Highlights
► Proteomic analysis of senescent secretome reveals upregulation of IGFBP-6 in fibroblasts. ► IGFBP-6 knockdown induces premature senescence in young fibroblasts. ► IGFBP-6 lentiviral overexpression delays replicative senescence in fibroblasts.
Abbreviations: IGF, insulin-like growth factor; IGFBP-6, insulin-like growth factor binding protein-6; LC-MS/MS, liquid chromatography tandem mass spectrometry; SASP, senescence-associated secretory phenotype
Keywords: IGFBP-6, Senescence, Secretome, Proteomics, Fibroblast
Abstract
Cellular senescence can be induced by a variety of mechanisms, and recent data suggest a key role for cytokine networks to maintain the senescent state. Here, we have used a proteomic LC-MS/MS approach to identify new extracellular regulators of senescence in human fibroblasts. We identified 26 extracellular proteins with significantly different abundance in conditioned media from young and senescent fibroblasts. Among these was insulin-like growth factor binding protein-6 (IGFBP-6), which was chosen for further analysis. When IGFBP-6 gene expression was downregulated, cell proliferation was inhibited and apoptotic cell death was increased. Furthermore, downregulation of IGFBP-6 led to premature entry into cellular senescence. Since IGFBP-6 overexpression increased cellular lifespan, the data suggest that IGFBP-6, in contrast to other IGF binding proteins, is a negative regulator of cellular senescence in human fibroblasts.
1. Introduction
During the process of human aging, cellular degeneration and a partial loss of physiological function occurs in various tissues. There is increasing evidence, that age-associated changes in tissue functions can be triggered by the appearance of senescent cells in various tissues, e.g. vascular tissue of elderly donors (Vasile et al., 2001). Cellular senescence is believed to be an important mechanism for preventing the cells from becoming malignant. However, senescent cells can also have detrimental effects on their microenvironment and neighboring cells due to the deregulation of their metabolism and changed composition of secreted proteins (for review see Campisi and d’Adda di Fagagna, 2007; Chen and Goligorsky, 2006). The altered secretome of senescent cells has been previously referred to as the senescence-associated secretory phenotype (SASP; Coppe et al., 2008) and the senescence-messaging secretome (SMS; Kuilman and Peeper, 2009), respectively. The secretome of senescent cells is complex and includes factors that are involved in senescence-associated proliferation arrest (IGF-I; Ferber et al., 1993 and IGFBPs; Wajapeyee et al., 2008), immune regulation (cytokines and chemokines; Maier et al., 1990), and extracellular matrix remodeling (MMP-1 and -3; Krizhanovsky et al., 2008). Fibroblasts play a central role in tissue processes, especially in the skin, not only because of their involvement in the reorganization of the extracellular matrix but also due to their role in the communication between different cell types, mediated mainly through the secretion of proteins with extracellular function, such as cytokines. Senescent fibroblasts have been shown to produce less collagen and to secrete increasing amounts of matrix-degrading enzymes during aging in vitro (Campisi, 1998; Toussaint et al., 2002). This is in agreement with in vivo findings, showing that the skin of aged individuals has a decreased content of collagen, leading to changes in skin morphology. In studies with skin biopsies from humans and non-human primates, an age-related increase of senescent cells was observed in vivo (Dimri et al., 1995; Herbig et al., 2006; Ressler et al., 2006), and this may account for the age-related changes in skin morphology. Therefore, identification of the nature of proteins secreted by senescent fibroblasts becomes important for a better understanding of aging processes and the development of age-related diseases.
In this communication, we used liquid chromatography tandem mass spectrometry (LC-MS/MS) proteomic technique to analyze the secretome of young and senescent human diploid fibroblasts (HDFs). We identified 26 extracellular proteins with differential abundance in supernatants of young and senescent cells. Among these, insulin-like growth factor binding protein-6 (IGFBP-6) was chosen for further analysis.
IGFBP-6 belongs to the family of six high affinity IGF binding proteins (IGFBP-1 to -6), which play an important role in the distribution and modulation of biological effects of IGFs (Firth and Baxter, 2002; Jones and Clemmons, 1995). IGFBP-6 is a relatively new member of the IGFBP family, with a unique about 50-fold preferential binding affinity for IGF-II relative to IGF-I (Bach, 1999; Roghani et al., 1989, 1991). This feature makes it a very potent inhibitor of IGF-II actions, which is of particular interest especially for growth inhibition of IGF-II-dependent tumors, such as neuroblastomas (Grellier et al., 1998), rhabdomyosarcomas (Gallicchio et al., 2001) and colon cancer (Kim et al., 2002). IGFBP-6 is expressed predominantly in quiescent, non-dividing cells (Ewton and Florini, 1995) and during differentiation of diverse cell types, such as neuroblastoma-derived cells or prostate cells (Chambery et al., 1998; Lipinski et al., 2005). IGF-independent effects of IGFBP-6 were also reported, including modulation of cell migration (Fu et al., 2007, 2010) and induction of apoptosis (Iosef et al., 2008). In addition to IGFBP-3 and -5, which both possess functional nuclear localization signals (NLS; Schedlich et al., 2000), NLS was also identified in the C-terminal domain of IGFBP-6. It was shown, that IGFBP-6 can be actively imported into the nuclei of rhabdomyosarcoma and HEK-293 cells, and nuclear translocation seems to be required for its IGF-independent apoptotic effects (Iosef et al., 2008). Many studies show regulation of IGFBP-6 predominantly in conditions consistent with its growth inhibitory and proapoptotic effects (for review see Bach, 2005). However, there are studies indicating that in some situations, IGFBP-6 is associated with proproliferative (Schmid et al., 1999) and/or protective effects (Beilharz et al., 1998); however, these IGFBP-6 actions are not clearly understood.
Insulin/IGF signaling plays a major role in determining the rate of aging in many species (Bartke, 2005; Rincon et al., 2005), although the precise role of IGFBPs in this process remains to be elucidated. In our previous work, we studied the role of IGFBP-3 in the senescence response of endothelial cells (Hampel et al., 2006; Muck et al., 2008), indicating that IGFBP-3 acts as an antiproliferative and premature senescence-inducing protein. Accumulation of IGFBP-3 in conditioned medium of senescent human fibroblasts was also reported (Goldstein et al., 1991; Moerman et al., 1993), where it contributes to the growth arrest phenotype of these cells (Grigoriev et al., 1995; Hampel et al., 2004, 2005). The role of IGFBP-6 in cellular senescence was not studied yet. However, increased levels of IGFBP-6 protein were detected in conditioned medium of human fibroblasts made prematurely senescent by exposure to H2O2 (Xie et al., 2005). In the same work, upregulation of IGFBP-6 protein was also observed in the plasma of aging mice and of young mice treated with doxorubicin, which leads to a premature senescence phenotype. In this communication, we focused on the role of IGFBP-6 in cellular senescence in HDFs. We depleted and/or overexpressed IGFBP-6 in primary human fibroblasts using a lentiviral approach. Our data suggest that IGFBP-6 depletion has detrimental effects on HDFs and leads to decreased proliferation, increased cell death and early occurrence of senescence. In contrast, IGFBP-6 overexpression had protective effects on HDFs and markedly delayed the onset of senescence.
2. Materials and methods
2.1. Chemicals
Urea, thiourea, tris, SDS, glycine, acrylamide, bis-acrylamide, TEMED, bromophenole blue, CHAPs, dithiothreitol (DTT) and iodoacetamide (IAA) were purchased from Bio-Rad. Sequencing grade trypsin was purchased from Promega. All other chemicals were purchased from Sigma unless indicated otherwise.
2.2. Cell culture
Two different pools of human primary diploid fibroblasts (HDFs) were obtained from ATCC (American Type Culture Collection). Each pool consisted of HDFs isolated from several different newborn donors. Cells were cultivated in Dulbecco's modified Eagle's medium (DMEM; Sigma), supplemented with 10% heat-inactivated fetal calf serum (FCS; Biochrom), 4 mM l-glutamine and 100 U/ml penicillin with 0.1 mg/ml streptomycin (Gibco Invitrogen). HDFs were serially passaged until they reached replicative senescence. Population doublings (PDL) were estimated using the following equation: n = (log10 F − log10 I)/0.301 (with n = population doublings, F = number of cells at the end of one passage, I = number of cells that were seeded at the beginning of one passage). After roughly 45 population doublings, the cells reached growth arrest. The maximal number of population doublings achieved by untreated HDF was set 100%, and used to calculate the percentage of lifespan completed for each culture. In particular, this procedure was used to determine the percentage of elapsed lifespan at the time when cells were transduced with vectors for IGFBP-6 overexpression and knockdown, respectively (see text). The senescent status was verified by in situ staining for senescence associated-β-galactosidase as described (Dimri et al., 1995). For production of lentiviral particles, HEK-293FT cells (human embryonic kidney 293 cells expressing the large T-antigen of SV40; Invitrogen) were maintained in DMEM (10% not-heat-inactivated FCS, 4 mM l-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin). U-2OS human osteosarcoma cells used for estimation of virus particle titer and the knockdown efficiency of individual IGFBP-6 shRNAs, were obtained from ATCC (American Type Culture Collection) and maintained in DMEM (10% heat-inactivated FCS, 4 mM l-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin). Cells were grown in an atmosphere of 5% CO2 at 37 °C and were subcultured by trypsinization with 0.05% trypsin–EDTA (Gibco Invitrogen) every 3–4 days.
2.3. Production of cellular supernatants
Young (18% of lifespan completed) and replicatively senescent (93% of lifespan completed) HDFs from pool 1 were seeded on 145 cm2 cell culture dishes in 10% FCS DMEM at the density of 2.5 × 106. After 24 h cells were washed twice with PBS and incubated with serum-free DMEM for 5 h to get rid of the residual FCS. Then the medium was exchanged and fresh serum-free DMEM was added to the cells. After 72 h the supernatants were harvested and filtered with a 0.45 μm Steritop Filter (Millipore). Supernatants were further concentrated in a Stericup filtration column with cut-off of 5 kDa (Millipore) and proteins were precipitated by a methanol/chloroform precipitation (Wessel and Flugge, 1984). Protein pellets were freeze-dried and stored at −80 °C. The whole procedure was repeated 3 times, in order to produce 3 independent technical replicates for both young and senescent HDFs.
2.4. Protein sample preparation
The freeze-dried samples were solubilized in cold lysis buffer (8 M urea, 4% (v/v) CHAPS, 40 mM Tris–base), then centrifuged under 4 °C, 15,000 × g for 60 min to remove insoluble fraction and transferred to a new tube. The protein concentration of each sample was quantified using Bradford kit (Bio-Rad). For each sample, about 100 μg of protein was subjected to in-solution digestion. The samples were reduced for 2.5 h at 37 °C by addition of 1 M dithiothreitol (DTT) to a final concentration of 2 mM DTT, and then alkylated for 45 min at room temperature by addition of 1 M iodoacetamide (IAA) to a final concentration of 10 mM IAA. Samples were precipitated using 1 ml of 50% acetone, 49.9% ethanol, 0.1% acetic acid and incubated at −20 °C for 16 h. The samples were then centrifuged at 15,000 × g, 4 °C for 45 min and the pellets were collected. The pellets were further washed twice with pure acetone and pure ethanol separately and repeated centrifugation. Finally, the pellets were resuspended in 100 mM NH4HCO3 and digested using 2 μg of modified trypsin (Promega) at 37 °C for 20 h. Before mass spectrometry analysis, the peptide mixtures were ultra-filtered using 10 kDa cut-off centrifugal filter units (Millipore) to remove trypsin and other undigested proteins.
2.5. Liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis
The peptide mixtures from each sample were separated by reverse phase high performance liquid chromatography RP-HPLC followed by tandem mass spectrometry analysis. RP-HPLC was performed on a surveyor LC system (Thermo Finnigan). The C18 column (RP, 180 μm × 150 mm) was obtained from Column Technology Inc. The pump flow was split 1:120 to achieve a column flow rate of 1.5 nL/min. Mobile phase A was 0.1% formic acid in water, and mobile phase B was 0.1% formic acid in acetonitrile. The tryptic peptide mixtures were eluted using a gradient of 2–98% B over 180 min. The LC system was interfaced to a LTQ linear ion trap mass spectrometer (Thermo Finnigan) using electro-spray ionization operated in positive mode. The mass spectrometer was operated in a data-dependent mode to automatically switch between MS and MS/MS acquisition. Following a full ion scan (from m/z 400–2000) the 10 most intense precursor ions with charge states larger than +1 were selected for fragmentation. Former target ions selected for fragmentation were dynamically excluded with the following settings: repeat count: 2; repeat duration: 0.5 min; exclusion duration: 1.5 min. The normalized collision energy was 35.0.
2.6. Data processing
All acquired MS/MS spectra were processed using Bioworks 3.1 (Thermo Finnigan) and searched against the Human International Protein Index protein sequence database (version 3.39, www.ebi.ac.uk/IPI), using the TurboSEQUEST program. All cysteine residues were searched as carboxamidomethycystein (+57.02 Da). Homemade Buildsummary software was used to delete the redundant data as previously reported (Tang et al., 2007). Additionally, to estimate the rate of incorrect identifications (false positives), all the filtered spectra were subjected to database searching against a composite database containing human protein sequences in both the forward (correct) and reverse (incorrect) orientation. Thresholds for Xcorr according to peptide false-discovery rate (FDR) lower than 1% were applied, with a fixed DeltaCn of more than 0.1. Further more proteins with less than 2 spectral counts were excluded and the final protein false discovery rate was lower than 5%.
2.7. Protein quantitation
A newly developed proteomic strategy of label-free quantification by spectral counts was utilized (Aebersold and Mann, 2003; Gramolini et al., 2008). Briefly, the raw peptide counts of each protein identified in each sample were used as a semi quantitative measure for the protein relative abundance. To reduce the systematic bias from the proteomics data, this abundance was then normalized using the “Global” normalization method (Callister et al., 2006). In this normalization method, the total raw spectral counts (T) in each LC-MS/MS run were set to an equal number (Tconstant). For protein (i), the normalized abundance () in the LC-MS/MS run (j) should be computed as the following formula: , where Xij is the original raw spectral counts for the protein. The protein abundance based on normalized spectral counts was then used to perform statistical analysis. Three independent technical replicates for each group were analyzed. Student t-test was used to compare the differences between young (18% of lifespan completed) and senescent (93% of lifespan completed) HDFs. p < 0.05 and fold changes >1.5 were considered statistically significant.
2.8. Lentiviral IGFBP-6 knockdown and overexpression
Production of lentiviral particles was carried out according to the manufacturer's protocol (Addgene). For lentiviral knockdown, lentiviral vector pLKO.1, containing 5 different IGFBP-6-specific shRNAs (small-hairpin RNA) and control shRNA respectively were purchased from Open Biosystems. Two IGFBP-6-shRNAs with the best level of knockdown were chosen for further experiments (shRNA1: 5′-GAGAATCCTAAGGAGAGTAAA-3′; shRNA2: 5′-GCCTGCTGTTGCAGAGGAGAA-3′). For lentiviral overexpression, IGFBP-6 cDNA was cloned into the pLenti6/CMV/V5-DEST Gateway system (Invitrogen). As a control empty vector and/or vector carrying green fluorescence protein (GFP) was used. For packaging of the lentivirus, HEK293FT cells were cultivated in 75 cm2 flasks to 90% confluence and transfected with a mixture of two packaging plasmids: 7.5 μg psPAX2 and 2.5 μg pMD2.G (Addgene) together with 3 μg pLenti6/CMV and/or specific shRNAs pLKO.1 by Lipofectamine 2000 (Invitrogen). Supernatants were harvested 48 h post transfection. Lentiviral particles were tittered on U-2OS cells. Lentiviral infection was carried out in young (21% of lifespan completed) HDFs pool 1 upon reaching 70–80% confluence, using lentiviral particles at multiplicity of infection of 4 in presence of 8 μg/ml hexadimethrine bromide (Sigma) as a transduction enhancer. 72 h post infection, transduced cells were selected by either puromycin (500 ng/ml) in the case of knockdown or blasticidin (10 μg/ml) in the case of overexpression.
2.9. Real time-PCR
Total RNA from HDFs was isolated with RNeasy Mini kit (Qiagen). IGFBP-6 gene expression levels in transduced cells were analyzed at 32% of lifespan completed. In addition, IGFBP-6 overexpressing cells were checked for IGFBP-6 gene expression also at latter passages (90% of lifespan completed, data not shown). 1 μg of RNA was reverse transcribed using Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science) and oligo(dT) primer. Primers for amplification of IGFBP-6 (Acc. No.: NM_002178) and housekeeper beta-2-microglobulin (B2M; Acc. No.: NM_004048) were designed using Primer3 software (IGFBP-6 forward: 5′-AAGGAGAGTAAACCCCAAGCA-3′, IGFBP-6 reverse: 5′-TTTGAGCCCCTCGGTAGAC-3′; B2M forward: 5′-GTGCTCGCGCTACTCTCTCT-3′; B2M reverse: 5′-TCAATGTCGGATGGATGAAA-3′). The cDNA equivalent of 5 ng of RNA was applied to PCR amplification in combination with 15 μl of LightCycler® 480 SYBR Green I Master (Roche Applied Science), a reaction mixture including FastStart Taq DNA Polymerase and SYBR Green I dye for product visualization. Real-time PCR was performed in triplicates for two independent measurements using the LightCycler® 480 Instrument (Roche Applied Science). Cycling conditions were as follows: 95 °C for 8 min (initial denaturation step) followed by 40 cycles of target amplification (95 °C for 15 s, 60 °C for 8 s and 72 °C for 15 s) and final melting (95 °C for 1 min, 60 °C for 30 s, 95 °C continuous with five acquisitions/°C). Crossing points for IGFBP-6 and housekeeper B2M were used for calculation of IGFBP-6 relative gene expression.
2.10. Western blot and ELISA
Cells and cell supernatants from young (18% of lifespan completed) and senescent (93% of lifespan completed) HDFs were harvested. Cells were lysed in lysis buffer containing 50 mM Tris–HCl, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% Na-deoxycholate, 0.2 mM phenylmethylsulfonyl-fluoride, 1 mM NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin (pH 7.5). Cell supernatants were centrifuged at 300 × g and IGFBP-6 levels and assayed by standard Western blot protocol using primary mouse monoclonal anti-human IGFBP-6 antibody (R&D Systems); goat anti-human Serpin E2 antibody (R&D Systems) and rabbit polyclonal anti-human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody (Santa Gruz Biotechnology). IGFBP-6 levels in supernatants were quantified by ELISA (Human IGFBP-6 DuoSet; R&D Systems) according to the manufacturer's protocol. Levels of p21Waf1/Cip1 in IGFBP-6 knockdown (32–42% of lifespan completed) and overexpressing cells (90–95% of lifespan completed) were assessed by Western blot using mouse anti-human p21Waf1/Cip1 antibody (BD Pharmingen). As a loading control, mouse monoclonal antibody for α-tubulin was used (Sigma).
2.11. Cell proliferation assay
Cell proliferation was assayed by the 5-bromo-2-deoxyuridine Labeling and Detection Kit I (Roche Applied Science) as described by the manufacturer. Cells were incubated with BrdU labeling reagent for approximately 60 min. After the staining procedure, the incorporation was visualized by fluorescence microscopy and in addition analyzed by FACS. The number of BrdU-positive cells was expressed as the percentage of total cell number. The measurements in IGFBP-6 knockdown cells were performed at 32–42% of lifespan completed, in IGFBP-6 overexpressing cells at 90–95% of lifespan completed.
2.12. Assessment of apoptotic cell death
For detection of apoptosis, Annexin V staining and propidium iodide (PI) staining, were used. For Annexin V staining, HDFs were detached and incubated with 5 μl of Annexin V-FITC (Pharmingen BD Biosciences) in Annexin V-buffer, containing 10 mM Hepes, 140 mM sodium chloride and 2.5 mM CaCl2 (pH 7.4) for 15 min. Cells were washed with PBS and Annexin V-FITC positive cells were measured using Flow cytometry (FACS). In the case of PI staining, cells were washed in PBS and resuspended in 0.1% propidium iodide (50 mg/ml) with 0.1% Triton X-100. The PI fluorescence of individual nuclei was measured by FACS. The measurements in IGFBP-6 knockdown cells were performed at 32–42% of lifespan completed, in IGFBP-6 overexpressing cells at 90–95% of lifespan completed.
2.13. Staining for senescence associated-β-galactosidase (SA-β-gal)
The senescent status was verified by in situ staining for SA-β-gal as described (Dimri et al., 1995). Briefly, cells were grown on 6-well cell culture dishes, washed three times with PBS and fixed with 2% formaldehyde, 0.2% glutaraldehyde in PBS for 5 min. After another washing step with PBS, the cells were incubated with SA-β-gal staining solution (150 mM NaCl, 2 mM MgCl2, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 40 mM citric acid, 12 mM sodium phosphate, pH 6.0, containing 1 mg/ml 5-bromo-4-chloro-3-indolyl-b-d-galactoside (X-gal)) for 24 h at 37 °C. The reaction was stopped by washing the cells with PBS. The number of SA-β-gal positive cells was calculated as the percentage of total cell number. The measurements in IGFBP-6 knockdown cells were performed at 32–42% of lifespan completed, in IGFBP-6 overexpressing cells at 90–95% of lifespan completed.
2.14. Starvation-mediated cell cycle arrest
Young (21% of lifespan completed) HDFs were seeded on 6-wells in 10% FCS DMEM at the density of 5 × 105. After 24 h cells were washed twice with PBS and incubated with serum-free DMEM for additional 24 h. Afterwards, the medium was exchanged and fresh serum-free DMEM was added to the cells. After 48 h the supernatants were harvested and stored at −80 °C. IGFBP-6 levels in supernatants were quantified by ELISA (Human IGFBP-6 DuoSet; R&D Systems) according to the manufacturer's protocol. As a control for cell cycle arrest, cell proliferation was assayed by the 5-bromo-2-deoxyuridine Labeling and Detection Kit I (Roche Applied Science) as described above.
2.15. ELISA measurements of human serum samples
IGFBP-6 levels in human serum were analyzed by ELISA (Human IGFBP-6 DuoSet; R&D Systems). The measurements were performed in 140 healthy volunteers, divided into 2 groups: the old age group (age: 66–92, n = 93) and the young/middle-age group (age: 25–41, n = 47). Blood draw was approved by the local ethics committee and all participants gave their written informed consent.
2.16. Ingenuity™ pathways analyses
Young (21% of lifespan completed) HDF were infected with IGFBP-6 shRNA1 and shRNA2 and/or SCR as described above. 20 days after lentiviral transduction (32% of lifespan completed) total mRNA was isolated by Trizol™ reagent (Invitrogen). Affymetrix microarray analysis using Human Genome U133 Plus 2.0 arrays was performed by the Microarray Facility Tübingen (www.microarray-facility.com). Results were further analyzed for relevant signaling pathways, using Ingenuity™ Pathways Analysis software (https://analysis.ingenuity.com; Ingenuity Systems, Inc.).
2.17. Statistical analysis
Student t-test was used to compare the differences between individual samples. p values below 0.05 were considered statistically significant. The graphs represent mean values ± standard error of mean (SEM).
3. Results
3.1. Upregulation of IGFBP-6 gene expression in fibroblast senescence
To address senescence-associated changes in the secretome of human diploid fibroblasts (HDFs), supernatants from young (18% of lifespan completed) and senescent (93% of lifespan completed) human foreskin fibroblasts were harvested and subjected to LC-MS/MS analysis. A total of 407 proteins were identified by 6 LC-MS/MS runs, and 37 differentially expressed proteins were selected, for which the fold change was >1.5 and the p-value was lower than 0.05. While the majority of the proteins identified in this analysis are well established as extracellular proteins (26 proteins in total, see Table 1), we also identified 10 differentially regulated proteins, which according to the literature are intracellular (data not shown) and one so far undescribed protein lacking annotations. The most likely explanation for the occurrence of intracellular proteins in cellular supernatants is leakage subsequent to cell death. An alternative possibility is that the proteins, which are primarily annotated as intracellular, may reach the supernatant by processes different from cellular leakage (e.g. ordered secretion), however, this possibility remains to be addressed. One of the proteins identified by differential secretome analysis was IGFBP-6, which was found upregulated in the supernatant of senescent cells (Fig. 1). Upregulation of IGFBP-6 in the supernatant of senescent cells was confirmed by Western blot (Fig. 2A) and ELISA (Fig. 2B), for both HDF pools we tested. IGFBP-6 was readily detected in supernatants, but only traces of IGFBP-6 were found in cellular lysates (Fig. 2A), suggesting that after synthesis IGFBP-6 is rapidly secreted. In order to confirm, that the upregulation of IGFBP-6 is related to cellular senescence, and does not simply reflect cell cycle-related changes, IGFBP-6 levels were analyzed in cells exposed to serum starvation for 72 h, which quantitatively arrested cells in the G(0) phase of the cell cycle but did not show any impact on IGFBP-6 expression relative to control cells (Fig. S1).
Table 1.
Differentially expressed secreted proteins. Quantitative proteomic analysis using LC-MS/MS combined with label free spectral counting approach identified 26 extracellular proteins that are differentially expressed by young (Y1–Y3; 18% of lifespan completed) and senescent (S1–S3; 93% of lifespan completed) fibroblasts (t-test, p < 0.05, fold change >1.5, n = 3). IPI – international protein index; s.c. – spectral counts.
| IPI | Name | Y1 s.c. |
Y2 s.c. |
Y3 s.c. |
S1 s.c. |
S2 s.c. |
S3 s.c. |
S/Y ratio |
t-test p value |
|---|---|---|---|---|---|---|---|---|---|
| IPI00296713 | GRN Isoform 1 of Granulins precursor | 0 | 1 | 0 | 2 | 3 | 5 | 12.489 | 0.0264 |
| IPI00295741 | CTSB Cathepsin B precursor | 4 | 3 | 6 | 11 | 17 | 27 | 5.061 | 0.0324 |
| IPI00008561 | MMP1 Interstitial collagenase precursor | 6 | 8 | 15 | 29 | 41 | 42 | 4.693 | 0.0043 |
| IPI00011229 | CTSD Cathepsin D precursor | 2 | 5 | 3 | 9 | 6 | 8 | 2.784 | 0.0075 |
| IPI00029235 | IGFBP6 Insulin-like growth factor-binding protein 6 precursor | 9 | 10 | 19 | 27 | 26 | 24 | 2.449 | 0.0049 |
| IPI00296099 | THBS1 Thrombospondin-1 precursor | 49 | 45 | 48 | 61 | 102 | 115 | 2.345 | 0.0344 |
| IPI00465439 | ALDOA Fructose-bisphosphate aldolase A | 1 | 1 | 2 | 3 | 2 | 2 | 2.104 | 0.0379 |
| IPI00844360 | COL4A1 Collagen alpha-1(IV) chain precursor | 3 | 6 | 3 | 7 | 7 | 5 | 1.921 | 0.0452 |
| IPI00302679 | LTBP1 latent transforming growth factor beta binding protein 1 isoform LTBP-1S | 3 | 2 | 2 | 4 | 3 | 4 | 1.852 | 0.0193 |
| IPI00013508 | ACTN1 Alpha-actinin-1 | 14 | 15 | 15 | 9 | 8 | 7 | 0.654 | 0.0025 |
| IPI00296537 | FBLN1 Isoform C of Fibulin-1 precursor | 79 | 67 | 83 | 48 | 27 | 30 | 0.545 | 0.0225 |
| IPI00029658 | EFEMP1 Isoform 1 of EGF-containing fibulin-like extracellular matrix protein 1 precursor | 6 | 11 | 10 | 2 | 4 | 3 | 0.407 | 0.0226 |
| IPI00218725 | LAMA2 laminin alpha 2 subunit isoform b precursor | 19 | 17 | 17 | 10 | 5 | 2 | 0.382 | 0.0226 |
| IPI00029568 | PTX3 Pentraxin-related protein PTX3 precursor | 35 | 23 | 46 | 12 | 10 | 11 | 0.378 | 0.0320 |
| IPI00296165 | C1R Complement C1r subcomponent precursor | 115 | 81 | 111 | 26 | 26 | 29 | 0.314 | 0.0066 |
| IPI00329573 | COL12A1 Isoform 1 of Collagen alpha-1(XII) chain precursor | 28 | 34 | 29 | 10 | 8 | 4 | 0.291 | 0.0010 |
| IPI00792115 | CLEC3B Putative uncharacterized protein DKFZp686H17246 | 5 | 10 | 10 | 1 | 2 | 1 | 0.197 | 0.0112 |
| IPI00012119 | DCN Isoform A of Decorin precursor | 17 | 30 | 41 | 2 | 5 | 4 | 0.153 | 0.0176 |
| IPI00783987 | C3 Complement C3 precursor (Fragment) | 6 | 10 | 10 | 0 | 2 | 0 | 0.098 | 0.0045 |
| IPI00013179 | PTGDS Prostaglandin-H2 d-isomerase precursor | 5 | 4 | 4 | 1 | 0 | 0 | 0.089 | 0.0035 |
| IPI00000860 | FMOD Fibromodulin precursor | 2 | 2 | 1 | 0 | 0 | 0 | Only in Y | 0.0097 |
| IPI00003865 | HSPA8 Isoform 1 of Heat shock cognate 71 kDa protein | 2 | 2 | 1 | 0 | 0 | 0 | Only in Y | 0.0097 |
| IPI00005292 | SPOCK1 Testican-1 precursor | 2 | 2 | 4 | 0 | 0 | 0 | Only in Y | 0.0128 |
| IPI00165972 | CFD Complement factor D preproprotein | 8 | 7 | 5 | 0 | 0 | 0 | Only in Y | 0.0035 |
| IPI00444378 | AEBP1 CDNA FLJ45634 fis, clone CHONS2002829, moderately similar to adipocyte enhancer binding protein 1 | 2 | 3 | 2 | 0 | 0 | 0 | Only in Y | 0.0012 |
| IPI00643034 | PLTP Isoform 1 of Phospholipid transfer protein precursor | 1 | 1 | 2 | 0 | 0 | 0 | Only in Y | 0.0128 |
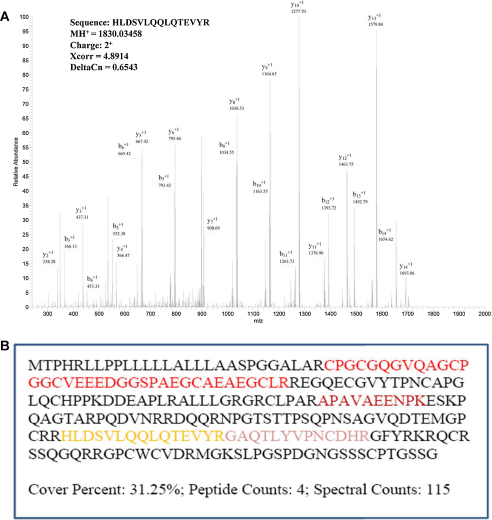
Fig. 1.
Identification of IGFBP-6 protein in supernatants from human diploid fibroblasts. A) MS/MS spectrum of HLDSVLQQLQTEVYR peptide assigned to IGFBP-6 protein. B) Sequence of IGFBP-6 protein showing all 4 peptides identified in quantitative LC-MS/MS analysis with total 31.25% sequence coverage and 115 spectral counts. Different peptides are highlighted with different colors. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
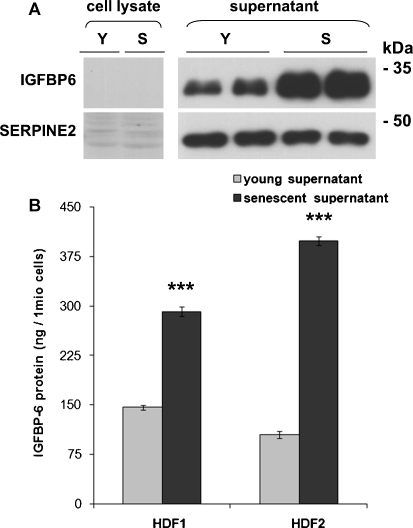
Fig. 2.
Levels of endogenous IGFBP-6 protein in HDFs. A) Representative Western blot of IGFBP-6 in cell lysates and supernatants of young (Y; 18% of lifespan completed) and senescent (S; 93% of lifespan completed) fibroblasts is shown. As a loading control, SERPINE2 with unchanged abundance in young and senescent supernatants was used. B) Concentration of IGFBP-6 in supernatants was measured by IGFBP-6 specific ELISA in two different HDF isolates (HDF1: p = 0.0011; HDF2: p = 0.0002; n = 3).
3.2. Depletion of IGFBP-6 reduces cell proliferation and induces apoptosis and senescence
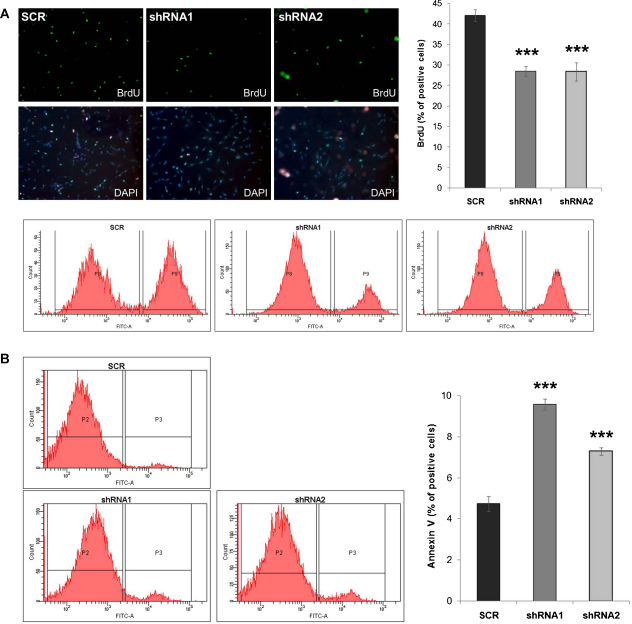
To address the functional significance of IGFBP-6 upregulation in HDF senescence, 5 different commercially available shRNAs against IGFBP-6 were tested by transient transfection in IGFBP-6 overexpressing U-2OS cells (data not shown). The two most potent shRNAs (referred to as shRNA1 and 2 below) were selected and lentiviral particles were produced, suitable for stable downregulation of IGFBP-6 expression in primary cells. Young fibroblasts (21% of lifespan completed) were infected with lentiviruses expressing shRNAs 1 and 2, and the effect on IGFBP-6 mRNA was determined 20 days after infection (32% of lifespan completed). We found that cells infected with both IGFBP-6 targeting shRNAs displayed a significant downregulation of IGFBP-6 mRNA (Fig. 3A) and protein (Fig. 3B), whereas infection with control shRNA had no effect. The analysis of transduced fibroblasts (32–42% of lifespan completed) revealed significant downregulation of cell numbers in cultures infected with viruses carrying shRNA 1 and 2, whereas control shRNA had no effect (Fig. 3C). The observed reduction in cell number could be due to a decreased rate of cell proliferation or an increased rate of cell death, or a combination of both. When cell proliferation was assessed by bromo-deoxyuridine (BrdU) incorporation assays, knockdown of IGFBP-6 led to a significant reduction in the percentage of BrdU-positive cells (Fig. 4A), suggesting that IGFBP-6 is required for the full proliferation capacity of these cells. When cell death was analyzed by Annexin V-staining and propidium iodide staining, we found a significant upregulation in the rate of apoptotic cells upon knockdown of IGFBP-6 (Fig. 4B and C), suggesting that IGFBP-6 might also protect fibroblasts from apoptosis. Together the data suggest that the reduced cell numbers obtained with fibroblasts depleted for IGFBP-6 is due to both a reduction of cell proliferation rate and an increase in cell death. To address a potential impact of IGFBP-6 knockdown on cellular senescence, cells were stained for senescence associated-β-galactosidase (SA-β-gal). This experiment revealed a significant increase in the percentage of SA-β-gal positive cells when IGFBP-6 was depleted by either shRNA1 or shRNA2, whereas control shRNA had no effect (Fig. 4D). The increase in the abundance of SA-β-gal positive cells was accompanied by a strong upregulation of p21Waf1/Cip1 protein (Fig. 4D) in IGFBP-6 depleted cells. These observations suggest that IGFBP-6 acts to prevent early onset of cellular senescence.
Fig. 3.
IGFBP-6 lentiviral knockdown in HDFs. A) Young HDFs (21% of lifespan completed) were infected with two different IGFBP-6 shRNAs (shRNA1 and shRNA2). IGFBP-6 knockdown levels were assessed by real-time PCR at 32% of lifespan completed. Results from two independent infections are shown (I, II) (p < 0.001; n = 6). B) Immunoblots show the IGFBP-6 protein levels in cell lysates and supernatants of knockdown cells compared to control shRNA (SCR). As a loading control, GAPDH and SERPINE2 were used. C) Growth curve of knockdown HDFs is represented as number of population doublings (PDL) after viral transduction.
Fig. 4.
Functional consequences of IGFBP-6 knockdown. A) Cell proliferation of infected HDFs was assessed by a BrdU incorporation assay and subsequent FACS analysis. Representative figures show BrdU positive cells (green) with DAPI counterstained nuclei (blue) along with corresponding FACS profiles. Summary BrdU graph represents the mean value ± SEM (shRNA1: p = 0.000004; shRNA2: p = 0.0005; n = 12). B) Cell death of HDFs with IGFBP-6 knockdown was determined by Annexin V-FACS. Representative FACS profiles are shown, along with summary graph (shRNA1: p = 0.00002; shRNA2: p = 0.0005; n = 9). C) The apoptosis frequency of cells treated as in panel B was independently assessed by propidium iodide staining (shRNA1: p = 0.05; shRNA2: p = 0.04; n = 4). D) Changes in the senescence status were monitored by senescence associated β-galactosidase staining (SA-β-gal: shRNA1: 0.000009; shRNA2: 0.000005; n = 8). Representative Western blot shows the levels of p21Waf1/Cip1 protein. As a control α-tubulin (α-Tub) was used. All measurements were performed at 32-42% of lifespan completed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.3. IGFBP-6 overexpression prevents apoptosis and delays senescence of human fibroblasts
The data obtained so far clearly document that IGFBP-6 is required to limit apoptotic cell death and to ensure the full proliferation potential of diploid human fibroblasts, suggesting that IGFBP-6 functions to delay cellular senescence in this cell type. To address this possibility further, we constructed lentiviral vectors for IGFBP-6 overexpression and infected young (21% of lifespan completed) HDFs with lentiviruses carrying this vector. As a control, lentiviruses with empty vector and GFP expressing vector were used. A few days after infection (32% of lifespan completed), a significant upregulation of IGFBP-6 mRNA (Fig. 5A) and protein (Fig. 5B) was observed. When infected cells were further passaged, the control cells started to reach senescence-associated growth arrest about 90 days after infection, whereas the IGFBP-6 expressing cells continued to proliferate. At the end of the lifespan, overexpression of IGFBP-6 enabled the cells to perform 4–5 additional population doublings (Fig. 5C). This was accompanied by a significant increase in the cell proliferation rate as measured by BrdU incorporation (Fig. 5D) and a significant decrease in apoptotic cell death determined by Annexin V-staining (Fig. 5E) and propidium iodide staining (Fig. 5F) at 90–95% of lifespan completed. 120 days after infection (ca. 95% of lifespan completed), nearly all the cells in the controls stained SA-β-gal positive, whereas roughly 50% of the IGFBP-6 expressing cells stained still SA-β-gal negative (Fig. 5G) and displayed reduced p21Waf1/Cip1 protein expression relative to controls (Fig. 5G). Together these data fully support the conclusion from the previous experiments, namely that IGFBP-6 can delay entry into the senescent phenotype in human diploid fibroblasts.
Fig. 5.
IGFBP-6 lentiviral overexpression in HDFs. A) Young HDFs (21% of lifespan completed) were infected with IGFBP-6 overexpressing lentiviruses. Levels of IGFBP-6 mRNA were quantified by real-time PCR at 32% of lifespan completed. As a control, cells were infected either with lentiviruses containing empty vector (Mock) and/or overexpressing green fluorescent protein (GFP). Results from two independent infections are shown (IGFBP6_1: p = 0.00004; IGFBP6_2: p = 0.0007; n = 6). B) Immunoblots show the IGFBP-6 protein levels in cell lysates and supernatants of HDFs with IGFBP-6 overexpression compared to Mock and GFP control cells. As a loading control, GAPDH and SERPINE2 were used. C) Growth curve of HDF with overexpression of IGFBP-6, Mock and/or GFP is represented as a number of population doublings (PDL) upon viral transduction. Data are shown in duplicates. D) Cell proliferation was estimated by BrdU incorporation assay. Representative figures show BrdU positive cells (green) with DAPI counterstained nuclei (blue) (p = 0.02; n = 8). E) Cell apoptosis was measured by Annexin V-FACS (p = 0.002; n = 4). F) The frequency of apoptosis of cells treated as in panel E was independently assessed by PI-FACS (p = 0.00045; n = 4). G) Changes in the percentage of senescent cells were monitored by SA-β-gal staining (p = 0.00005; n = 8). Representative Western blot shows the levels of p21Waf1/Cip1 protein. As a control α-tubulin (α-Tub) was used. All functional assays were performed at 90–95% of lifespan completed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.4. Gene expression changes induced by IGFBP-6 depletion
Next we addressed potential mechanisms, underlying the actions of IGFBP-6. RNA was prepared from IGFBP-6 knockdown cells and analyzed for changes in the gene expression profile, using Affymetrix™ genome-wide mRNA profiling technology. The gene expression pattern was further analyzed for relevant signaling pathways, using Ingenuity™ Pathways Analysis software and led to identification of NFkB pathway as potential functional target for IGFBP-6 (Fig. S2). Thus, we observed significant downregulation of NFkB-regulated genes coding for cytokines such as CCL2, CXCL1, CXCL5, and IL-8 in IGFBP-6 depleted cells (Fig. S2). These findings imply potential role for IGFBP-6 in the regulation of NFkB activity.
3.5. Upregulation of IGFBP-6 levels in serum from elderly donors
To link our observations made in cell culture to aging phenotypes, IGFBP-6 levels were determined in serum of healthy probands in different age groups. We found that IGFBP-6 levels were significantly increased in serum from elderly donors (Fig. 6), suggesting that IGFBP-6 upregulation may be relevant for human aging.
Fig. 6.
IGFBP-6 levels in human serum. IGFBP-6 protein levels were quantified by ELISA in serum samples derived from healthy volunteers in the old age group (age: 66–92, n = 93) and young/middle-age group (age: 25–41, n = 47; p = 0.000009).
4. Discussion
The data obtained in this study suggest that IGFBP-6 is upregulated, when HDFs undergo replicative senescence. This has been discovered by an unbiased proteomic analysis of cellular supernatants and confirmed by Western blot and ELISA. When IGFBP-6 gene expression was downregulated by shRNA-mediated gene silencing, cell proliferation was inhibited and apoptotic cell death was increased. Furthermore, downregulation of IGFBP-6 led to premature entry into cellular senescence. Since IGFBP-6 overexpression led to a slight but significant increase in cellular lifespan, the data suggest that IGFBP-6, in contrast to other IGF-binding proteins, is a negative regulator of cellular senescence in human fibroblasts.
4.1. Role of the IGF/IGFBP axis in cellular senescence
Signaling through the insulin/IGF signal transduction pathway is known to contribute to the modulation of lifespan in all metazoan organisms, where this has been studied (Bartke, 2005; Rincon et al., 2005). IGF-binding proteins are essential modulators of IGF signaling. They do so by regulating the availability of IGF for its receptors, and IGFBP-6 might play a particular role because its affinity for IGF-II is approximately 50-fold higher than for IGF-I. Studies of cellular senescence have revealed an important contribution of IGF-binding proteins to the regulation of cellular lifespan. Thus, it was shown that IGFBP-3 is upregulated in senescent fibroblasts, endothelial cells and other cell types (Goldstein et al., 1991; Moerman et al., 1993) and IGFBP-3 prevents mitogenic signaling through extracellular IGF in senescent fibroblasts (Grigoriev et al., 1995) and thereby induces cellular senescence (Muck et al., 2008; Kim et al., 2007a). IGFBP-5 is also upregulated in cellular models of replicative senescence (Hampel et al., 2006) and was shown to induce senescence in HUVEC (Kim et al., 2007b). IGFBP-5 was also shown to contribute to fibrosis in vivo (Yasuoka et al., 2006). IGFBP-6 was found upregulated in stress-induced cellular senescence (Xie et al., 2005), but its regulation in replicative senescence of human fibroblasts has not been studied so far.
Among the members of the IGFBP gene family, IGFBP-3, -5 and -6 seem to share additional functions besides IGF binding, since these three proteins contain nuclear localization sequences. Accordingly, a nuclear function was suggested for IGFBP-3, which seems to be independent of its capability to sequester IGF. Thus, it was shown that IGFBP-3 can interact physically and functionally with retinoid X receptor (Liu et al., 2000; Schedlich et al., 2004; for recent review, see Yamada and Lee, 2009), leading to changes in gene expression. Recent studies also suggested that IGFBP-3 can inhibit transcriptional activation by NFkB (Williams et al., 2007; Han et al., 2011), a family of structurally related transcription factors that regulate immune and inflammatory responses, development, cell growth, and apoptosis. There is increasing evidence that NFkB belongs to the key regulators of the senescence-associated secretome, and Acosta et al. (2008) have shown NFkB-dependent activation of several cytokines in oncogene-induced senescence. Hence, NFkB may play an active role in at least some forms of cellular senescence.
4.2. Role of IGFBP-6 in regulation of cell survival, proliferation, and senescence
Consistent with the here reported antiapoptotic activity of IGFBP-6 in human fibroblasts, several studies support a specific role for IGFBP-6 for cell survival in the nervous system, as IGF-II is the most abundantly expressed IGF in the adult CNS (Naeve et al., 2000). Robust upregulation of IGFBP-6 mRNA in lesioned motoneurons suggests that this binding protein may be of special relevance for severed neuronal cells (Hammarberg et al., 1998). Beilharz et al. (1998) have shown that IGFBP-6 was significantly upregulated in injured hemisphere following a severe hypoxic-ischemic (HI) injury. Since treatment of an HI brain injury with IGF-II was shown to worsen outcome and block the neuroprotective effects of IGF-I (Guan et al., 1996), it can be hypothesized that IGFBP-6 may potentiate the neuroprotective actions of endogenous IGF-I (Beilharz et al., 1998) by a so far unknown mechanism.
Several previous studies showed that IGFBP-6 is accompanied mainly with antiproliferative phenotype due to its binding of IGF-II and blocking the IGF/insulin signaling (Bach, 2005), and IGFBP-6 was reported as tumor suppressor in nasopharyngeal carcinoma (Kuo et al., 2010). Moreover, IGFBP-6 gene expression is upregulated by agents which lead to the development of prematurely senescent phenotype, such as 5AZA (Kulaeva et al., 2003), doxorubicin (Chang et al., 2002; Xie et al., 2005) and H2O2 (Xie et al., 2005). However, some studies show upregulation of IGFBP-6 in situations accompanied by increased cell proliferation and survival. Thus, a Gli1 binding element was found in the promoter of IGFBP-6 (Yoon et al., 2002) and recently, it was shown that, transcription factor Gli1 maintained cell survival by binding the promoter regions and facilitating transcription of Bcl-2 genes along with IGFBP-6 in a parallel manner in pancreatic cancer cells (Xu et al., 2009). IGFBP-6 expression was also shown to be increased in invasive but not non-invasive mammary carcinoma cell lines (Evtimova et al., 2003). Finally, direct mitogenic and antiapoptotic effects of IGFBP-6 were described in the human osteoblastic osteosarcoma cell line Saos-2/B-10 (Schmid et al., 1999). The studies reported here clearly suggest that IGFBP-6, unlike other members of the IGFBP family, delays cellular senescence in diploid human fibroblasts, and this activity seems to involve regulation of the cdk inhibitor p21Waf1/Cip1. A significant upregulation of p21Waf1/Cip1 protein levels was observed in IGFBP-6 depleted cells, which contrasts with IGFBP-6 overexpressing cells, where the expression of p21Waf1/Cip1 was strongly downregulated. It has been shown, that overexpression of p21Waf1/Cip1 is able to induce a senescence-like growth arrest in some cells (McConnell et al., 1998; Chang et al., 2000), while deletion of p21Waf1/Cip1 can postpone senescent arrest (Medcalf et al., 1996; Brown et al., 1997). It may be interesting to note that in both cases the levels of p21Waf1/Cip1 mRNA were not regulated (Micutkova et al., unpublished results), indicating that regulation of p21Waf1/Cip1 gene expression by IGFBP-6 occurs at the posttranscriptional level, consistent with several previous publications reporting posttranscriptional regulation of p21Waf1/Cip1 under various conditions (Li et al., 1996; Gong et al., 2003).
Circumstantial evidence presented here may also support the NFkB pathway as target for IGFBP-6 action. Using Affymetrix™ genome-wide mRNA profiling technology in combination with RT-qPCR (Laschober et al., 2010), we found a significant downregulation of NFkB-driven genes coding for cytokines such as CCL2, CXCL1, CXCL5, and IL-8 in IGFBP-6 depleted cells (see Fig. S2), suggesting that IGFBP-6 can influence NFkB activity; however, more work will be required to substantiate these findings.
We think that the increased production of IGFBP-6 by senescent cells may reflect an adaptive mechanism by which the cells avoid/delay senescence-associated growth arrest. Along these lines, we found that IGFBP-6 expression level is increased in serum from elderly donors, suggesting that IGFBP-6 upregulation may be relevant for human aging.
Acknowledgements
We acknowledge excellent technical support by Michael Neuhaus and Hans-Peter Viertler. The work was supported by the European Union (Integrated Project PROTEOMAGE). The work in PJD's laboratory was also supported by a grant from the Austrian Science Funds (L342-B05).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.mad.2011.07.005.
Appendix A. Supplementary data
References
- Acosta J.C., O’Loghlen A., Banito A., Guijarro M.V., Augert A., Raguz S., Fumagalli M., Da Costa M., Brown C., Popov N., Takatsu Y., Melamed J., d’Adda di Fagagna F., Bernard D., Hernando E., Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Aebersold R., Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- Bach L.A. Insulin-like growth factor binding protein-6: the “forgotten” binding protein? Horm. Metab. Res. 1999;31:226–234. doi: 10.1055/s-2007-978723. [DOI] [PubMed] [Google Scholar]
- Bach L.A. IGFBP-6 five years on; not so ‘forgotten’? Growth Horm. IGF Res. 2005;15:185–192. doi: 10.1016/j.ghir.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- Beilharz E.J., Russo V.C., Butler G., Baker N.L., Connor B., Sirimanne E.S., Dragunow M., Werther G.A., Gluckman P.D., Williams C.E., Scheepens A. Co-ordinated and cellular specific induction of the components of the IGF/IGFBP axis in the rat brain following hypoxic-ischemic injury. Brain Res. Mol. Brain Res. 1998;59:119–134. doi: 10.1016/s0169-328x(98)00122-3. [DOI] [PubMed] [Google Scholar]
- Brown J.P., Wei W., Sedivy J.M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- Callister S.J., Barry R.C., Adkins J.N., Johnson E.T., Qian W.J., Webb-Robertson B.J., Smith R.D., Lipton M.S. Normalization approaches for removing systematic biases associated with mass spectrometry and label-free proteomics. J. Proteome Res. 2006;5:277–286. doi: 10.1021/pr050300l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. The role of cellular senescence in skin aging. J. Investig. Dermatol. Symp. Proc. 1998;3:1–5. [PubMed] [Google Scholar]
- Campisi J., d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Chambery D., de Galle B., Babajko S. Retinoic acid stimulates IGF binding protein (IGFBP)-6 and depresses IGFBP-2 and IGFBP-4 in SK-N-SH human neuroblastoma cells. J. Endocrinol. 1998;159:227–232. doi: 10.1677/joe.0.1590227. [DOI] [PubMed] [Google Scholar]
- Chang B.D., Watanabe K., Broude E.V., Fang J., Poole J.C., Kalinichenko T.V., Roninson I.B. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4291–4296. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B.D., Swift M.E., Shen M., Fang J., Broude E.V., Roninson I.B. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc. Natl. Acad. Sci. U. S. A. 2002;99:389–394. doi: 10.1073/pnas.012602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Goligorsky M.S. Premature senescence of endothelial cells: Methusaleh's dilemma. Am. J. Physiol.: Heart Circ. Physiol. 2006;290:H1729–H1739. doi: 10.1152/ajpheart.01103.2005. [DOI] [PubMed] [Google Scholar]
- Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O., Smith J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evtimova V., Zeillinger R., Weidle U.H. Identification of genes associated with the invasive status of human mammary carcinoma cell lines by transcriptional profiling. Tumour Biol. 2003;24:189–198. doi: 10.1159/000074429. [DOI] [PubMed] [Google Scholar]
- Ewton D.Z., Florini J.R. IGF binding proteins-4, -5 and -6 may play specialized roles during L6 myoblast proliferation and differentiation. J. Endocrinol. 1995;144:539–553. doi: 10.1677/joe.0.1440539. [DOI] [PubMed] [Google Scholar]
- Ferber A., Chang C., Sell C., Ptasznik A., Cristofalo V.J., Hubbard K., Ozer H.L., Adamo M., Roberts C.T., Jr., LeRoith D., Dumenil G., Baserg R. Failure of senescent human fibroblasts to express the insulin-like growth factor-1 gene. J. Biol. Chem. 1993;268:17883–17888. [PubMed] [Google Scholar]
- Firth S.M., Baxter R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Fu P., Thompson J.A., Bach L.A. Promotion of cancer cell migration: an insulin-like growth factor (IGF)-independent action of IGF-binding protein-6. J. Biol. Chem. 2007;282:22298–22306. doi: 10.1074/jbc.M703066200. [DOI] [PubMed] [Google Scholar]
- Fu P., Liang G.J., Khot S.S., Phan R., Bach L.A. Cross-talk between MAP kinase pathways is involved in IGF-independent, IGFBP-6-induced Rh30 rhabdomyosarcoma cell migration. J. Cell. Physiol. 2010;224:636–643. doi: 10.1002/jcp.22156. [DOI] [PubMed] [Google Scholar]
- Gallicchio M.A., Kneen M., Hall C., Scott A.M., Bach L.A. Overexpression of insulin-like growth factor binding protein-6 inhibits rhabdomyosarcoma growth in vivo. Int. J. Cancer. 2001;94:645–651. doi: 10.1002/ijc.1519. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Moerman E.J., Jones R.A., Baxter R.C. Insulin-like growth factor binding protein 3 accumulates to high levels in culture medium of senescent and quiescent human fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 1991;88:9680–9684. doi: 10.1073/pnas.88.21.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Ammanamanchi S., Ko T.C., Brattain M.G. Transforming growth factor beta 1 increases the stability of p21/WAF1/CIP1 protein and inhibits CDK2 kinase activity in human colon carcinoma FET cells. Cancer Res. 2003;63:3340–3346. [PubMed] [Google Scholar]
- Gramolini A.O., Kislinger T., Alikhani-Koopaei R., Fong V., Thompson N.J., Isserlin R., Sharma P., Oudit G.Y., Trivieri M.G., Fagan A., Kannan A., Higgins D.G., Huedig H., Hess G., Arab S., Seidman J.G., Seidman C.E., Frey B., Perry M., Backx P.H., Liu P.P., MacLennan D.H., Emili A. Comparative proteomics profiling of a phospholamban mutant mouse model of dilated cardiomyopathy reveals progressive intracellular stress responses. Mol. Cell. Proteomics. 2008;7:519–533. doi: 10.1074/mcp.M700245-MCP200. [DOI] [PubMed] [Google Scholar]
- Grellier P., De Galle B., Babajko S. Expression of insulin-like growth factor-binding protein 6 complementary DNA alters neuroblastoma cell growth. Cancer Res. 1998;58:1670–1676. [PubMed] [Google Scholar]
- Grigoriev V.G., Moerman E.J., Goldstein S. Overexpression of insulin-like growth factor binding protein-3 by senescent human fibroblasts: attenuation of the mitogenic response to IGF-I. Exp. Cell Res. 1995;219:315–321. doi: 10.1006/excr.1995.1234. [DOI] [PubMed] [Google Scholar]
- Guan J., Williams C.E., Skinner S.J., Mallard E.C., Gluckman P.D. The effects of insulin-like growth factor (IGF)-1, IGF-2, and des-IGF-1 on neuronal loss after hypoxic-ischemic brain injury in adult rats: evidence for a role for IGF binding proteins. Endocrinology. 1996;137:893–898. doi: 10.1210/endo.137.3.8603600. [DOI] [PubMed] [Google Scholar]
- Hammarberg H., Risling M., Hokfelt T., Cullheim S., Piehl F. Expression of insulin-like growth factors and corresponding binding proteins (IGFBP 1–6) in rat spinal cord and peripheral nerve after axonal injuries. J. Comp. Neurol. 1998;400:57–72. [PubMed] [Google Scholar]
- Hampel B., Malisan F., Niederegger H., Testi R., Jansen-Durr P. Differential regulation of apoptotic cell death in senescent human cells. Exp. Gerontol. 2004;39:1713–1721. doi: 10.1016/j.exger.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Hampel B., Wagner M., Teis D., Zwerschke W., Huber L., Jansen-Durr P. Apoptosis resistance of senescent human fibroblasts is correlated with the absence of nuclear IGFBP-3. Aging Cell. 2005;4:325–330. doi: 10.1111/j.1474-9726.2005.00180.x. [DOI] [PubMed] [Google Scholar]
- Hampel B., Fortschegger K., Ressler S., Chang M.W., Unterluggauer H., Breitwieser A., Sommergruber W., Fitzky B., Lepperdinger G., Jansen-Durr P., Voglauer R., Grillari J. Increased expression of extracellular proteins as a hallmark of human endothelial cell in vitro senescence. Exp. Gerontol. 2006;41:474–481. doi: 10.1016/j.exger.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Han J., Jogie-Brahim S., Harada A., Oh Y. Insulin-like growth factor-binding protein-3 suppresses tumor growth via activation of caspase-dependent apoptosis and cross-talk with NF-κB signaling. Cancer Lett. 2011 doi: 10.1016/j.canlet.2011.04.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Herbig U., Ferreira M., Condel L., Carey D., Sedivy J.M. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- Iosef C., Gkourasas T., Jia C.Y., Li S.S., Han V.K. A functional nuclear localization signal in insulin-like growth factor binding protein-6 mediates its nuclear import. Endocrinology. 2008;149:1214–1226. doi: 10.1210/en.2007-0959. [DOI] [PubMed] [Google Scholar]
- Jones J.I., Clemmons D.R. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Schaffer B.S., Kang Y.H., Macdonald R.G., Park J.H. Decreased production of insulin-like growth factor-binding protein (IGFBP)-6 by transfection of colon cancer cells with an antisense IGFBP-6 cDNA construct leads to stimulation of cell proliferation. J. Gastroenterol. Hepatol. 2002;17:563–570. doi: 10.1046/j.1440-1746.2002.02703.x. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Kim M.S., Seu Y.B., Chung H.Y., Kim J.H., Kim J.R. Regulation of replicative senescence by insulin-like growth factor-binding protein 3 in human umbilical vein endothelial cells. Aging Cell. 2007;6:535–545. doi: 10.1111/j.1474-9726.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Seu Y.B., Baek S.H., Kim M.J., Kim K.J., Kim J.H., Kim J.R. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol. Biol. Cell. 2007;18:4543–4552. doi: 10.1091/mbc.E07-03-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V., Yon M., Dickins R.A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S.W. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T., Peeper D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- Kulaeva O.I., Draghici S., Tang L., Kraniak J.M., Land S.J., Tainsky M.A. Epigenetic silencing of multiple interferon pathway genes after cellular immortalization. Oncogene. 2003;22:4118–4127. doi: 10.1038/sj.onc.1206594. [DOI] [PubMed] [Google Scholar]
- Kuo Y.S., Tang Y.B., Lu T.Y., Wu H.C., Lin C.T. IGFBP-6 plays a role as an oncosuppressor gene in NPC pathogenesis through regulating EGR-1 expression. J. Pathol. 2010;222:299–309. doi: 10.1002/path.2735. [DOI] [PubMed] [Google Scholar]
- Laschober G.T., Ruli D., Hofer E., Muck C., Carmona-Gutierrez D., Ring J., Hutter E., Ruckenstuhl C., Micutkova L., Brunauer R., Jamnig A., Trimmel D., Herndler-Brandstetter D., Brunner S., Zenzmaier C., Sampson N., Breitenbach M., Frohlich K.U., Grubeck-Loebenstein B., Berger P., Wieser M., Grillari-Voglauer R., Thallinger G.G., Grillari J., Trajanoski Z., Madeo F., Lepperdinger G., Jansen-Durr P. Identification of evolutionarily conserved genetic regulators of cellular aging. Aging Cell. 2010;9:1084–1097. doi: 10.1111/j.1474-9726.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.S., Rishi A.K., Shao Z.M., Dawson M.I., Jong L., Shroot B., Reichert U., Ordonez J., Fontana J.A. Posttranscriptional regulation of p21WAF1/CIP1 expression in human breast carcinoma cells. Cancer Res. 1996;56:5055–5062. [PubMed] [Google Scholar]
- Lipinski R.J., Cook C.H., Barnett D.H., Gipp J.J., Peterson R.E., Bushman W. Sonic hedgehog signaling regulates the expression of insulin-like growth factor binding protein-6 during fetal prostate development. Dev. Dyn. 2005;233:829–836. doi: 10.1002/dvdy.20414. [DOI] [PubMed] [Google Scholar]
- Liu B., Lee H.Y., Weinzimer S.A., Powell D.R., Clifford J.L., Kurie J.M., Cohen P. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosis. J. Biol. Chem. 2000;275:33607–33613. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- Maier J.A., Voulalas P., Roeder D., Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990;249:1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- McConnell B.B., Starborg M., Brookes S., Peters G. Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr. Biol. 1998;8:351–354. doi: 10.1016/s0960-9822(98)70137-x. [DOI] [PubMed] [Google Scholar]
- Medcalf A.S., Klein-Szanto A.J., Cristofalo V.J. Expression of p21 is not required for senescence of human fibroblasts. Cancer Res. 1996;56:4582–4585. [PubMed] [Google Scholar]
- Moerman E.J., Thweatt R., Moerman A.M., Jones R.A., Goldstein S. Insulin-like growth factor binding protein-3 is overexpressed in senescent and quiescent human fibroblasts. Exp. Gerontol. 1993;28:361–370. doi: 10.1016/0531-5565(93)90063-j. [DOI] [PubMed] [Google Scholar]
- Muck C., Micutkova L., Zwerschke W., Jansen-Durr P. Role of insulin-like growth factor binding protein-3 in human umbilical vein endothelial cell senescence. Rejuvenation Res. 2008;11:449–453. doi: 10.1089/rej.2007.0628. [DOI] [PubMed] [Google Scholar]
- Naeve G.S., Vana A.M., Eggold J.R., Verge G., Ling N., Foster A.C. Expression of rat insulin-like growth factor binding protein-6 in the brain, spinal cord, and sensory ganglia. Brain Res. Mol. Brain Res. 2000;75:185–197. doi: 10.1016/s0169-328x(99)00262-4. [DOI] [PubMed] [Google Scholar]
- Ressler S., Bartkova J., Niederegger H., Bartek J., Scharffetter-Kochanek K., Jansen-Durr P., Wlaschek M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5:379–389. doi: 10.1111/j.1474-9726.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- Rincon M., Rudin E., Barzilai N. The insulin/IGF-1 signaling in mammals and its relevance to human longevity. Exp. Gerontol. 2005;40:873–877. doi: 10.1016/j.exger.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Roghani M., Hossenlopp P., Lepage P., Balland A., Binoux M. Isolation from human cerebrospinal fluid of a new insulin-like growth factor-binding protein with a selective affinity for IGF-II. FEBS Lett. 1989;255:253–258. doi: 10.1016/0014-5793(89)81101-9. [DOI] [PubMed] [Google Scholar]
- Roghani M., Segovia B., Whitechurch O., Binoux M. Purification from human cerebrospinal fluid of insulin-like growth factor binding proteins (IGFBPs). Isolation of IGFBP-2, an altered form of IGFBP-3 and a new IGFBP species. Growth Regul. 1991;1:125–130. [PubMed] [Google Scholar]
- Schedlich L.J., Le Page S.L., Firth S.M., Briggs L.J., Jans D.A., Baxter R.C. Nuclear import of insulin-like growth factor-binding protein-3 and -5 is mediated by the importin beta subunit. J. Biol. Chem. 2000;275:23462–23470. doi: 10.1074/jbc.M002208200. [DOI] [PubMed] [Google Scholar]
- Schedlich L.J., O’Han M.K., Leong G.M., Baxter R.C. Insulin-like growth factor binding protein-3 prevents retinoid receptor heterodimerization: implications for retinoic acid-sensitivity in human breast cancer cells. Biochem. Biophys. Res. Commun. 2004;314:83–88. doi: 10.1016/j.bbrc.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Schmid C., Keller C., Gosteli-Peter M., Zapf J. Mitogenic and antiapoptotic effects of insulin-like growth factor binding protein-6 in the human osteoblastic osteosarcoma cell line Saos-2/B-10. Biochem. Biophys. Res. Commun. 1999;263:786–789. doi: 10.1006/bbrc.1999.1451. [DOI] [PubMed] [Google Scholar]
- Tang L.Y., Deng N., Wang L.S., Dai J., Wang Z.L., Jiang X.S., Li S.J., Li L., Sheng Q.H., Wu D.Q., Zeng R. Quantitative phosphoproteome profiling of Wnt3a-mediated signaling network: indicating the involvement of ribonucleoside-diphosphate reductase M2 subunit phosphorylation at residue serine 20 in canonical Wnt signal transduction. Mol. Cell. Proteomics. 2007;6:1952–1967. doi: 10.1074/mcp.M700120-MCP200. [DOI] [PubMed] [Google Scholar]
- Toussaint O., Royer V., Salmon M., Remacle J. Stress-induced premature senescence and tissue ageing. Biochem. Pharmacol. 2002;64:1007–1009. doi: 10.1016/s0006-2952(02)01170-x. [DOI] [PubMed] [Google Scholar]
- Vasile E., Tomita Y., Brown L.F., Kocher O., Dvorak H.F. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J. 2001;15:458–466. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- Wajapeyee N., Serra R.W., Zhu X., Mahalingam M., Green M.R. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flugge U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Williams A.C., Smartt H., AM H.Z., Macfarlane M., Paraskeva C., Collard T.J. Insulin-like growth factor binding protein 3 (IGFBP-3) potentiates TRAIL-induced apoptosis of human colorectal carcinoma cells through inhibition of NF-kappaB. Cell Death Differ. 2007;14:137–145. doi: 10.1038/sj.cdd.4401919. [DOI] [PubMed] [Google Scholar]
- Xie L., Tsaprailis G., Chen Q.M. Proteomic identification of insulin-like growth factor-binding protein-6 induced by sublethal H2O2 stress from human diploid fibroblasts. Mol. Cell. Proteomics. 2005;4:1273–1283. doi: 10.1074/mcp.M500032-MCP200. [DOI] [PubMed] [Google Scholar]
- Xu X.F., Guo C.Y., Liu J., Yang W.J., Xia Y.J., Xu L., Yu Y.C., Wang X.P. Gli1 maintains cell survival by up-regulating IGFBP6 and Bcl-2 through promoter regions in parallel manner in pancreatic cancer cells. J. Carcinog. 2009;8:13. doi: 10.4103/1477-3163.55429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada P.M., Lee K.W. Perspectives in Mammalian IGFBP-3 biology: local vs. systemic action. Am. J. Physiol.: Cell Physiol. 2009;296:C954–C976. doi: 10.1152/ajpcell.00598.2008. [DOI] [PubMed] [Google Scholar]
- Yasuoka H., Jukic D.M., Zhou Z., Choi A.M., Feghali-Bostwick C.A. Insulin-like growth factor binding protein 5 induces skin fibrosis: a novel murine model for dermal fibrosis. Arthritis Rheum. 2006;54:3001–3010. doi: 10.1002/art.22084. [DOI] [PubMed] [Google Scholar]
- Yoon J.W., Kita Y., Frank D.J., Majewski R.R., Konicek B.A., Nobrega M.A., Jacob H., Walterhouse D., Iannaccone P. Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J. Biol. Chem. 2002;277:5548–5555. doi: 10.1074/jbc.M105708200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.