Abstract
The importance of cues signaling reward, threat or danger would suggest that they receive processing privileges in the neural systems underlying perception and attention. Previous research has documented enhanced processing of motivationally salient cues, and has pointed to the amygdala as a candidate neural structure underlying the enhancements. In the current study, we examined whether the amygdala was necessary for this emotional modulation of attention to occur. Patients with unilateral amygdala lesions and matched controls completed an emotional attentional blink task in which emotional distractors impair the perception of subsequent targets. Emotional images proved more distracting across all participant groups, including those with right or left amygdala lesions. These data argue against a central role for the amygdala in mediating all types of attentional capture by emotional stimuli.
Keywords: amygdala, attention, emotion, lesion study
Introduction
Under optimal viewing conditions, an individual has time to examine each item within his or her visual environment, to decide which items are most important for a particular goal, and to plan an appropriate response for achieving that goal. However, such conditions rarely occur; instead, individuals often have to allocate limited attentional resources, within a limited time, to particular stimuli at the expense of others. In these attentionally competitive situations, emotional stimuli tend to enter, capture, or hold attention to a greater degree than do non-emotional stimuli (e.g. Anderson, 2005; Arnell, Killman, & Fijavz, 2007; Barnard, Scott, Taylor, May, & Knightley, 2004; West, Anderson, & Pratt, 2009). This emotional modulation of attention allows for the preferential processing of emotional stimuli, thereby increasing the likelihood that these stimuli will be perceived and elicit an adaptive motor response (Anderson, 2005; Lang, Davis, & Öhman, 2000; Vuilleumier, Armony, Driver, & Dolan, 2001).
Neuroimaging studies have delineated much of the neural architecture underlying the facilitated processing of emotional stimuli. Both conditioned and intrinsically affective stimuli elicit increased activity concurrently in the amygdala and visual regions for faces, scenes and words compared to similar neutral items (Hamann, Ely, Hoffman, & Kilts, 2002; Isenberg et al., 1999; Morris, Buchel, & Dolan, 2001; Morris et al., 1998; Padmala & Pessoa, 2008), consistent with neuroanatomical analyses demonstrating robust amygdalar projections to areas along the visual hierarchy (Amaral, Behniea, & Kelly, 2003). Electrophysiological studies have provided further evidence consistent with an amygdalar modulation of visual cortex based on the specific time-course of perceptual enhancement (Münte et al., 1998; Pizzagalli et al., 2002). Critically, amygdala damage abolishes emotionally enhanced activity in perceptual regions, at least for face stimuli (Morris et al., 1998; Sabatinelli, Bradley, Fitzsimmons, & Lang, 2005; Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004). What is unclear from these studies, however, is whether the amygdala is necessary for the prioritized processing of emotional stimuli to occur, or if this structure is simply activated without playing a critical causative role in this process.
Data from neuropsychological patients suggests that the amygdala plays a crucial role in the emotional modulation of attention. Anderson and Phelps (2001) assessed attentional effects of emotional stimuli in patients with unilateral and bilateral amygdala damage using an “attentional blink” paradigm, where participants typically search for targets within a rapid serial visual presentation (RSVP) and have difficulty perceiving a second target if it appears less than 500 ms after the first target (see Broadbent & Broadbent, 1987; Raymond, Shapiro, & Arnell, 1992). Participants were shown words within RSVP streams, and they were asked to detect two green words embedded among black words. In neurologically healthy individuals, aversive or taboo verbal stimuli broke through the attentional blink: they were perceived even when they appeared soon after the first target. However, patients with damage to the left amygdala did not show this effect, suggesting that the left amygdala plays a critical role in allowing emotional stimuli to preferentially gain access to attention (Anderson & Phelps, 2001). This study is notable in that the effects emerged following unilateral left, but not right amygdala lesions, suggesting the existence of an asymmetrical amygdalar influence on attention, at least in the case of affective word processing.
Preferential processing of emotional stimuli is not always beneficial. Such stimuli can capture attention even when they are irrelevant to current goal-directed behavior, as demonstrated by the emotional blink of attention (EBA) paradigm (Most, Chun, Widders, & Zald, 2005; Most, Smith, Cooter, Levy, & Zald, 2007). In this paradigm a RSVP stream of landscape pictures is presented with a single target per trial. If an emotionally arousing picture is introduced into the stream prior to the target, it spontaneously disrupts the subjects’ ability to detect the target. That is, the emotional distractors capture attention, resulting in an attentional blink even though these distractors are irrelevant to the subject’s task goals.
Given the proposed role of the amygdala in modulating attention, it might be predicted that the amygdala would play a causal role in directing the capture of attention in the EBA paradigm. However, recent studies have raised questions about the generalizability of the amygdala’s role in directing attention. Two recent studies indicate that the amygdala is not essential for the preferential detection of threat-related stimuli during visual search (Piech, McHugo, et al., 2010; Tsuchiya, Moradi, Felsen, Yamazaki, & Adolphs, 2009). In addition, Pessoa and Adolphs (2010) recently challenged the established view of the amygdala’s role in evaluating biological significance in general, and suggested that a number of structures and pathways might be responsible for such functions.
Consistent with this multiple pathways hypothesis, a recent fMRI study by Schwabe and colleagues (Schwabe et al., 2011), emphasized two principally different processes involved in emotional modulation of attentional blink. They compared the capability of emotional stimuli to break through an existing attentional blink (‘capturing attention’, as described by Anderson and Phelps (2001)*) to the potential of emotional stimuli to create and prolong a blink after their presentation (similar to the EBA paradigm used here). They found that while amygdala activity correlated with the breaking through effect, prolonged holding of attention by emotional stimuli was associated with activity in cortical regions including the anterior cingulate, insula, and orbitofrontal cortex.
As such, it is unclear whether the amygdala plays a necessary role in all aspects of attentional modulation by emotional stimuli. In order to specifically test the role of the amygdala in the capture of attention by emotional stimuli, we examined the performance of patients with unilateral lesions of the amygdala on an EBA paradigm, similar to that described by Most and colleagues (2005). If the amygdala is critical in mediating the attentional capture observed in the EBA, lesions of the amygdala should substantially reduce this effect. In contrast, if amygdala lesion patients show an EBA, then it would suggest that this type of attentional capture does not depend upon the amygdala, and refinements will be necessary for theories attempting to explain how emotional stimuli gain preferential access to attention.
Methods
Participants
Participants in the study belonged to three groups: participants with lesions to the left amygdala (‘left resection’, n=11), participants with lesions to the right amygdala (‘right resection’, n=15), and a control group of healthy participants matched for age, education, and sex (‘healthy controls’, n=21). Table 1 contains participant demographics. Patients were recruited from the Vanderbilt University Medical Center Epilepsy Surgery Program; these patients had undergone neurosurgery in order to alleviate the symptoms of pharmacologically intractable medial temporal lobe epilepsy. The neurosurgical procedure consisted of either a selective resection of the amygdala and anterior parts of the hippocampus using a transcortical approach (left resection patients n=7; right resection patients n=5), or a temporal lobectomy that included the amygdala, as well as the temporal pole, and anterior temporal cortex (left resection patients n=4; right resection patients n=10). Patients with brain damage outside the focus area, neuropsychiatric conditions other than epilepsy, and with general cognitive impairment (IQ<80) were excluded from the study. Control participants were recruited from the Nashville community, primarily through web listings for paid research volunteers with specific age, sex and education (matched to the surgery patients). The study was approved by the Vanderbilt University Institutional Review Board, and all participants gave written informed consent to take part in the study.
Table 1.
Participant demographics and lesion volumes. The groups do not differ in respect to age (p=.230), the mean length of education (p=.187), the age at epilepsy onset for patients (p=.557), or the time between surgery and testing (p=.146). IQ values differ significantly for the groups (F(2,41)=12.22, p<.0005), with planned comparisons indicating that healthy controls had a higher mean IQ than right resection patients (t(41)=3.1, p=.003), and right resection patients a marginally higher IQ than left resection patients (t(41)=1.9, p=.059). Performance impairment due to emotional distractors did not correlate with the age at epilepsy onset in patients (r=−.154; p=.452 and r=−.017; p=.934 for aversive and erotic distractors). It also did not correlate with time between surgery and testing (r=.108; p=.606 and r=−.098; p=.640 for aversive and erotic distractors).
On the day of testing, the patients were taking the following anticonvulsant medications: levetiracetam (14 patients), lamotrigine (11), oxcarbazepine (7), carbamazepine (6), clonazepam (2), gabapentin (2), pregabalin (2), topiramate (6), zonisamide (1), other (14).
| Group | Sex (m/f) | Age in Years (SD) | Education in Years (SD) | Age at onset of epilepsy | Time since surgery (weeks) | IQ | Left Amygdala Volume | Right Amygdala Volume | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) (mm3) | Range (mm3) | Mean (SD) (mm3) | Range (mm3) | |||||||
| Healthy Controls | (7/14) | 37.9 (10.0) | 14.8 (1.8)* | 114.9 (13.1)* | ||||||
| Right Resection | (2/13) | 40.8 (8.2) | 14.8 (2.3) | 9.2 (7.4) | 106 (65) *** | 102.6 (10.5) | 1580 (195) | 688 | 126 (234) | 657 |
| Left Resection | (5/6) | 34.3 (9.3) | 13.5 (1.8) | 7.4 (7.4) | 65 (68) | 93.2 (8.6)** | 307 (300) | 823 | 1389 (171) | 485 |
available for
n = 20,
n = 9,
n=14
Structural MRI Data Acquisition and Analysis
High-resolution T1-weighted images (TR=8.97 ms; TE=4.6 ms; in-plane resolution=1 mm2; slice thickness=1 mm) were acquired on a 3T Philips Intera Achieva scanner and used to identify the resected area and determine the remaining amygdala volumes in the resection groups (Table S1). The structural image for one participant was acquired on a 1.5T Philips scanner (in-plane resolution=1 mm2; slice thickness=1.2 mm). Structural images from 15 controls in the current study and 33 additional healthy adults who participated in other studies were used in the lesion identification analysis (total N=48). Images were normalized to MNI space at 1 mm3 resolution using the unified segmentation and normalization procedure in SPM5 (Ashburner & Friston, 2005), as this method has been shown to outperform other techniques for normalizing lesioned brains (Crinion et al., 2007). In the unified segmentation and normalization step we used the following parameters: warp regularization = 1, warp frequency cutoff = 25, bias regularization = 0.0001 and bias full with at half maximum = 60. For lesion identification, the normalized, segmented gray and white matter images were smoothed with an 8 mm full width at half maximum kernel.
Amygdala Volumetric Analysis
Amygdala volumes were traced on the normalized image of each resection participant using FSLView (http://www.fmrib.ox.ac.uk/fsl/fslview/index.html) based on criteria modified from Pruessner et al. (2000) and Honeycutt et al. (1998) (see supplementary Figure S2). The superior border was identified in the coronal plane using a line drawn between the superolateral aspect of the optic tract and the fundus of the circular sulcus of the insula. The posterior, lateral and medial boundaries of the amygdala were defined in the axial plane with reference to the coronal and sagittal planes as necessary. The alveus of the hippocampus (excluding the alveus itself) was used as the posterior border. The lateral boundary was defined as 1 mm from the most medial adjacent white matter. In superior slices of the amygdala, the medial border was defined as 1 mm from the ambient cistern and the white matter separating the amygdala from the entorhinal cortex served as the medial border in slices inferior to the level of the uncus. The anterior boundary of the amygdala was defined as 1 mm from the subarachnoid space in the axial plane or the coronal slice just posterior to the anterior commissure. If the above boundaries were not identifiable on the resected side of an image, voxels in the amygdala were marked with reference to the corresponding non-resected slice excluding one layer of voxels from the resected area in any plane.
Lesion Identification Analysis
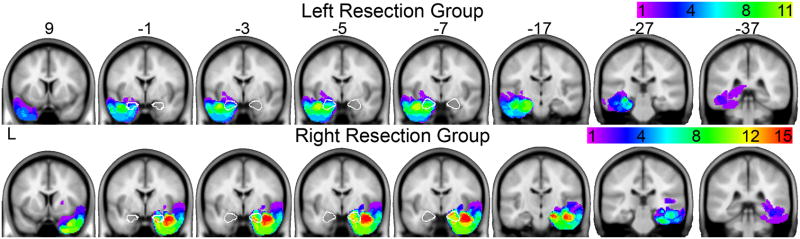
Lesions (resected areas) were identified on each participant’s T1 structural image using an outlier detection algorithm implemented in Matlab based on Seghier et al. (2008). The following steps were applied separately to the smoothed, normalized gray and white matter images. First, the image from each resection participant was merged with the images from all healthy controls using FSLmerge (Smith et al., 2004). For every voxel of each participant in the merged image, the value of that voxel was then compared to the mean value of that voxel across all participants in the image to calculate a similarity metric (equation 2 in Seghier et al. 2008). Next, the similarity value of each voxel in the resection participant’s image was compared the similarity values from all healthy controls to identify voxels in the resection participant image that were unlikely to belong to the given tissue class (“lesion maps”; equation 3 in Seghier et al. 2008). The gray and white matter lesion maps for each resection participant were then combined (equation 4 in Seghier et al. 2008) and thresholded at a value of 0.3. Each lesion map was manually inspected and voxels identified outside the temporal lobe were excluded. To identify the amygdala on the lesion maps, the manually defined intact amygdalae from each resection participant were left-right flipped and summed to create an amygdala map. The outline of this amygdala map is shown over the lesion maps (see Figure 1).
Figure 1.
Lesion maps from resection groups displayed on coronal slices from SPM5 MNI template brain. The color of each voxel indicates the number of subjects within each group that have a voxel identified as resected. Purple voxels indicate areas in which few participants have overlapping voxels; Red indicates areas in which many participants have overlapping voxels. The amygdala has been outlined in white on slices MNI y = −1, −3, −5 and −7.
Emotional Blink of Attention Task
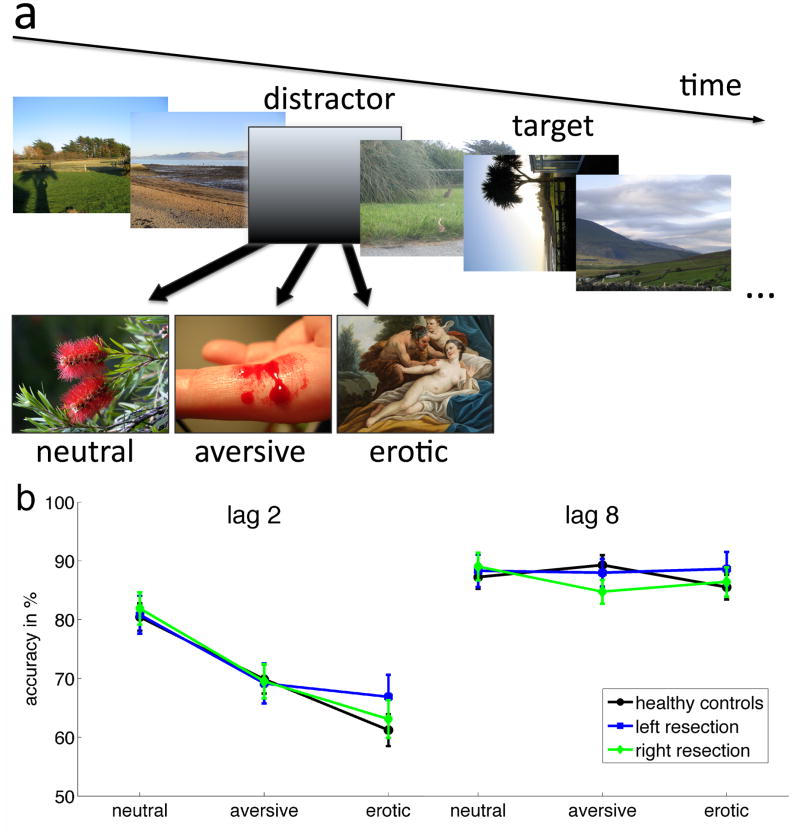
Participants viewed rapidly presented images of landscapes (or buildings, see Fig. 2a). Between the landscape images, we embedded two other types of images: distractors and targets. The participants’ aim was to detect the target. The targets were defined by their orientation: they were also landscapes, but lying on their side (the images were rotated by 90 degrees, while maintaining the same dimensions as the non-rotated images). Crucially, each task trial also included a distractor image, which appeared before the target. Distractors could either have neutral, aversive, or erotic content.
Figure 2.
The Emotional Blink of Attention Task: schematic (panel a) and performance (panel b). a) The Emotional Blink of Attention can occur if a target must be detected shortly (200 ms) after a distractor image. A rotated landscape is the target. Distractors can be neutral or emotionally salient (aversive or erotic*). b) Performance on trials when the target was presented 200 ms (lag 2) or 800 ms (lag 8) after a distractor. The y-axis displays the percentage of correctly identified targets after the respective distractors. Both categories of motivationally salient distractors (aversive and erotic images) are more distracting than neutral images and impair performance significantly. That is true for control participants as well as both patient groups with amygdala lesions. Error bars represent one standard error of the mean. (Analysis of erotic distractors performance. A post-hoc comparison of the experimental groups for erotic distractors at lag 2 (panel b) revealed no differences (F(2,44)=.752, p=.477)).
* In the actual experiment, the erotic pictures depicted real couples engaged in erotic scenes, not classical paintings as shown here for illustrational purposes only.
Participants responded by pressing right or left arrow keys to indicate target rotation at the end of each trial. They were instructed that the target items would always be rotated landscapes, and that images of other objects may also appear, but should be ignored.
The task consisted of 168 trials divided into 6 blocks. Each trial contained 17 images, which were presented for 100 ms each, with no interval between the images. Target images appeared either two or eight presentations after the distractor (referred to as lag 2 and lag 8). We expected impaired detection of targets at lag 2, but a recovery of performance at lag 8. Distractors were positioned as the 4th, 6th, or 8th image in each trial. Distractor categories and positions were counterbalanced. Trial sequence was randomized for each participant. Before the main task, participants completed 16 practice trials.
Distractors with neutral content, aversive content (scenes of violence and medical trauma), and images with erotic scenes of male-female couples were either drawn from the IAPS database (Lang, Bradley, & Cuthbert, 2008), or from other publicly available sources. Each distractor was only used once.
Although the emotional and neutral distractors in this task differ in several non-affective respects, such as the number of objects in the picture, or color, we have previously demonstrated that the EBA reflects emotional effects over and above physical picture properties (Most et al., 2005; Most et al., 2007).
Results
The processing enhancement of emotional images in the three participant groups is shown in Figure 2. Distractor pictures clearly produced EBA effects consistent with those seen in previous studies with healthy participants (Most et al., 2005; Most et al., 2007): when targets were closely preceded (at lag 2) by emotional distractors, target detection performance was impaired. Both types of emotional distractors, aversive and erotic, led to accuracies lower than that after neutral distractors. Contrary to our expectations, participants with resected amygdalae in either hemisphere showed the same pattern of results as participants with two functioning amygdalae: that is the performance impairment induced by emotional distractors was present to the same degree in patients as in healthy controls.
To analyze the data, we first conducted a mixed effects ANOVA with three factors: Lag (lag 2 or lag 8), Distractor Type (neutral, aversive, erotic), and Group (healthy matched controls, patients with a left amygdala resection, patients with a right amygdala resection). This showed a main effect for Lag, (F(1,44)=159.2, p<.0005), meaning that a significant EBA effect at lag 2 was obtained with the task. It also showed an interaction between the Lag and Distractor Type factors (F(2,43)=24.9, p<.0005), indicating that the EBA effect was related to affective content. There was no effect of Group (F(2,44)=.146, p=.865). To illuminate the significance of the interaction effect, we conducted two separate analyses for lag 2 and lag 8.
In an analysis for lag 2 only, a mixed effects ANOVA with factors Group and Distractor Type, showed a main effect of Distractor Type (F(2,43)=43.4, p<.0005). Planned comparisons of Distractor Types revealed that performance after aversive distractors was impaired compared to neutral ones (p<.0005) and that performance after erotic distractors was even more impaired than after aversive ones (p=.028). There was no effect of Group, (F(2,44)=.153, p=.859), nor an interaction of Group and Distractor Type (F(4,88)=.578, p=.679), indicating that performance of the three experimental groups did not differ for any of the three distractor types, be they neutral or emotional.
In an equivalent analysis for lag 8 (with trials with distractor and target separated by seven standard images), target detection performance was high irrespective of distractor type (F(2,43)=.544, p=.582). The groups did not differ in performance (F(2,44)=.144, p=.866), nor did the distractor type interact with the participant group (F(4,88)=1.592, p=.183).
Due to the nature of the surgical procedure, the amygdalae were not completely removed in a number of subjects, with an average of 76.0% volume loss in the left amygdala (range = 28.6–100%), or 92.5% volume loss in the right amygdala (range = 63.8–100%). While the functional status of this residual tissue is not clear (as many of the inputs and outputs will have been severed during the resection of neighboring tissue), it is possible that some subjects have enough residual tissue to still contribute to an emotional modulation of attention. To test this possibility, we analyzed whether performance drop due to aversive emotional distractors correlated with the degree of amygdala tissue remaining in the resected temporal lobe for each post-surgery group. The residual amygdala volume was not correlated with the emotional modulation of performance in either patient group (R2 left resection group = 0.04, p=.540; R2 right resection group = 0.04, p =.453, see supplementary materials, Figure S1), making it unlikely that the preservation of emotional modulation is related to the spared portions of the amygdala in subjects with smaller lesions.
In summary, the profound effect of emotional distractors on target detection performance is preserved in patients with lesions of either the left or the right amygdala. There is no indication of a potential reduction of the effect in patients.
Discussion
The present data challenge the established belief that the amygdala is generally necessary for emotional stimuli to capture attention. If amygdalar processing is a mandatory condition for the emotional modulation of attention to occur, then one would expect an attenuation of such effects in patients with amygdala damage. However, in an EBA paradigm, emotional images captured attention and disrupted subsequent target detection to a similar degree in neurologically intact individuals and patients with unilateral damage to either the left or right amygdala. These data, combined with recent findings of spared emotional influences on visual-search performance in patients with amygdala damage (Piech, McHugo, et al., 2010; Tsuchiya et al., 2009) and with evidence that attentional blinks created by emotional stimuli correlate predominantly with cortical activity (Schwabe et al., 2011), indicate that emotional influences on attention do not generically depend upon the amygdala.
An obvious alternative explanation for the present results is that given the unilateral nature of the damage, the remaining intact amygdala was sufficient to elicit emotional effects. This criticism is tempered by the fact that several previous studies have identified deficits in emotion related processing following unilateral amygdala lesions (Adolphs, Tranel, & Damasio, 2001; Akiyama et al., 2007; Anderson, Spencer, Fulbright, & Phelps, 2000; Bechara et al., 1995; Benuzzi et al., 2004; Coppens, van Paesschen, Vandenbulcke, & Vansteenwegen, 2010; Frank & Tomaz, 2003; LaBar, LeDoux, Spencer, & Phelps, 1995). Although deficits after unilateral lesions are not always as severe as those found after bilateral damage, these studies support the notion that unilateral damage can be sufficient to disrupt normal emotional processing. Crucially for our argument, in Anderson and Phelps’ (2001) seminal paper, the attenuation of emotional effects was indeed found in left- but not right-amygdala lesion patients. In contrast, we observed no effects of lesions in either hemisphere.
The present results suggest a need for greater specificity in defining the amygdala’s contributions to attention. In respect to differences between groups, our results show a null finding. While null findings often raise criticisms that are overlooked for studies with positive results, they are important in that they help define the boundaries of phenomena. Because both the present study and the study by Anderson and Phelps (2001) make use of attentional blink paradigms, they might be assumed to both capitalize on the same process. However, Anderson and Phelps probed the ability of actively sought emotional words to “break through” the attentional blink caused by a neutral target. In contrast, we assessed the ability of emotional pictures to capture attention and cause a spontaneous attentional blink. It is possible that the neural substrates underlying the intentional, task-related perception of emotional stimuli differ from those underlying the automatic capturing of attention by task-irrelevant emotional images. In fact, Schwabe and colleagues (2011) support this view with their neuroimaging data. Using words in both conditions, they showed amygdala activity to be related to “breaking through”, and paralimbic cortical activity to creating and prolonging a blink. It is also possible that the neural architecture underlying the perception of highly arousing visual stimuli is more extensive than that underlying the encoding of emotional words, thereby providing redundant systems that could influence attention even in the absence of one or both amygdalae. This latter possibility could also explain the preserved emotional influences on visual search following amygdala damage. Another possibility is that even break through effects are mediated by pathways outside the amygdala. Lim and colleagues (2009) showed cortical activations associated with breaking through. Furthermore, Bach and colleagues (2011) documented preserved breaking through effects in two patients with bilateral amygdala lesions. They used a word-based RSVP task much like Anderson & Phelps (2001), and thus their results appear to contradict the original Anderson and Phelps finding.
We found that the attentional effect of the erotic stimuli was more pronounced than that of aversive stimuli. This may be due to a parametric difference in their impact, or an indicator of distinct underlying processes and pathways. At this point little evidence is present to argue for either explanation. In a previous study (Piech, Pastorino, & Zald, 2010), we observed an unexpected performance improvement at lag 8 (after recovery from blink effects) after the presentation of erotic distractors, suggesting some unique properties for the processing of erotic (or generally positive) material.
If the amygdala is not always critical for the emotional modulation of attention, then it is necessary to identify alternative structures that could influence this process. Schwabe and colleagues (2011) suggest the anterior cingulate, insula, and orbitofrontal cortices could be involved in the prolonging of attentional blinks by emotional words. This partially converges with a model of top-down facilitation of object recognition by Bar (2003). According to this model, the orbitofrontal cortex (OFC) formulates predictions about what objects are likely to exist in the visual field based on low spatial-frequency information transmitted from early visual regions, and uses these predictions to modulate neural processing in object-related ventral visual areas in order to focus processing resources on the items most likely to be present. Given that neurons in the OFC are sensitive to emotional valence (Morrison & Salzman, 2009), the OFC would be positioned to modulate attention based on emotion. Alternatively, De Martino and colleagues (2009) suggest that the rostral anterior cingulate might play a critical role in attentional modulation, as this region showed activity specific to trials when an emotional face broke through the period of normal suppression during an fMRI study of the attentional blink. (Although the rostral anterior cingulate receives little direct visual sensory input, this information may be relayed through the OFC (Saleem, Kondo, & Price, 2008).) Importantly, De Martino and colleagues (2009) failed to detect amygdalar activations related to task performance, further suggesting the need to consider other mechanisms mediating the ability of emotional stimuli to gain preferential access to awareness (e.g. Pessoa & Adolphs, 2010).
Supplementary Material
Research Highlights.
We investigated attentional capture by emotional stimuli
Our sample included surgery patients with unilateral amygdala lesions
Emotional enhancement was present in controls and patients
The amygdala may not be necessary for all types of such enhancement
Acknowledgments
This research was supported by grants from the National Institutes of Health 5R01MH74567-4, the Manitoba Health Research Council, and the Vanderbilt Institute for Clinical and Translational Research (1-UL1-RR024975 NCRR/NIH).
Footnotes
Anderson and Phelps (2001) refer to this ‘breaking through’ as capturing attention. To avoid confusion, we will refer to the phenomenon described in the present study, attentional capture that prevents detection of subsequent stimuli, as attentional capture, and to the phenomenon described by Anderson and Phelps as ‘breaking through’.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. 2001;15(3):396–404. doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Kato M, Muramatsu T, Umeda S, Saito F, Kashima H. Unilateral amygdala lesions hamper attentional orienting triggered by gaze direction. Cerebral cortex (New York, NY: 1991) 2007;17(11):2593–2600. doi: 10.1093/cercor/bhl166. [DOI] [PubMed] [Google Scholar]
- Amaral D, Behniea H, Kelly J. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118(4):1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Anderson A. Affective influences on the attentional dynamics supporting awareness. Journal of experimental psychology General. 2005;134(2):258–280. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- Anderson A, Spencer D, Fulbright R, Phelps E. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;14(4):526–536. doi: 10.1037//0894-4105.14.4.526. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Killman KV, Fijavz D. Blinded by emotion: target misses follow attention capture by arousing distractors in RSVP. Emotion. 2007;7:465–477. doi: 10.1037/1528-3542.7.3.465. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bach DR, Talmi D, Hurlemann R, Patin A, Dolan RJ. Automatic relevance detection in the absence of a functional amygdala. Neuropsychologia. 2011;49(5):1302–1305. doi: 10.1016/j.neuropsychologia.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. Journal of cognitive neuroscience. 2003;15(4):600–609. doi: 10.1162/089892903321662976. [DOI] [PubMed] [Google Scholar]
- Barnard PJ, Scott S, Taylor J, May J, Knightley W. Paying attention to meaning. Psychological Science. 2004;15:179–186. doi: 10.1111/j.0956-7976.2004.01503006.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio A. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science (New York, NY) 1995;269(5227):1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Benuzzi F, Meletti S, Zamboni G, Calandra-Buonaura G, Serafini M, Lui F, et al. Impaired fear processing in right mesial temporal sclerosis: a fMRI study. Brain research bulletin. 2004;63(4):269–281. doi: 10.1016/j.brainresbull.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Broadbent MHP. From detection to identification: Response to multiple targets in rapid serial visual presentation. Perception & Pscychophysics. 1987;42:105–113. doi: 10.3758/bf03210498. [DOI] [PubMed] [Google Scholar]
- Coppens E, van Paesschen W, Vandenbulcke M, Vansteenwegen D. Fear conditioning following a unilateral anterior temporal lobectomy: reduced autonomic responding and stimulus contingency knowledge. Acta neurologica Belgica. 2010;110(1):36–48. [PubMed] [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. NeuroImage. 2007;37(3):866–875. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Kalisch R, Rees G, Dolan R. Enhanced Processing of Threat Stimuli under Limited Attentional Resources. Cerebral Cortex (New York, NY) 2009;19(1):127–133. doi: 10.1093/cercor/bhn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Tomaz C. Lateralized impairment of the emotional enhancement of verbal memory in patients with amygdala-hippocampus lesion. Brain and cognition. 2003;52(2):223–230. doi: 10.1016/s0278-2626(03)00075-7. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychological science: a journal of the American Psychological Society/APS. 2002;13(2):135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Honeycutt NA, Smith PD, Aylward E, Li Q, Chan M, Barta PE, et al. Mesial temporal lobe measurements on magnetic resonance imaging scans. Psychiatry Research: Neuroimaging. 1998;83(2):85–94. doi: 10.1016/s0925-4927(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattie B, et al. Linguistic threat activates the human amygdala. Proc Natl Acad Sci USA. 1999;96(18):10456–10459. doi: 10.1073/pnas.96.18.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K, LeDoux J, Spencer D, Phelps E. Impaired fear conditioning following unilateral temporal lobectomy in humans. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15(10):6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lang PJ, Davis M, Öhman A. Fear and anxiety: animal models and human cognitive psychophysiology. Journal of Affective Disorders. 2000;61(3):137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Morris J, Buchel C, Dolan R. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. NeuroImage. 2001;13(6 Pt 1):1044–1052. doi: 10.1006/nimg.2000.0721. [DOI] [PubMed] [Google Scholar]
- Morris J, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Morrison S, Salzman C. The convergence of information about rewarding and aversive stimuli in single neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(37):11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most S, Chun M, Widders D, Zald D. Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin and Review. 2005;12(4):654. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- Most S, Smith S, Cooter A, Levy B, Zald D. The naked truth: Positive, arousing distractors impair rapid target perception. Cognition & Emotion. 2007;21(5):964–981. [Google Scholar]
- Padmala S, Pessoa L. Affective learning enhances visual detection and responses in primary visual cortex. The Journal of Neuroscience. 2008;28(24):6202. doi: 10.1523/JNEUROSCI.1233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11(11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piech R, McHugo M, Smith S, Dukic M, Van Der Meer J, Abou-Khalil B, et al. Fear-enhanced visual search persists after amygdala lesions. Neuropsychologia. 2010;48(12):3430–3435. doi: 10.1016/j.neuropsychologia.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piech R, Pastorino M, Zald D. All I saw was the cake. Hunger effects on attentional capture by visual food cues. Appetite. 2010;54(3):579–582. doi: 10.1016/j.appet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10(4):433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. NeuroImage. 2005;24(4):1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. The Journal of comparative neurology. 2008;506(4):659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Merz C, Walter B, Vaitl D, Wolf O, Stark R. Emotional modulation of the attentional blink: The neural structures involved in capturing and holding attention. Neuropsychologia. 2011;49(3):416–425. doi: 10.1016/j.neuropsychologia.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Ramlackhansingh A, Crinion J, Leff AP, Price CJ. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. NeuroImage. 2008;41(4):1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R. Intact rapid detection of fearful faces in the absence of the amygdala. Nature neuroscience. 2009;12(10):1224–1225. doi: 10.1038/nn.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of Attention and Emotion on Face Processing in the Human Brain:: An Event-Related fMRI Study. Neuron. 2001;30(3):829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson M, Armony J, Driver J, Dolan R. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature neuroscience. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- West GL, Anderson AA, Pratt J. Motivationally significant stimuli show visual prior entry: evidence for attentional capture. Journal of Expimental Psychology: Human Perception and Performance. 2009;35:1032–1042. doi: 10.1037/a0014493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.