745-004-1207-5. [DOI] [PubMed] [Google Scholar]
- 194.Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, Watson AD, Faull KF, Fogelman AM, Berliner JA. Arterioscler Thromb Vasc Biol. 2000;20:2248. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 195.Imbusch R, Mueller MJ. Free Radic Biol Med. 2000;28:720. doi: 10.1016/s0891-5849(00)00154-4. [DOI] [PubMed] [Google Scholar]
- 196.Gao L, Yin H, Milne GL, Porter NA, Morrow JD. J Biol Chem. 2006;281:14092. doi: 10.1074/jbc.M601035200. [DOI] [PubMed] [Google Scholar]
- 197.Roberts LJ, 2nd, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. J Biol Chem. 1998;273:13605. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 198.Durand T, Bultel-Ponce V, Guy A, Berger S, Mueller MJ, Galano JM. Lipids. 2009;44:875. doi: 10.1007/s11745-009-3351-1. [DOI] [PubMed] [Google Scholar]
- 199.Mueller MJ. Prostaglandins Leukot Essent Fatty Acids. 2010;2-3:71. doi: 10.1016/j.plefa.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 200.Kim HY, Akbar M, Kim YS. Prostaglandins Leukot Essent Fatty Acids. 2010:165. doi: 10.1016/j.plefa.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Song JH, Fujimoto K, Miyazawa TJ. Nutr. 2000;130:3028. doi: 10.1093/jn/130.12.3028. [DOI] [PubMed] [Google Scholar]
- 202.Yin H, Musiek ES, Gao L, Porter NA, Morrow JD. J Biol Chem. 2005;280:26600. doi: 10.1074/jbc.M503088200. [DOI] [PubMed] [Google Scholar]
- 203.Musiek ES, Cha JK, Yin H, Zackert WE, Terry ES, Porter NA, Montine TJ, Morrow JD. J Chromatogr A. 2004;799:95. doi: 10.1016/j.jchromb.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 204.Montine TJ, Quinn JF, Montine KS, Kaye JA, Breitner JC. J Alzheimers Dis. 2005;8:359. doi: 10.3233/jad-2005-8405. [DOI] [PubMed] [Google Scholar]
- 205.Brown JE, Zeiger SL, Hettinger JC, Brooks JD, Holt B, Morrow JD, Musiek ES, Milne G, McLaughlin B. J Neurosci. 2010;30:5242. doi: 10.1523/JNEUROSCI.6366-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bernoud-Hubac N, Davies SS, Boutaud O, Montine TJ, Roberts LJ., II J Biol Chem. 2001;276:30964. doi: 10.1074/jbc.M103768200. [DOI] [PubMed] [Google Scholar]
- 207.Bernoud-Hubac N, Roberts LJ., 2nd Biochemistry. 2002;41:11466. doi: 10.1021/bi0257383. [DOI] [PubMed] [Google Scholar]
- 208.Bernoud-Hubac N, Davies SS, Boutaud O, Montine TJ, Roberts LJ., 2nd J Biol Chem. 2001;276:30964. doi: 10.1074/jbc.M103768200. [DOI] [PubMed] [Google Scholar]
- 209.Taber DF, Hoerrner RS, Herr RJ, Gleave DM, Kanai K, Pina R, Jiang Q, Xu M. Chem Phys Lipids. 2004;128:57. doi: 10.1016/j.chemphyslip.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 210.Durand T, Guy A, Henry O, Roland A, Bernad S, Fangour SE, Vidal JP, Rossi JC. Chem Phys Lipids. 2004;128:15. doi: 10.1016/j.chemphyslip.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 211.Zanoni G, Brunoldi EM, Porta A, Vidari G. J Org Chem. 2007;72:9698. doi: 10.1021/jo701719f. [DOI] [PubMed] [Google Scholar]
- 212.Musiek ES, Brooks JD, Joo M, Brunoldi E, Porta A, Zanoni G, Vidari G, Blackwell TS, Montine TJ, Milne GL, McLaughlin B, Morrow JD. J Biol Chem. 2008;283:19927. doi: 10.1074/jbc.M803625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.VanRollins M, Woltjer RL, Yin H, Morrow JD, Montine TJ. J Lipid Res. 2008;49:995. doi: 10.1194/jlr.M700503-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bang HO, Dyerberg J, Sinclair HM. Am J Clin Nutr. 1980;33:2657. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 215.Gillum RF, Mussolino M, Madans JH. J Clin Epidemiol. 2000;53:237. doi: 10.1016/s0895-4356(99)00149-3. [DOI] [PubMed] [Google Scholar]
- 216.Kromhout D, Bosschieter EB, de Lezenne Coulander CN. Engl J Med. 1985;312:1205. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 217.Kromhout D, Feskens EJ, Bowles CH. Int J Epidemiol. 1995;24:340. doi: 10.1093/ije/24.2.340. [DOI] [PubMed] [Google Scholar]
- 218.Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RBN. Engl J Med. 1997;336:1046. doi: 10.1056/NEJM199704103361502. [DOI] [PubMed] [Google Scholar]
- 219.Daviglus ML, Stamler J, Greenland P, Dyer AR, Liu K. Eur Heart J. 1997;18:1841. doi: 10.1093/oxfordjournals.eurheartj.a015186. [DOI] [PubMed] [Google Scholar]
- 220.Albert CM, Hennekens CH, O'Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE. J Am Med Assoc. 1998;279:23. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 221.Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE. J Am Med Assoc. 2002;287:1815. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 222.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. Am J Clin Nutr. 2003;77:319. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 223.GISSI Lancet. 1999;354:447. [Google Scholar]
- 224.Yokoyama Y, Saito M, Saito T, Yuguchi T, Sawataishi M, Sakamoto T, Tazawa K, Tsukada K. Hum Cell. 2000;13:23. [PubMed] [Google Scholar]
- 225.Yokoyama M, Origasa H. Am Heart J. 2003;146:613. doi: 10.1016/S0002-8703(03)00367-3. [DOI] [PubMed] [Google Scholar]
- 226.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Lancet. 2007;369:1090. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 227.Eritsland J, Arnesen H, Gronseth K, Fjeld NB, Abdelnoor M. Am J Cardiol. 1996;77:31. doi: 10.1016/s0002-9149(97)89130-8. [DOI] [PubMed] [Google Scholar]
- 228.von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. Ann Intern Med. 1999;130:554. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- 229.Serhan CN, Arita M, Hong S, Gotlinger K. Lipids. 2004;39:1125. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 230.Serhan CN. J Periodontol. 2008;79:1520. doi: 10.1902/jop.2008.080231. [DOI] [PubMed] [Google Scholar]
- 231.Gao L, Yin H, Milne GL, Porter NA, Morrow JD. J Biol Chem. 2006;281:14092. doi: 10.1074/jbc.M601035200. [DOI] [PubMed] [Google Scholar]
- 232.Pratico D, Smyth EM, Violi F, FitzGerald GA. J Biol Chem. 1996;271:14916. doi: 10.1074/jbc.271.25.14916. [DOI] [PubMed] [Google Scholar]
- 233.Song WL, Paschos G, Fries S, Reilly MP, Yu Y, Rokach J, Chang CT, Patel P, Lawson JA, FitzGerald GA. J Biol Chem. 2009;284:23636. doi: 10.1074/jbc.M109.024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kulkarni PS, Srinivasan BD. Invest Ophthalmol Vis Sci. 1985;26:1178. [PubMed] [Google Scholar]
- 235.Brooks JD, Milne GL, Yin H, Sanchez SC, Porter NA, Morrow JD. J Biol Chem. 2008;283:12043. doi: 10.1074/jbc.M800122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gao L, Wang J, Sekhar KR, Yin H, Yared NF, Schneider SN, Sasi S, Dalton TP, Anderson ME, Chan JY, Morrow JD, Freeman ML. J Biol Chem. 2007;282:2529. doi: 10.1074/jbc.M607622200. [DOI] [PubMed] [Google Scholar]


