1. Introduction
The critical role of inflammatory processes in health and disease has long been recognized,1 yet the detailed molecular mechanisms and biological events that regulate the progression and resolution of inflammation remain of interest. A number of recent investigations have provided strong evidence that the resolution of inflammation is not a passive process, as believed earlier.2–4 Instead, resolution is a biosynthetically active process, regulated by biochemical mediators and receptor-signaling pathways, and driven by specialized pro-resolving mediators (SPM). In particular, following a number of findings by Serhan and his group, the authors and their collaborators introduced and systematically investigated a number of SPM derived from polyunsaturated fatty acids (PUFA), including lipoxins, E-series resolvins, D-series resolvins, protectins/neuroprotectins, and, most recently, maresins. This review summarizes efforts on the resolvins and protectins with an emphasis on the corresponding biochemical pathways. Additional reviews covering different aspects of these anti-inflammatory and pro-resolving lipid mediators,5 including immunology,6,7 pathology,8 biochemistry,9 pharmacology10 and chemistry11 are also available.6–9,12
1.1 The Inflammatory Response and the Resolution of Inflammation
Localized acute inflammation is part of the host’s normal protective response to tissue injury and infection by invading microbial pathogens.1 Although this inflammatory response to a range of harmful stimuli is protective to the host, if kept uncontrolled it can result in a wide range of acute, chronic and systemic inflammatory disorders. Indeed, some of the most common and difficult to treat diseases are linked to excessive, uncontrollable, or chronic inflammation, including: cardiovascular disease, rheumatoid arthritis, periodontal disease, asthma, diabetes, inflammatory bowel disease (IBD), as well as neurological disorders such as Alzheimer’s disease and age-related macular degeneration (AMD).1,13,14 Although the involvement of inflammatory pathways in the initiation of all of these diseases is well established, the specific role by which inflammation contributes to their pathogenesis is not fully understood. The recent findings that the resolution of inflammation is an active process2–4,15 have provided new insights and created new paradigms for understanding and treating these conditions.
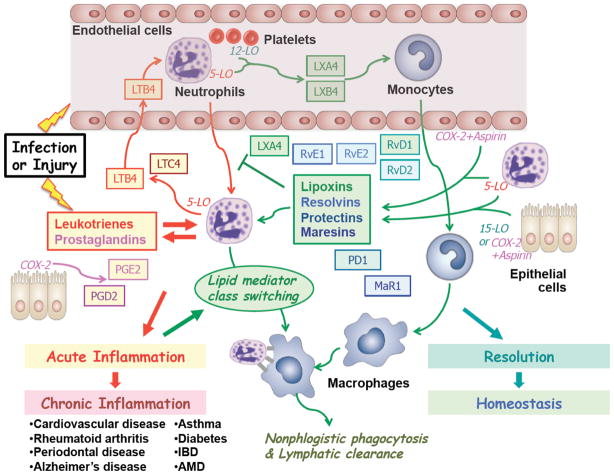
The key role of a number of lipid mediators in the initiation of the inflammatory response and the subsequent progression towards resolution is illustrated in Fig. 1. Among the first signaling events following microbial infection or tissue injury, is the release of pro-inflammatory lipid mediators, such as leukotrienes (LTs) and prostaglandins (PGs) that launch a series of signaling cascades with the ultimate goal of destroying the invading pathogens and repairing the damaged tissue.16–18 Thus, the biosynthesis and release of the potent chemotactic agent leukotriene B4 (LTB4) promotes the recruitment of neutrophils (PMNs) to the inflamed tissue, while the formation of prostaglandins E2 and D2 (PGE2 and PGD2)15 further accelerates the inflammatory process, ultimately resulting in a condition of acute inflammation. Despite its critical host-protective function, acute inflammation is not sustainable over a prolonged period of time, giving rise to disruptive conditions of chronic inflammation that may be responsible for the pathogenesis of a wide range of diseases, that can be attributed to a failure of resolution.19 Typically, the therapeutic treatment of such conditions involves the inhibition of pro-inflammatory mediators, but in many cases such approaches are often not very effective.
Figure 1.
From initiation of acute inflammation to resolution: The inflammatory response to microbial infection and tissue injury, and the role of selected cell types and specialized pro-resolving lipid mediators.
The recognition of the proactive nature of the resolution of inflammation has revealed alternative therapeutic paradigms based on resolving acute inflammation and preventing the onset of chronic inflammation.19 Indeed, a number of endogenous lipid mediators identified are able to act in this manner, suggesting a lipid mediator class switching3 from the initial actions of pro-inflammatory lipid mediators, i.e. leukotriene and prostaglandins, to the anti-inflammatory and pro-resolving actions of lipoxins, resolvins, protectins and maresins, which are discussed in this review. Each family of these pro-resolving mediators exert specialized actions, including blocking neutrophil recruitment, promoting the recruitment and activation of monocytes, as well as mediating the nonphlogistic phagocytosis and lymphatic clearance of apoptotic neutrophils by activated macrophages. Eventually, through the combined actions of these mediators, the resolution of inflammation is completed and homeostasis is reached. In this regard, it was important to introduce precise definitions for resolution mechanisms and indices19–21 that also permitted the first information that certain agents can be resolution-toxic.22–24
1.2 Lipid Mediator Pathways in Inflammation and Resolution: The Roles of Polyunsaturated Fatty Acids
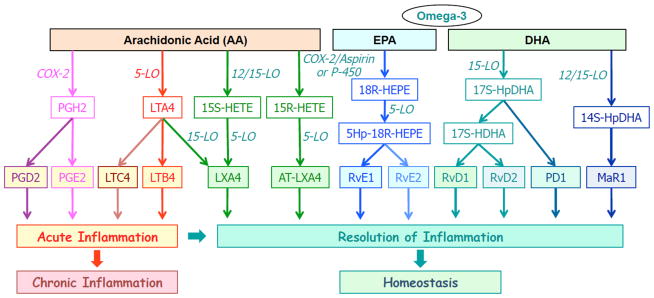
The key role of several new classes of lipid mediators for regulating the resolution of inflammation has recently emerged by several recent discoveries,5–9,11,12 including extensive collaborative efforts.25–64 In particular, it was discovered that a number of novel lipid mediators derived from polyunsaturated fatty acids (PUFA) that are endogenously generated during inflammation have potent anti-inflammatory actions and serve as specialized pro-resolving lipid mediators (SPM)8,10 and are able to promote the resolution of inflammation.7,12,15 Several families of new lipid-derived SPM have been recently identified and characterized. The biosynthetic and signaling pathways of these pro-resolving molecules, as well as their relationships with selected pro-inflammatory mediators are outlined in Fig. 2.
Figure 2.
Biosynthetic cascades and actions of selected lipid mediators derived from arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
1.2.1 Pro-inflammatory Lipid Mediators from Arachidonic Acid
At the onset of the inflammatory response, phospholipase enzymes (e.g. cPLA2) acting on phospholipids mediate the release of free polyunsaturated fatty acids (PUFA), including arachidonic acid (AA) and the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These fatty acids initiate a series of biosynthetic pathways involving lipoxygenases (human 5-LO, 65,66 12-LO and 15-LO) and cyclooxygenases, particularly the inflammation-induced isoform COX-2. Among the resulting PUFA-derived hydroxylated metabolites are several series of potent lipid mediators that exert their specialized inflammation-related actions by acting on specific G-protein coupled receptors (GPCR). Activation of these receptors by these lipids directly affects the expression levels of multiple enzymes, chemokines, cytokines, and growth factors that play dominant roles in inflammation and resolution.
Arachidonic acid has long been known to be involved in the initiation phase of inflammation, by forming pro-inflammatory leukotrienes (LTB4 and LTC4) and prostaglandins (PGE2 and PGD2) that govern the early events in host defense.16 Each of these potent molecules acts via its perspective GPCRs that are present in the membranes of the relevant cell types, and triggers the expression of inflammatory enzymes (5-LO,65,66 COX-2,67–70), chemokines and cytokines (IL-6, IL-8) that initiate and accelerate inflammation. Thus, activation of BLT1/2 by LTB4 leads to increased neutrophil recruitment, while activation of CysLT1/2 by LTC4 leads to airway smooth muscle contraction. Similarly, activation of the EP1–4 receptors by PGE2 and the DP1/2 receptors by PGD2 leads to multiple pro-inflammatory actions. The inhibition of key pro-inflammatory enzymes (e.g. 5-LO,65,66, COX-267–70) and the development of antagonists for key pro-inflammatory receptors (e.g. BLT1/2, CysLT1/2)71 has been extensively investigated as a means of developing therapeutics for inflammatory disorders, some of which are now in clinical use.
1.2.2 Anti-inflammatory and Pro-resolving Lipid Mediators from Arachidonic Acid
Notably, despite its central role in the initiation and progression of inflammation, AA is also involved in the biosynthesis of anti-inflammatory and pro-resolving lipid mediators.72,73 Most prominent are the lipoxins5,11 (LXA4 and LXB4), which are formed in humans to a large extent via transcellular biosynthesis through the sequential actions of 5-LO and 12-LOX with LTA4 as an intermediate or 15-LOX-initiated interactions with 5-LOX-bearing cells. Of interest, blocking or reducing LTA4 hydrolase given an increase in endogenous LXA4 biosynthesis and an anti-inflammatory response.74 The epimeric aspirin-triggered lipoxins,29,33,34 (AT-LXA4 and AT-LXB4) have similar actions as the lipoxins, but are formed from COX-2 in the presence of aspirin or via the P450 pathway. The biosynthetic pathways and biological functions of the lipoxins (LX) and aspirin-triggered lipoxins (ATL) have been described in several reviews.5,11,75 For reviews on the lipoxins and their analogs, design and synthesis, see Petasis et al.11 and Guilford et al.76 The discovery that LX undergo inactivation catalyzed by the enzyme 15-prostaglandin dehydrogenase (15-PGDH), led us to the first rational design and synthesis of a series of stable lipoxin analogs11,58 that retain all of the biological actions and sub-nanomolar potency of the lipoxins. These LX analogs served as key molecular probes for extensive investigations on the biological actions of the lipoxins, and provided the basis for the first LX-based lead compounds for clinical development.11,76 Both LXA4 and AT-LXA4 act on ALXR/FPR2 (for a recent review, see Ref. 77) and GPR32, and exhibit potent anti-inflammatory and pro-resolving actions.63 A prostaglandin-type pro-resolving lipid mediator is 15-deoxy-prostaglandin J2 (15dPGJ2),24,78 which is formed from PGD2. PGE2 and PGD2 can both switch the phenotype of human neutrophils from leukotrienes to lipoxins and resolution.3,20
1.2.3 New Pro-resolving Lipid Mediators from Omega-3 Fatty Acids
While enzymatic oxygenation of AA, an omega-6 fatty acid, generates both pro-inflammatory and pro-resolving lipid mediators, analogous pathways with the corresponding omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) generally lead to the formation of anti-inflammatory, pro-resolving and cytoprotective lipid mediators. Using an unbiased lipidomics and systems approach, the enzymatic oxygenation of omega-3 fatty acids, Serhan and collaborators identified, characterized and elucidated families of novel pro-resolving lipid mediators from EPA and DHA.6–9,12 These include the E-series resolvins (RvE1 and RvE2) 2,45 derived EPA, as well as the D-series resolvins (RvD1, RvD2, RvD3, RvD4, RvD5, RvD6),4,55,60 the neuroprotectins/protectins (NPD1/PD1),51,79–81 and the maresins (MaR1),82 which are derived from DHA. Additional series of lipid mediators formed in the presence of aspirin were also discovered, including the D-series resolvins4 and protectins and their aspirin-triggered forms,79 all of which are biosynthesized from PUFA and aspirin-acetylated COX-2 that acts as a modified dioxygenase inserting a molecule of oxygen with opposite stereochemistry.
1.3 The Beneficial Roles of Omega-3 Fatty Acids in Inflammation and Resolution
The discovery of resolvins, protectins and maresins is of particular significance, since these potent lipid mediators provided the first molecular basis for the many health benefits attributed to the omega-3 fatty acids EPA and DHA, which are abundant in fish and are widely used as dietary supplements. These essential fatty acids have long been associated with beneficial effects in human health and in the prevention of various diseases,83 including inflammation,84 immunomodulation,85 autoimmune diseases,84 rheumatoid arthritis,86 cardiovascular diseases,87,88 Alzheimer’s disease and other neurodegenerative diseases, type-2 diabetes,89 and cancer.90
Although the specific molecular mechanisms for these health benefits have remained unclear, the recent collaborative efforts by our team have provided the first detailed mechanistic insights for the likely pathways involved. The distinct properties of EPA and DHA to form primarily pro-resolving lipid mediators may explain their well-known beneficial health effects. These novel pathways may also explain some of the beneficial effects of aspirin, since they generate epimeric lipid mediators that are more metabolically stable and longer lasting.
1.4 Time-course of the Pro-inflammatory and Pro-resolving Lipid Mediator Pathways
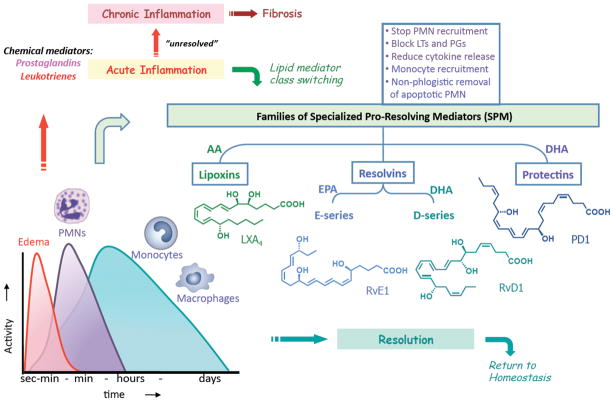
During the inflammatory response, the biosynthesis of PUFA-derived lipid mediators increases with time (Fig. 3). The initial mediators (prostaglandins and leukotrienes) are produced within seconds and minutes and regulate the edema and postcapillary events at the site following the recruitment of neutrophils. With time (hours up to days), increasing leukocyte recruitment with the non-phlogistic appearance of monocytes and macrophages leads to a growing amount of mediators involving these cells (Fig. 3). The net result of this timeline is that initially mostly pro-inflammatory mediators (LTs, PGs) are produced, while at a later time, a lipid mediator class switching results in the production of pro-resolving mediators (LXs, Rvs, PDs) that promote resolution. For recent reviews on the cellular mechanisms, see Refs. 73,91,92 The release of AA and mobilization of EPA and DHA from circulation,57 and the formation of the pro-resolving mediators by these substrates also follows a time course that favors initial inflammatory response and host defense, followed by resolution and return to homeostasis. In the event that the pro-resolving lipid mediators are not produced or are defective, the acute inflammatory process remains unresolved and escalates into a state of chronic inflammation that promotes fibrosis and is associated with the pathogenesis of multiple diseases. Table 1 lists the many in vivo systems where a key enzyme is genetically manipulated in the biosynthesis of proresolving mediators including AA-derived LX and DHA-derived PD1 (see below).
Figure 3.
Inflammatory response and resolution time course: Roles of pro-resolving lipid mediators.
Table 1.
Genetic Manipulation of Human 15-Lipoxygenase and Mouse 12/15-Lipoxygenase Regulates Mediator Profiles and Disease Outcomes in Animal Models
| Animal models/cellular systems | Alox15 gene modification | Phenotypes | References |
|---|---|---|---|
| Periodontitis | Alox15 transgenic rabbit | Decrease PMN-mediated tissue degradation and bone loss | Serhan et al.42 |
| Isolated Th2 cells | Alox15 type 1 siRNA knockdown | Decrease PD1 production | Ariel et al.155 |
| Cornea injury | Alox 12/15-deficient mice | Increase inflammation | Gronert et al.a |
| Decrease wound healing | Biteman et al.b | ||
| Decrease corneal re-epithelialization | |||
| Decrease endogenous LXA4 production | |||
| Decrease heme-oxygenase 1 | |||
| LXA4 rescues exacerbated inflammation and impaired wound healing in Alox15-deficient mice | |||
| Atherosclerosis | Alox 12/15 transgenic mice | Delayed atherosclerosis | Merched et al.c |
| Decrease IL-17, CCL5, PGE2 | |||
| Increase PD1 | |||
| Alox 12/15-deficient mice* | Accelerated atherosclerosis | Merched et al.d | |
| Increase IL-12p40, CCL5 | |||
| Decrease LXA4 production in macrophages | |||
| Retinal pigment epithelial cells | Alox15 siRNA knockdown | Increase sensitivity to oxidate stress-induced apoptosis | Calandria et al.62 |
| NPD1 rescues Alox15-silenced cells from oxidative stress-induced apoptosis | |||
| Arthritis | Alox 12/15-deficient mice | Increase inflammatory gene expression | Krönke et al.e |
| Decrease LXA4 in inflamed synovia and macrophages | |||
| Human colon cancer | Silencing of 15-LOX type I | May lead to cancer cell proliferation | Shureiqi et al.f |
Gronert, K. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 221.
Biteman, B.; Hassan, I. R.; Walker, E.; Leedom, A. J.; Dunn, M.; Seta, F.; Laniado-Schwartzman, M.; Gronert, K. FASEB J. 2007, 21, 2257.
Merched, A.; Ko, K.; Gotlinger, K. H.; Serhan, C. N.; Chan, L. FASEB J. 2008, 22, 3595.
Merched, A. J.; Serhan, C. N.; Chan, L. J. Nutrigenet. Nutrigenomics 2011, 4, 12.
Kronke, G.; Katzenbeisser, J.; Uderhardt, S.; Zaiss, M. M.; Scholtysek, C.; Schabbauer, G.; Zarbock, A.; Koenders, M. I.; Axmann, R.; Zwerina, J.; Baenckler, H. W.; van den Berg, W.; Voll, R. E.; Kuhn, H.; Joosten, L. A.; Schett, G. J. Immunol. 2009, 183, 3383.
Shureiqi, I.; Zuo, X.; Broaddus, R.; Wu, Y.; Guan, B.; Morris, J. S.; Lippman, S. M. FASEB J. 2007, 21, 743.
Assimes, T. L.; Knowles, J. W.; Priest, J. R.; Basu, A.; Borchert, A.; Volcik, K. A.; Grove, M. L.; Tabor, H. K.; Southwick, A.; Tabibiazar, R.; Sidney, S.; Boerwinkle, E.; Go, A. S.; Iribarren, C.; Hlatky, M. A.; Fortmann, S. P.; Myers, R. M.; Kuhn, H.; Risch, N.; Quertermous, T. Atherosclerosis 2008, 198, 136.
Poeckel, D.; Zemski Berry, K. A.; Murphy, R. C.; Funk, C. D. J. Biol. Chem. 2009, 284, 21077.
It should be noted that 12/15-ALO-deficient mice have given controversial findings in atherosclerosis models.e,g,h These differences likely reflect diet of the mice (see ref. d), where changes in diet even in genetically-defined mice overexpressing 12/15-ALOX can give or lead to loss of vascular protections. Also, the cell types expressing the mouse 12/15-LOX might be different in deficient mouse models, as the human 12-LOX and 15-LOX type I are distinct enzymes and may also yield separate functions in humans vs. mouse models. Nonetheless, resolvins, lipoxins and other SPM are protective and anti-inflammatory-proresolving in these systems.
1.5 Specialized Pro-resolving Lipid Mediators: Potential Therapeutics for Inflammation
Given the biological profiles of the new pro-resolving mediators, the pathways that drive the formation and actions of these molecules provide a new paradigm for treating inflammatory diseases. Indeed, our recent efforts on the identification, structural characterization, total synthesis, and biological investigation of selected members and structural analogs of these PUFA-derived lipid mediators have revealed their potent actions (nM and pM range) in a variety of cell types in vitro, as well as in many in vivo models of inflammatory diseases.7 These include: dermal inflammation,29,30 dorsal air pouch,2,4,33 peritonitis,21,44 periodontitis,36,42,50 colitis and intestinal inflammation,31,45 asthma and airway inflammation,93,94 cystic fibrosis,95,96 acute lung injury,59 ischemia-reperfusion injury,27 kidney injury,97 glomerulonephritis,38,98 ischemic-stroke,80 retinal degeneration,62,64 and Alzheimer’s disease.99 Resolvins of the D series and PD1 have been found to be important in protecting the host from obesity-induced insulin resistance and hepatic steatosis in murine models in vivo.100 Also, RvD1 improves insulin sensitivity by resolving the chronic inflammation that is associated with obesity. In this murine system, Hellmann et al found that RvD1 increased adiponectin production and decreased IL-6 as well as reduced the presence of crown-like structures within adipose tissue.101 Together, these results point to an important action of resolvins and protectins in the regulation of inflammation in fat tissues, which is an emerging area of global public health and concern.100,101 RvE1 also reduces inflammation in ocular tissues, reducing the number of macrophages in dry eye murine models and in the symptoms of dry eye.102 As a result of these studies, several synthetic SPM derived from our work are currently in clinical trials, including lipoxin analogs, as well as resolvin E1 and RvE analogs for ocular103–105 and systemic indications including lung, kidney, skin and bowel diseases.45,106–109 RvE1 and RvD1 each regulate and reduce pain110,111, as do the lipoxins112, which opens new means to control pain signals.113 Taken together, these findings demonstrate the profound importance and great therapeutic potential of these novel lipid mediator molecules, listed in Table 2.
Table 2.
Specialized Pro-resolving Lipid Mediators in Disease Models
| Disease | Reference |
|---|---|
| Cardioprotective | Spite and Serhana |
| Keyes et al.b | |
| Reno-protection | Hassan and Gronert108 |
| Atherosclerosis | Merched et al.c |
| Corneal wound healing | Gronert et al.d |
| Ocular indications | Connor et al.103 |
| Li et al.102 | |
| Zhang et al.105 | |
| Stroke | Marcheselli et al.80 |
| Ye et al.e | |
| Ischemia-reperfusion (kidney and lung) | Duffield et al.97 |
| Kasuga et al.57 | |
| Asthma, ALI | Haworth et al.106 |
| Fukunaga et al.f | |
| Aoki et al.g | |
| Hisada et al.h | |
| Obesity-induced insulin resistance and hepatic steatosis | Clària et al.i |
| Inflammatory pain | Xu et al.111 |
| Parturition | Maldonado-Pèriz et al.j |
Spite, M.; Serhan, C. N. Circulation Res. 2010, 107, 1170.
Keyes, K. T.; Ye, Y.; Lin, Y.; Zhang, C.; Perez-Polo, J. R.; Gjorstrup, P.; Birnbaum, Y. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H153.
Merched, A.; Ko, K.; Gotlinger, K. H.; Serhan, C. N.; Chan, L. FASEB J. 2008, 22, 3595.
Gronert, K.; Maheshwari, N.; Khan, N.; Hassan, I. R.; Dunn, M.; Schwartzman, M. L. J. Biol. Chem. 2005, 280, 15267.
Ye, X.-H.; Wu, Y.; Guo, P.-P.; Wang, J.; Yuan, S.-Y.; Shang, Y.; Yao, S.-L. Brain Res. 2010, 1323, 174.
Fukunaga, K.; Kohli, P.; Bonnans, C.; Fredenburgh, L. E.; Levy, B. D. J. Immunol. 2005, 174, 5033.
Aoki, H.; Hisada, T.; Ishizuka, T.; Utsugi, M.; Kawata, T.; Shimizu, Y.; Okajima, F.; Dobashi, K.; Mori, M. Biochem. Biophys. Res. Commun. 2008, 367, 509.
Hisada, T.; Ishizuka, T.; Aoki, H.; Mori, M. Expert Opin. Ther. Targets 2009, 13, 513.
Clària, J.; Titos, E.; López-Vicario, C.; González-Périz, A. Drug Discov. Today Dis. Mech. 2010, 7, e219.
Maldonado-Pèriz, D.; Golightly, E.; Denison, F. C.; Jabbour, H. N.; Norman, J. E. FASEB J. 2011, 25, 569.
2. Resolvins from Eicosapentaenoic Acid (EPA)
Eicosapentaenoic acid (EPA), a major component of fish oil and related nutritional supplements, has long been considered to have multiple beneficial effects, but the molecular basis for these properties remained unknown.83,114 The first molecular-level evidence for the health-promoting contributions of EPA was provided by the discovery that EPA generates E-series resolvins 2,4 that have stereochemically defined structures acting via specific GPCRs,44,115,116 and show potent anti-inflammatory and pro-resolving actions.21,45,50,111
2.1 Biosynthesis of E-series Resolvins
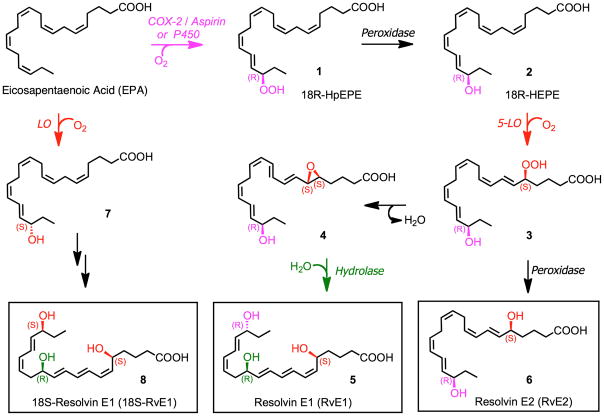
The biosynthetic pathways involved in the conversion of EPA to resolvin E1 (RvE1) and resolvin E2 (RvE2) are shown in Fig. 4. Oxygenation of EPA catalyzed by acetylated COX-2 (formed in the presence of aspirin) or via a cytochrome P450 pathway, generates 18R-hydroperoxy-eicosapentaenoic acid (18R-HpEPE, 1) which is reduced via a peroxidase to 18R-HEPE (2). A second lipoxygenation catalyzed by 5-LO forms hydroperoxide 3, which is transformed to epoxide 4 that undergoes enzymatic hydrolysis via a hydrolase enzyme to produce RvE1 (5). Alternatively, reduction of 3 via a peroxidase leads to RvE2 (6).
Figure 4.
E-series resolvin biosynthesis and metabolic inactivation.
RvE1 was monitored in healthy human volunteers given EPA and aspirin via liquid chromatography-tandem mass spectrometry (LC-MS/MS)54 using MS3 data acquisition mode and LC-MS/MS with m/z 291 transition > m/z 229. The plasma values ranged from 0.1 to 0.4 ng/ml in 6 donors. The biosynthesis of RvE1 is consistent with the proposed scheme that endothelial cells (Figs. 1, 2, 4) expressing COX-2 treated with aspirin transform vascular EPA to produce and release 18R-HEPE as reported earlier.2 When human leukocytes and endothelial cell interact within the vasculature, 18R-HEPE is rapidly converted to RvE1 via transcellular biosynthesis that in turn evokes potent actions.44 It is important to note that the precursor 18R-HEPE is not bioactive, nor is EPA, in the dose and concentration range of RvE1. In recent studies using chiral LC-MS/MS, it was shown that the isomeric 18S-RvE1 (8) is also produced in vivo in healthy human subjects.117
2.2 Resolvin E1 (RvE1)
2.2.1 Total Synthesis and Assignment of the Complete Stereochemistry of RvE1
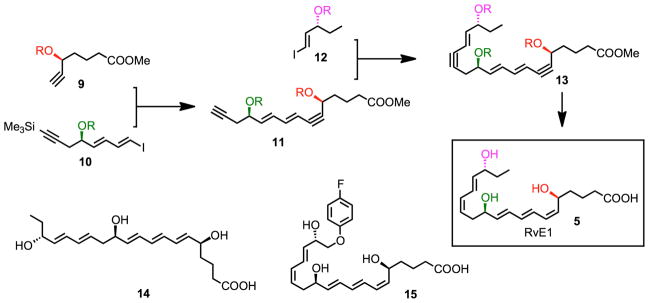
With the isolation, basic structure, and actions of RvE1 reported,2 its complete stereochemical assignment remained to be established. In order to assign the complete stereochemistry of RvE1 and establish its biological activities, RvE1 was prepared enzymatically2 and its structure was matched by its chromatographic, physical and biological properties44 with stereochemically pure synthetic material (5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid) ) that we prepared by total organic synthesis44,118 (Fig. 5). Our synthetic approach118 relied on iterative cross-coupling of isomerically pure building blocks (8, 10, 12) to form the bis-acetylenic precursor of RvE1 (13), which was reduced to RvE1 (5) via stereocontrolled hydrogenation. Several geometrical and stereochemical isomers (e.g. 14) were also prepared by organic synthesis from stereochemically defined precursors. An alternative synthetic approach was also recently reported.119 Since RvE1 is produced in subnanogram amounts in vivo, in order to assign its complete stereochemistry it was necessary to prepare both synthetic and biogenic materials for matching of their physical properties using UV spectroscopy, LC-MS/MS, GC-MS, and, most importantly, their direct comparisons to biologically isolated RvE1 and its defined biological activities. Of the isomers prepared, the matching synthetic compound eluted beneath a single peak in HPLC with UV absorbance maxima 271 nm and 234 nm, indicative of conjugated triene and diene in the molecule. MS/MS fragmentation ions were essentially identical with biologic RvE1 showing the parent ion at m/z 349 = [M-H]− and diagnostic product ions at m/z =291 and 195. Based on matching of physical and biological properties, the 18R series RvE1, a potent anti-inflammatory mediator, was assigned the complete structure 5S,12R,18R-trihydroxy-6Z, 8E, 10E, 14Z, 16E-eicosapentaenoic acid.44
Figure 5.
Design and synthesis of RvE1, RvE1 isomers and metabolically stable RvE1 analogs.
2.2.2 Biological Actions of RvE1
With the availability of larger quantities of synthetic RvE1 it became possible to identify a number of potent biological actions exhibited by RvE1. Administration of as little as 100 ng/mouse of synthetic RvE1 stopped PMN infiltration into inflammatory loci by 50~70 % in TNF-α-induced dorsal air pouch. By comparison, local administration of dexamethasone (10 mg/mouse) gives 60% inhibition and aspirin (1.0 mg/mouse) gives 70% inhibition of leukocyte recruitment.33 These results indicated that RvE1 at nanomolar level is as potent as higher doses of dexamethasone or aspirin in stopping leukocyte infiltration. In addition, another widely used anti-inflammatory, indomethacin (100 ng/mouse), gave 25% inhibition and RvE1 (100 ng/mouse) gave 50~60% inhibition of leukocyte recruitment in zymosan-induced peritonitis.44 The18S isomer gave essentially equivalent activity as RvE1 containing 18R, whereas the 6-trans,14-trans isomer showed reduced potency (~70%) for reducing leukocyte infiltration in zymosan-induced peritonitis. This is of interest because, in recent studies with chiral LC-MS/MS, 18S-RvE1 is produced in vivo in healthy human subjects.117 Additional reported anti-inflammatory and pro-resolving bioactions of RvE1 include: reduction of pro-inflammatory gene expression and protection against colitis,45 promotion of macrophage ingestion of apoptotic PMNs, 21 protection against osteoclast-mediated bone destruction and prevention of periodontitis,50 selective counterregulation of platelets and leukocytes,120 and reduction of inflammatory pain.111
RvE1 was also monitored using liquid chromatography-tandem mass spectrometry (LC-MS/MS) in healthy human volunteers given EPA and aspirin using MS3 data acquisition mode and LC-MS/MS with m/z 291 transition > m/z 229. The plasma values ranged from 0.1 to 0.4 ng/ml in 6 donors. The biosynthesis of RvE1 is consistent with the proposed scheme that endothelial cells (Figure 4) expressing COX-2 treated with aspirin transform vascular EPA to produce and release 18R-HEPE as reported earlier.2 When human leukocytes and endothelial cell interact within the vasculature, 18R-HEPE is rapidly converted to RvE1 via transcellular biosynthesis that in turn evokes potent actions.44 It is important to note that the 18R-HEPE precursor is not bioactive, nor is EPA, in the dose and concentration range of RvE1.
Similarly to other potent lipid mediators (LTs, PGs, LXs), the biological actions of resolvins are also mediated via GPCRs. For RvE1, in particular, two such receptors have been identified, ChemR2344 and BLT1.115 To determine RvE1 binding to candidate receptors identified using an NFκB screening system, tritium-labeled RvE1 was prepared by catalytic hydrogenation from synthetic bis-acetylenic RvE1 (Fig. 5, compound 13). The integrity of the synthetic 3H-RvE1 was confirmed by HPLC; radioactive compound co-eluted with synthetic RvE1 beneath a single peak in HPLC. Its characteristic UV absorbance maxima at 271 nm and 234 nm were indicative of the conjugated triene and diene in RvE1 structure. Analysis of 3H-labeled RvE1 specific binding and Scatchard transformation showed that [3H]RvE1 bound to an apparent single site on ChemR23 transfectants with high affinity (Kd=11.3±5.4 nM, Bmax=4200±1050 binding sites per cell). The RvE1 biosynthesis precursors, EPA and 18R-HEPE, did not compete for specific [3H]RvE1 binding.44 The ChemR23 RvE1 receptor is expressed in several human tissues at the mRNA level including leukocytes, gastrointestinal, testis, prostate, heart, aorta, brain, kidney, liver and lung. The role of RvE1 in many of these tissues remains to be established. [3H]RvE1 also binds to the LTB4 receptor denoted BLT1 and gives a Ki ~ 70 nM.115 RvE1 blocks the actions of LTB4 at the BLT1 receptor and reduces LTB4-stimulated responses with human PMN. For a recent International Union of Basic and Clinical Pharmacology Committee review on leukotriene and related receptors, see Bäck et al.121
2.2.3 RvE1 Metabolome: Metabolic inactivation and RvE1 analogs
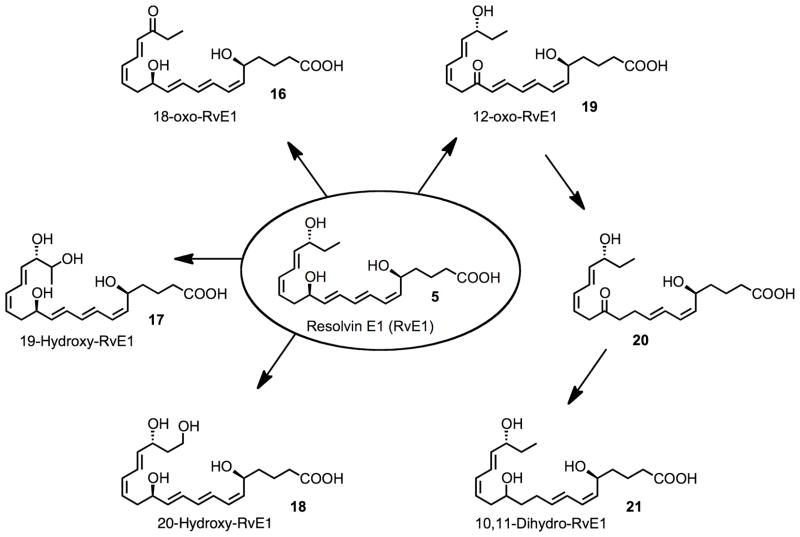
Using an LC-MS/MS approach, we found at least four separate pathways for further metabolism of RvE1 (18) are present in mammalian tissues (Fig. 6).122 These pathways appear to be species-, organ- and cell type-specific and generate the RvE1 metabolites 16–21. Although 10,11-dihydro-12-oxo-RvE1 (13) could not be directly isolated and identified in mammalian tissues studied to date, it is likely to be an intermediate in a 12-oxo-dehydrogenation-initiated route of RvE1 further metabolism. Given that the product 10,11-dihydro-RvE1 (21) is essentially biologically inactive, it is possible that this metabolite of RvE1 may serve as an inactive biomarker of RvE1 transient formation in vivo. It is of interest to point out that the 20-hydroxy-RvE1 product of RvE1 made via omega carbon 20 oxidation (Fig. 6) retains some of the activity of RvE1, namely, in vivo anti-inflammatory actions and pro-resolving actions, namely accelerating the phagocytic uptake of zymosan by macrophages. This point demonstrates that not all further metabolites of RvE1 or potentially other resolvins can be assumed to be biologically inactive. It is possible that further metabolites can retain activity and/or possibly possess new actions, whereas others are clearly pathways of RvE1 inactivation. The omega-1 hydroxylation of RvE1 to 19-hydroxy RvE1 (17) and reduction of a conjugated double bond to 10,11-dihydro-RvE1 are novel metabolic pathways identified that inactivate RvE1.122
Figure 6.
Metabolites of RvE1.
By modifying the omega portion of RvE1 via the introduction of a p-fluorophenoxy moiety (Fig. 5, compound 15), we have shown that it is possible to block the three metabolic inactivation pathways that convert RvE1 to 16–18.49 This modification of the RvE1 structure without attenuating its anti-inflammatory and pro-resolving activities could be one means to develop RvE1-based therapeutics that can serve as agonists of resolution. Moreover, identification of the RvE1 metabolome may be useful in qualifying suitable biomarkers relevant in omega-3 fatty acid supplementation studies in humans as well as monitoring their relation to endogenous biosynthesis and actions of the E-series resolvins in human tissues.
3.Resolvins from Docosahexaenoic Acid (DHA)
Docosahexaenoic acid (DHA), is the second major component of fish oil and omega-3 nutritional supplements, and its multiple beneficial effects have been long recognized,83,114 although the molecular basis for these properties have remained unknown. The recent discoveries of the endogenous conversion of DHA to D-series resolvins,79 protectins/neuroprotectins,79–81 and more recently to the maresins,82 have provided a new basis for the health benefits of DHA.
3.1 Biosynthesis of D-Series and Aspirin-Triggered D-Series Resolvins
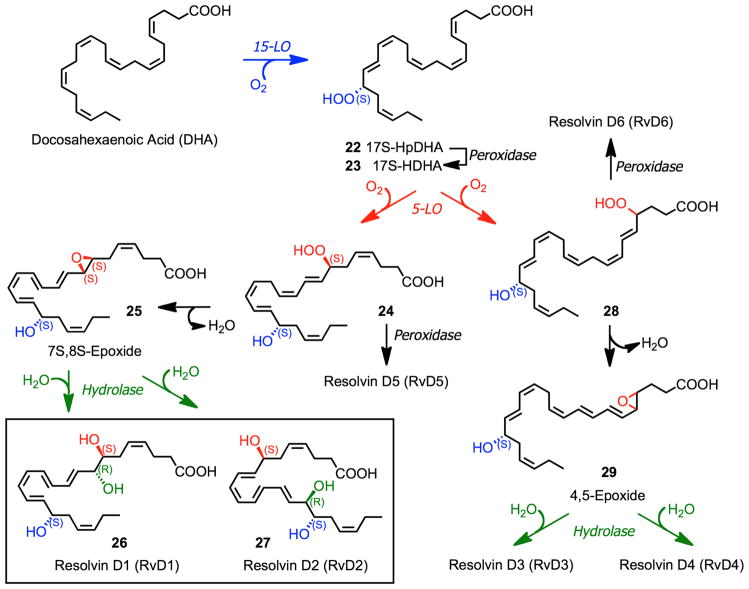
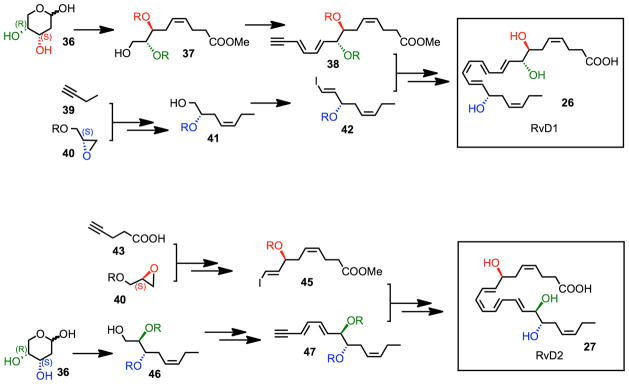
The biosynthetic conversion of DHA to D-series resolvins (RvD1 – RvD6) involves two iterative lipoxygenation steps (Fig. 7).4 Initial conversion of DHA to 17S-HpDHA (22) catalyzed by 15-lipoxygenase (15-LO) followed a second lipoxygenation via 15-LO at the C-7 position gives a peroxide intermediate (24) that is transformed to the 7S,8S-epoxide (25). Enzymatic hydrolysis of 25 generates the trihydroxylated products RvD1 (26) and RvD2 (27), while reduction of 24 forms RvD5. Alternatively, 5-LO lipoxygenation at the C-4 position forms 28 that is similarly converted to RvD3, RvD4 and RvD6.
Figure 7.
Pathways and enzymes in the biosynthesis of D-series resolvins.
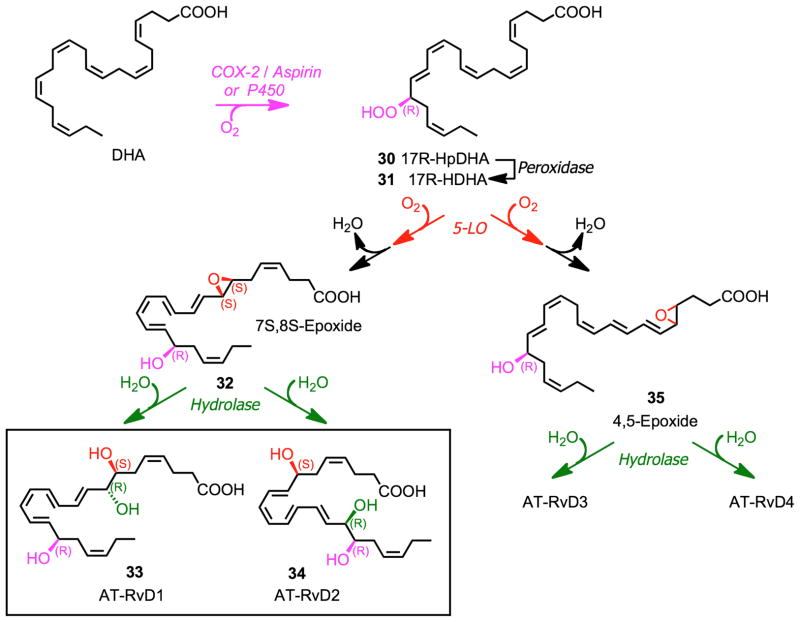
An aspirin-triggered 17R D-series resolvins were also identified (Fig. 8).79 Initial oxygenation at C-17 to form 17R-HpDHA (30) takes place in the presence of aspirin via acetylated COX-2, or via a P450 pathway. Subsequent enzymatic transformations lead to AT-RvD1 (33), AT-RvD2 (34), and other AT-resolvins.
Figure 8.
Pathways and enzymes involved in the biosynthesis of aspirin-triggered D-series resolvins.
3.2 Resolvin D1 (RvD1)
Resolvin D1 (RvD1) is biosynthesized in situ from DHA by two sequential oxygenations at the carbon 7 and 17 positions incorporating molecular oxygen predominately in the S configuration in both positions,4 while the third alcohol group at position 8 is proposed to be generated via enzymatic opening of an epoxide-containing intermediate (Fig. 7). Given the conjugated double bond system produced when C7 and C17 are oxygenated, RvD1 could theoretically be generated by either a 1,10 addition into the conjugated system involving a possible 16,17 epoxide-containing intermediate from DHA or via an enzyme-directed hydrolysis of a 7,8 epoxide intermediate (25).55 In addition to the soybean LOX-mediated generation of RvD1 utilized55, RvD1 is also produced by incubating 17-H(p)DHA (22), generated via 15-LO lipoxygenation of DHA, with activated human PMNs.79 Since this two-step biosynthesis is thought to occur in vivo via cell-cell interactions within resolving inflammatory exudates,4 evidence was sought to establish the role of an epoxide intermediate in RvD1 biosynthesis and determine which routes predominate in the biosynthesis. To this end, 17S-hydrox(peroxy) intermediate from DHA (22, 23) was incubated with human PMNs, followed by incubation with acidic methanol to trap epoxide intermediates. LC-MS-MS analysis of the trapping products obtained from these incubations revealed two major components when monitored at m/z = 389, the M-H for methoxy-trapping products. MS/MS analysis was consistent with methoxy RvD1 trapping products by the presence of the following prominent daughter ions: m/z 374 [a-CH3], 371 [a-H2O]; 357 [a-MeOH]; 345 [389-CO2]; 339 [a-MeOH-H2O]; 302 [320-H2O]; 275 [290-CH3]; 257 [275-H2O]; and 231 [275-CO2]. The presence of these ions is consistent with the formation of a methoxy-containing trapping product formed from an epoxide-containing intermediate derived from the 17S-H(p)DHA precursor in human PMN. The material in Ib gave essentially the same prominent ions obtained for Ia.55 The identification of these ions in MS-MS from both trapping products established the role of an epoxide intermediate. In these, both were consistent with a 7S-hydroxy-8-methoxy-containing trapping product as well as a 7S-hydroxy-16-methoxy-containing trapping product. Specific ions in both products identified that distinguished a preference in the formation of 8-methoxy- versus a 16-methoxy-containing trapping product generated from a 7S,8S-epoxy-17S-containing intermediate were not evident. Thus, it is likely that a carbonium cation is produced in the H+, MeOH trapping conditions, giving both 16-methoxy and 8-methoxy trapping products. In this regard, 17S-HDHA gave similar trapping products with activated human PMN but in lower amounts than with 17S-H(p)DHA as substrate, and the isolated 15-LOX also gave the methoxy-trapping products.55 The identification of these methoxy trapping products (both 8-methoxy- and 16-methoxy-containing) provides direct evidence for the formation of an epoxide intermediate shown in the biosynthesis of RvD1 and RvD2 in Fig. 7.
3.2.1 Total Synthesis and Stereochemical Assignment of RvD1
To assign the complete stereochemistry of natural RvD1 and determine whether RvD1 as well as its aspirin-triggered form AT-RvD1 (Figs. 7 and 8) share biological properties, as well as match their reported properties and actions,4,79 it was essential to first establish the spectroscopic and physical properties of the synthetic materials.55 The structure and stereochemistry of synthetic RvD1 and AT-RvD1 was unambiguous on the basis of their total synthesis from chiral starting materials of known stereochemistry (Fig. 9). The R/S configurations of C-7, C-8 and C-17 were directly derived from starting materials of the same chirality. Let us consider, for example, the C-17 S alcohol chirality was retained from a protected (S)-(-)-glycidol derivative (40), while and the C-17R chirality was produced via the enantiomeric glycidol starting material. The 7S,8R diol moiety was introduced by utilizing a carbohydrate starting material with the same stereochemistry (36). Iterative coupling and stereoselective alkyne hydrogenation of these isomerically pure building blocks produced isomerically pure RvD1 (26). The Z/E configuration of the double bonds was determined and confirmed using 1H-NMR (COSY). RvD1 and AT-RvD1 each give similar spectra, yet displayed different chromatographic behaviors with RP-HPLC.55
Figure 9.
Synthetic scheme for RvD1 and RvD2.
Liquid chromatography analysis gave UV chromatograms when monitored at 301 nm with major peak AT-RvD1 at 13.9 min separating and eluting before RvD1 at 15.1 min. The column and mobile phase parameters used here permitted separation of these two diastereomers by ~ 1.2 minutes. The carboxy-methyl esters of RvD1 and AT-RvD1 also resolved using essentially identical conditions. Further analysis of both RvD1 and AT-RvD1 demonstrated the major anion for both MS spectra was at m/z 375, which represents [M-H] for both RvD1 and AT-RvD1. These were consistent with the original mass spectra and structural elucidation of these resolvins produced in vivo in mouse exudates and with isolated human leukocytes 4. As expected, analysis of the MS-MS spectrum obtained for both RvD1 and AT-RvD1 gave essentially the same fragmentation patterns, and matched the ions reported earlier for endogenous RvD1 and AT-RvD1 isolated from murine inflammatory exudates and human PMN.4
Having established the physical properties of synthetic RvD1, we next determined whether it was identical to the biologically active enzymatically generated material as well as assign the geometry of its conjugated double bonds and chirality of the alcohol group at the carbon 8 position that remained to be established.4,79 It was essential to take this approach because the nanogram amounts obtained from human cells and inflammatory exudates permitted assigning the basic structure and bioactions of RvD1 but precluded direct determination of the complete stereochemistry of the biologically derived RvD1.4 To this end, RvD1 was prepared by incubation of DHA with isolated 15-LOX type I (i.e., soybean LOX type IV) using the one-pot incubation procedure that employed micellar substrate presentation.79,123,124 Liquid chromatographic analysis of this material resulted in one major component at 7.5 min monitored at 301 nm and two minor ones at 6.1 min and 6.5 min. Each of these three components exhibited a triplet UV spectrum with λmax at 301 nm with shoulders at 289 nm and 315 nm, characteristic of a conjugated tetraene system. Similar analysis with the synthetic material resulted in a major component at 7.6 min and a minor at 6.6 min when monitored at 301 nm. Both of these demonstrated a UV spectrum identical to that of the biogenic/enzymatic material: λmax at 301 nm with shoulders at 289 nm and 315 nm. Human leukocyte-derived RvD1 coeluted with the major product, obtained with isolated LOX, and further analysis of the biogenic and synthetic mixtures resulted in co-elution at 7.5 min. These results demonstrate that the predominant component of the RvD1 mixture generated by the isolated LOX incubations also matched synthetic RvD1.55
LC-MS-MS analysis of the enzymatically generated RvD1 matched that of the RvD1 prepared by total organic synthesis.55 To obtain more evidence for matching, GC-MS analysis was carried out as in the original identification and basic structural elucidation.4 RvD1 was treated with diazomethane and converted to its corresponding trimethylsilyl derivative and subject to GC-MS. Based on retention time, the C-value was determined to be 25.9 ± 0.2. This fragmentation pattern, UV spectrum, and C-value are consistent with those obtained for GC-MS analysis of biogenic RvD1 and those prepared by total organic synthesis, thereby firmly establishing the overall stereochemistry.
3.2.2 RvD1 Metabolome: Metabolic Inactivation of RvD1 and AT-RvD1
D series resolvins are generated and act locally at sites of inflammation,79,80 much like their arachidonic acid-derived cousins, LXs. LXs are rapidly inactivated by eicosanoid oxidoreductases (EORs).125,126 Along these lines, further enzymatic conversion of the D series resolvins remained of interest. Therefore, we sought to determine whether RvD1 and AT-RvD1 were substrates for the EOR-initiated further metabolism. To this end, RvD1, AT-RvD1, or, for purposes of direct comparison, LXA4 (~ 20 μM) was incubated with EOR (0.05 μg) and the reactions were monitored by the formation of NADH, an essential cofactor for the enzyme.55 While LXA4 was converted most readily, RvD1 was converted to a similar extent within 25 min in these incubations. The kinetics for conversion, however, were slower for RvD1 than LXA4 as can be seen by the statistically significant difference in NADH formation at 5 min (~10 μM NADH formation versus ~ 6 μM NADH formation with LXA4 and RvD1 as substrates, respectively, p < 0.05, two-tailed Student’s t-test). Interestingly, AT-RvD1, which differs from RvD1 only in the configuration of the 17-hydroxyl group, namely 17R for AT-RvD1, was essentially resistant to rapid conversion.
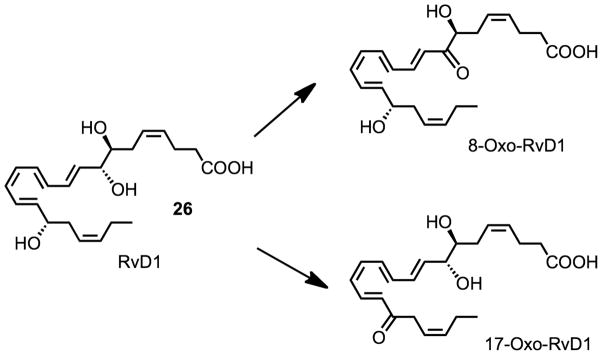
Since RvD1 was converted by the EOR, we determined the structure and bioactions of the metabolite(s). Using reverse phase (RP)-HPLC analysis, the UV absorbance spectra of RvD1-ME and the metabolites were measured. The presence of a conjugated tetraene within RvD1 is responsible for its characteristic triplet chromophore with a λmax of 301 nm. LC analysis of the metabolites yielded 2 distinct products that eluted at 15.9 min and 17.6 min, both containing a single broad UV absorbance at λmax = 351 nm. Since the EOR utilized, i.e., 15-prostaglandin dehydrogenase, oxidizes alcohols to their respective ketones as in the inactivation of PGE2 reviewed in Ref.127 and LXA4,125, the potential single oxo-containing products generated might be 7-oxo-RvD1, 8-oxo-RvD1 or 17-oxo-RvD1. Formation of a 7-oxo product from RvD1 would retain the same conjugated tetraene structure; the absence of this chromophore indicated that 7-oxo-RvD1 is not likely to be generated by this EOR. Both 8-oxo and 17-oxo-RvD1 would extend the conjugation of the tetraene system to the corresponding carbonyl group, thereby lowering the difference in energy (ΔE) between the ground and excited electronic states. Lowering of the ΔE results in a red-shift of the absorption maximum analogous to the shift in 15-oxo-LXA4.125 Therefore, the presence of this red-shifted UV absorbance for compounds I and II suggests that the two main metabolites were 8-oxo-RvD1 and 17-oxo-RvD1 (Fig. 10). The 17-oxo-RvD1 was also produced from RvD1 in murine lung and identified using the same criteria and prominent ions in its MS-MS spectrum.55
Figure 10.
Metabolites of RvD1.
Having identified these further metabolic products of RvD1, we next determined whether 8-oxo-RvD1 and 17-oxo-RvD1 also displayed the in vivo anti-inflammatory actions characteristic of their precursor RvD1. The 8-oxo-RvD1 at 10 ng/mouse limited PMN infiltration in murine peritonitis by a statistically significant 41% (p < 0.05, two-tailed unpaired Student’s t-test) that was comparable to RvD1 (44%, p < 0.05). In sharp contrast, 17-oxo-RvD1 did not decrease PMN infiltration in vivo in a statistically significant manner. When compared to the actions of RvD1, 8-oxo-RvD1 was as effective (~93% the activity of RvD1) while 17-oxo-RvD1 was significantly less bioactive (~ 10%, p < 0.05, two-tailed unpaired Student’s t-test). These results indicated that 17-oxo-RvD1 is the inactivation product of RvD1.55
3.2.3 Biological Actions of RvD1 and AT-RvD1
The actions of RvD1 and AT-RvD1 on human PMN transendothelial migration, the first event in acute inflammation,4,128,129 were compared by exposing PMN to either RvD1 or AT-RvD1 (0–1000 nM) to assess their impact with fMLP-stimulated (100 nM) transendothelial migration. RvD1 and AT-RvD1 each stopped PMN transmigration in a concentration-dependent fashion (p<0.01 for both AT-RvD1 and RvD1). While both molecules potently reduced PMN transmigration by as much as 65±8% at 1μM concentrations of AT-RvD1 (EC50 ~30 nM), no statistically significant differences were noted between RvD1 or its aspirin-triggered form ATRvD1 at the concentrations tested (p = not significant by ANOVA). An approximately 50% reduction in PMN transmigration was obtained with concentrations as low as 10 nM.
Earlier results demonstrated that both 17S and 17R D series resolvins (RvD and AT-RvD respectively) significantly reduce PMN infiltration in vivo as effectively as equivalent doses of indomethacin.79 Because of the presence of additional resolvins within the preparations of isolated RvD and AT-RvD series,4,79 the relative potencies of RvD1 remained to be established in vivo. Using purified synthetic RvD1 and AT-RvD1, we determined their potency in vivo. Both RvD1 and AT-RvD1 limited total leukocytic infiltration at each dose tested with the maximal decrease in total leukocytes with as little as a 100 ng dose per mouse. At the 10 ng dose, AT-RvD1 reduced leukocytic infiltration to a greater extent than RvD1 (~ 23 % and ~ 8–10 % respectively, p < 0.05, two-tailed Student’s t-test). Both RvD1 and AT-RvD1 exhibited a dose-dependent reduction in PMN infiltration with similar potency and efficacy (maximal inhibition of ~ 35% at dose of 100 ng/mouse). Both RvD1 and its AT form were bioactive at the lowest dose administered (1 ng/mouse), which indicates the very potent actions of these DHA-derived mediators.
Recently, we demonstrated that RvD1 activates the lipoxin receptor (ALX/FPR2) as well as the GPR32 receptor, an orphan GPCR. The actions of RvD1 on human PMN are pertussis toxin sensitive, decreased actin polymerization and block LTB4 regulated adhesion molecules (b2 integrins). Synthetic [3H]-RvD1 was prepared, which revealed specific RvD1 recognition sites on human leukocytes. Screening systems to identify receptors for RvD1 gave 2 candidates – ALX, a lipoxin A4 receptor, and GPR32, an orphan – that were confirmed using a β arrestin based ligand receptor system. Nuclear receptors including RXRα and PPAR-α, δ, γ were not activated by either RvE1 or RvD1 at bioactive nM concentrations, important in the resolution mechanism. RvD1 enhanced macrophage phagocytosis of zymosan and apoptotic PMN that was increased with overexpression of human ALX and GPR32 and reduced with selective knockdown of these GPCRs. The surface expression of both receptors ALX and GPR32 in human monocytes was upregulated by zymosan and GM-CSF. Thus, RvD1 specifically interacts with both ALX and GPR32 on phagocytes63.
3.3 Resolvin D2 (RvD2)
Resolvin D2 (RvD2, 27) was originally identified during resolution,4 and it is biosynthesized from docosahexaenoic acid (DHA) via sequential lipoxygenation and epoxide hydrolysis (Fig. 7). Recently,60,130 we established the complete stereochemistry and actions of RvD2, and also investigated whether it preserves host immune function to facilitate resolution of inflammatory sepsis.
3.3.1 Total Synthesis and Stereochemical Assignment of RvD2
The complete stereochemistry of endogenous RvD2 was determined by physical matching with compounds prepared by total organic synthesis (Fig. 9) from isomerically pure starting materials (36, 40), and in accordance with the basic structure determined in resolving exudates.4 This approach was needed, as pointed out above, because the nanogram amounts of endogenous RvD2 isolated precluded direct NMR analysis. The double bond geometry of synthetic material was validated by 1H NMR.60 The biosynthesis of RvD2 (Fig. 7) proceeds via 17-lipoxygenation of DHA to 17S-hydroperoxy-4Z, 7Z, 10Z, 13Z, 15E, 19Z-docosahexaenoic acid (17S-HpDHA) (22), which is enzymatically transformed to a 7S,8S-epoxide-containing intermediate (25)4,79 by human leukocytes. This enzymatic transformation and activity involves 5-lipoxygenase (LO) and its epoxide generating activity.131 These steps can occur within a single cell type or via transcellular biosynthesis. Human eosinophils are rich in 15-LO and can convert DHA to 17-HpDHA, for example, which polymorphonuclear neutrophils (PMNs) can convert to RvD2. Actively phagocytosing polymorphonuclear neutrophils (PMN) converted resolvin precursor 17-HpDHA to RvD2 as determined by LC-MS/MS-based lipidomics. A total ion chromatogram (m/z 375 [M-H]) of human leukocyte-derived RvD2 showed that a conjugated tetraene UV-chromophore was present. Synthetic material showed an exclusive and prominent peak with retention time (RT) and UV-spectrum essentially identical to leukocyte-derived RvD2. Co-injection of synthetic and leukocyte-derived RvD2 led to an increase in intensity and co-elution.60
To establish the physical properties, their tandem mass spectra (MS) were analyzed, with essentially identical MS fragmentation, and diagnostic ions in agreement with original assignments for endogenous RvD2.4 To validate the biosynthetic pathway, activated human PMN were incubated with deuterium labelled 17S-HpDHA-d5 or DHA-d5; RvD2 containing the d5 label was biosynthesized. In these experiments, the parent ion increased to m/z 380 [M-H], and neutral loss ions were reflective of d5-containing fragments. To further provide evidence for the structural assignment,4,132 derivatized RvD2 was subjected to GC/MS. Derivatized RvD2 C-value was 25.2 ± 0.1 and its spectrum (Ref. 60, supplementary materials) showed diagnostic ions at m/z 479, 435, 229 and 171.4 Collectively, matching of synthetic and leukocyte-derived RvD2 (by RT, diagnostic MS ions and derivatization) established that the complete stereochemistry and double bond geometry of endogenous RvD2 is 7S, 16R, 17S-trihydroxy-4Z, 8E, 10Z, 12E, 14E, 19Z-docosahexaenoic acid.
3.3.2 Biological Actions of RvD2
RvD2 displayed potent actions in microbial peritonitis, with a drastic ~70% reduction in zymosan-stimulated PMN infiltration at doses as low as 10 pg. Importantly, Δ10-trans-RvD2 isomer was essentially inactive, indicating that specific geometry of endogenous RvD2 is required for bioactivity. To determine whether RvD2 decreases leukocyte-endothelial interactions, cremasteric microcirculation was analyzed. Platelet activating factor (PAF; 100 nM)131,132 superfusion caused increased leukocyte adherence and emigration that was markedly reduced by 1 nM RvD2. Representative microcirculation before and after RvD2 superfusion are in supplementary movies. These potent RvD2 actions were recapitulated with human cells to identify potential cellular directed actions (i.e. endothelial cells and PMN). RvD2 potently reduced PAF-stimulated capture and adhesion of PMN by HUVECs under flow (see Ref. 60, supplementary materials).133 Consistent with the impact of RvD2 on leukocyte:endothelial interactions, RvD2 diminished PAF-stimulated CD62L shedding on isolated human PMN, and CD18 surface expression. RvD2 alone did not alter PMN adhesion molecule expression.
To obtain further evidence for direct actions of RvD2 on human leukocytes, we monitored reactive oxygen species (ROS). Importantly, RvD2 did not stimulate extracellular superoxide, while it potently reduced C5a-stimulated extracellular superoxide generation. We also assessed the contribution of nitric oxide (NO), an established anti-adhesive mediator134,135 in RvD2-reduced leukocyte adherence in post-capillary venules. The non-selective nitric oxide synthase inhibitor, L-NAME, before addition of RvD2, partially reversed the decreased leukocyte adherence and emigration. To obtain additional evidence for NO generation by RvD2 in vivo, vascular fluorescence was monitored.60 Topical administration of RvD2 (100 pg/ear) increased fluorescence intensity, whereas lower doses (1 and 10 pg) were ineffective. L-NAME given before topical RvD2 application abolished this response, indicating that RvD2-stimulated vascular responses at this dose were NO-dependent. Of note, i.v. injection of RvD2 at doses that inhibited PMN infiltration in peritonitis (10 pg) did not increase fluorescence intensity indicating that only local elevated doses of RvD2 stimulated vascular responses. Additionally, RvD2 superfusion (1 nM) does not cause an increase in vascular permeability. Topical RvD2 (10 or 100pg) did not induce leukocyte infiltration into ear skin compared to chemoattractant leukotriene B4. Hence, high dose/focal delivery of RvD2 stimulates rapid NO release consistent with its anti-adhesive effects but not produced at levels that could be pro-inflammatory.
Corroboratory results were obtained with HUVECs whereby RvD2 dose-dependently stimulated NO generation,60 suggesting topical actions were likely mediated via endothelial nitric oxide synthase (eNOS). RvD2 also stimulated vasoprotective prostacyclin, as monitored by the stable metabolite 6-keto-PGF1α (a hydration product of prostacyclin); this dose-response proved bell-shaped like other lipid mediators.7,24,136 RvD2-stimulated prostacyclin and NO were pertussis-toxin sensitive implicating a role for G-protein coupled receptor(s). Thus, RvD2 regulates leukocyte adherence via both direct actions on PMN (vide supra) and endothelial vasoactive substances.
The anti-inflammatory and pro-resolving actions of RvD2 were next evaluated in cecal ligation and puncture (CLP), an established murine microbial sepsis that closely resembles human pathology.137,138. Omega-3 fatty acids are beneficial in some inflammatory conditions, including sepsis,139–141 although the mechanistic basis underlying protection is still emerging. To this end, RvD2 significantly reduced the amount of live aerobic bacteria in both blood and peritoneum at 12 h post-CLP, while Δ10-trans-RvD2 was essentially not active. This was associated with a significant reduction in total leukocytes and specifically, PMN infiltration into the peritoneum.60 Intraperitoneal delivery of RvD2 at 1h post-CLP also reduced both blood and peritoneal bacteria. Given the role of macrophages in clearance of bacteria, cellular debris and apoptotic PMNs to facilitate inflammation-resolution,7,24 RvD2 treatment promoted phagocyte-dependent bacterial clearance observed in inguinal lymph nodes.60 Evidence for direct macrophage actions were obtained in vitro, where RvD2 potently enhanced macrophage phagocytosis of opsonized-zymosan.60 Cytokines were monitored during CLP both locally (peritoneum) and systemically (plasma). RvD2 drastically reduced levels of pro-inflammatory cytokines associated with poor outcomes in sepsis,138 namely IL-6, IL-1β, IL-23 and TNF-α.60 RvD2 reduced cytokine levels while enhancing bacterial clearance, a response also observed for macrophage scavenger receptor A.142 Thus, it is plausible that RvD2 prevents persistent amplification signals downstream of pattern-recognition receptors, dampening responses of classically activated macrophages.143,144
RvD2 also decreased IL-17, as well as IL-10, which is of interest in light of its detrimental impact on survival in sepsis.145 Thus, RvD2 differs in action from lipoxin A4. Lipoxin A4 stimulates IL-10.146 High levels of both pro- and anti-inflammatory cytokines, including IL-10, the “cytokine storm”, are predictive of early mortality in sepsis, and diminishing cytokine levels as well as those of IL-10 appear to be beneficial in human sepsis.147,148 Pro-inflammatory mediators, including prostaglandin E2 (PGE2) and LTB4 were also decreased in peritoneum by RvD2.60 Of interest, RvD2 directly enhanced PMN E. coli phagocytosis that was accompanied by an increase in intracellular ROS. However, RvD2 does not possess direct antibacterial activity. Accordingly, RvD2 dramatically increased survival rates among CLP-operated mice and activity levels12h post-CLP were resumed.
RvD2 proved to be a potent D-series resolvin that protects from excessive leukocyte infiltration and overzealous cytokine production, as well as enhanced clearance of microbes, preventing sepsis-induced lethality. Sepsis remains a clinical challenge, with high mortality rates and increasing prevalence.149,150. Given its uncontrolled inflammatory pathogenesis, anti-inflammatory therapies are used for sepsis management, but have ultimately failed due primarily to sustained immunosuppression.149 These results indicate that RvD2 is a potent endogenous mediator that actively promotes resolution. Also, they suggest new approaches to treat sepsis that do not compromise host-defence via immune suppression, because the resolvins are not immunosuppressive.
4. Protectins from Docosahexaenoic Acid (DHA)
The results reviewed above provide a critical biological framework for the fundamental biologically important actions of DHA derived mediators. In this section, we consider results obtained from several independent lines of investigation required to address the structure of the natural potent bioactive protectin D1/neuroprotectin D1 (NPD1/PD1). For readers interested in the neuroscience aspects and role of DHA and NPD1; see the recent review from Bazan et al.151
4.1 Biosynthesis of Protectin D1/Neuroprotectin D1 (PD1/NPD1)
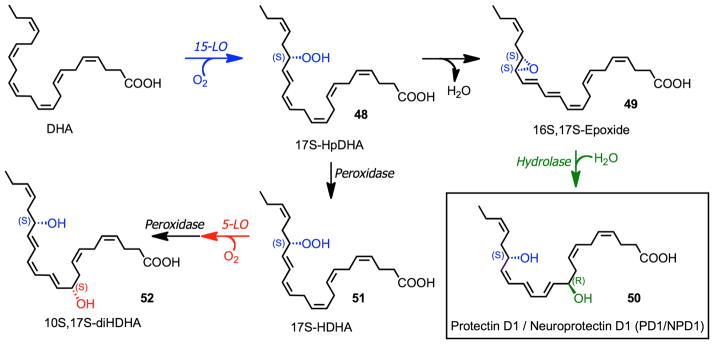
The biosynthetic scheme for NPD1/PD1 is shown in Fig. 11 from results reported in Ref. 51. Following 17S-HpDHA (48) formation from 15-LO action on DHA, an epoxide intermediate (49) is formed that requires enzymatic transformation to obtain the correct double bond geometry, namely cis, trans, trans present in NPD1/PD1 (50). This double bond geometry and chirality of the carbon 10 position and the R configuration were established from the matching of synthetic compounds of defined chirality. Of interest, the double dioxygenation products 7S,17S-diHDHA (resolvin D5) and 10S,17S-diHDHA (52) appear to be less active than endogenous or synthetic NPD1/PD1. Without endogenous biosynthesis studies or assessment of biological actions, it is surprising that a claim152 could be made for assessment of the complete stereochemistry of NPD1 based only on NMR results obtained for 10S,17S-diHDHA from plant lipoxygenase-prepared material with DHA.
Figure 11.
Pathways and enzymes involved in the biosynthesis of protectin (PD1/NPD1) and 10S,17S-diHDHA.
4.2 Total Synthesis and Stereochemical Assignments of PD1/NPD1
As with other potent lipid mediators, such as the prostaglandins and leukotrienes the arachidonate derived eicosanoids,16 it is important to establish the stereochemistry of the mediator of biological functions because many structural related isomers can be less active, inactive or even in some cases display opposing biologic actions as a result of subtle changes in stereochemistry that are recognized by cellular receptors in biologic systems. Subtle changes in, for example, the stereochemistry of lipoxin A4 can dramatically reduce potency of this molecule (i.e. from R to S and cis to trans double bonds).153 To confirm the proposed basic structure and establish the complete stereochemistry these studies on the 10,17S-docosatriene termed NPD1/PD1 included results obtained from i) biosynthesis studies ii) matching of materials prepared by total organic synthesis with defined stereochemistry and iii) the actions of these and related compounds in other biological systems.4,51,64,79,154,155 We also consider here the chronology in which these appeared with the goal of providing a rigorous account of the evidence that supports the structure of the bioactive NPD1/PD1. Investigations along these lines were essential in order to establish the complete structure of the potent NPD1/PD1 and related endogenous products biosynthesized from DHA in vivo/in situ because of the small amounts of NPD1/PD1 (nanograms-micrograms) attainable from biological systems that precluded at the time direct stereochemical analyses of the products by NMR. Thus in this section, we focus on the evidence for NPD1/PD1 structural elucidation using the approach for structural elucidation of the resolvins and their complete stereochemical assignments as outlined above; for additional details see other recent reviews.7,12,151
The first evidence for the conversion of labeled DHA to metabolites was obtained from REP cells was reported and using available inhibitors of the time Bazan et al suggested a role for lipoxygenase in the biosynthesis of these structurally previously unknown DHA-derived products.156 The structure of 10,17-docosatriene was first disclosed while reporting on the characterization of the novel bioactive resolvins identified using an unbiased systems approach with resolving inflammatory exudates and LC-MS-MS-based lipid mediator metabolomics.4 These new bioactive molecules (resolvins and docosatrienes) were biosynthesized from omega-3 essential fatty acids during the resolution phase of acute inflammatory reactions in vivo that promote resolution of inflammation in vivo (see Figure 8 in Ref. 4 and related text). Since the DHA derived compounds were identified in self-limited or resolving inflammatory exudates additional evidence was obtained for their biosynthesis from both murine brain and human vascular endothelial cells and human leukocytes.4
These investigations focused initially on aspirin and its impact in the biosynthesis of 17R-hydroxy-containing resolvins and related structures.2,4 The results indicated that new DHA-derived molecules reduced cytokine IL-1β production by human glioma cells stimulated with the cytokine TNFα and importantly that DHA stored in the brain is converted to the new molecules. It is noteworthy that exudates obtained from mice given DHA, following extraction and transferred to other mice with peritonitis reduced neutrophil infiltration in vivo in the recipient mouse indicating the presence of potent bioactive products from DHA within the original inflammatory exudates.4 In parallel, experiments with isolated primary human cells were carried out to examine potential biosynthetic routes involved in the biosynthesis of these mediators. In this context, hypoxic endothelial cells exposed to growth factors and inflammatory stimuli in vitro converted ω-3 essential fatty acids to intermediates taken up by human leukocytes. The intermediate was further converted to bioactive products that showed potent activities relevant in the control of inflammation in a process known as transcellular biosynthesis.2,4,44
In these experiments with incubations or inflammatory exudates taken without aspirin treatment, 17S-HDHA and corresponding 17S-hydroxy-containing dihydroxy- and tri-hydroxy- products were uncovered in both murine exudates and isolated human cells.4 The formation of some of these was modeled in vitro and formed by sequential lipoxygenation reactions. These products were investigated with 15-lipoxygenase and included the double dioxygenation products namely, 7S,17S-diHDHA and 10,17S-diHDHA were isolated and identified as well as tri-hydroxy-containing products formed via epoxide-containing intermediates.4 The well-established lipoxygenase reaction mechanism implicated that new products were 7S,17S-diHDHA (coined resolvin D5) and 10,17S-diHDHA, which could easily be produced in microgram amounts in vitro. Both products contained 2 diene conjugated double bond systems each in a trans, cis geometry. Along these lines consider for example, the earlier studies with 5S,12S-diHETE formed from arachidonate via double dioxygenation which is an isomer of the potent chemoattractant LTB4.16 This isomer 5S,12S-diHETE separates in SP-HPLC from LTB4 yet has very similar mass spectra to each other. However, 5S,12S-diHETE displays little if any of the potent actions or chemotactic activity for its isomer LTB4 (Ref. 157 and refs. within). Given the size of the report in Ref. 4 and new composition of matter,123,124 these pathways, biosynthesis and actions of these new compounds were submitted for publication in another manuscript which appeared later.79 Thus, in addition to the 17S-series resolvins, DHA is also precursor to a novel family of endogenous molecules that possess a conjugated triene system, hence the term docosatrienes, which are biosynthesized in blood, leukocytes, brain and glial cells.79
The main bioactive member of the docosatrienes from the 17S-hydroxy-containing intermediates proved to be 10,17S-docosatriene in addition to the resolvins.79 Human PMN convert 10,17S-docosatriene to its omega-22 hydroxy product from DHA that is likely an inactivation route for this pathway. As with the other new bioactive products from omega-3 precursors the basic structures and proposed stereochemical assignments reported were based on results of biosynthesis studies and given as tentative stereochemical assignments (see below). At the time, some of the newly identified compounds were matched to reference compounds prepared with plant lipoxygenases. These included, for example, 17S-HDHA and resolvin D5 (7S,17S-diHDHA), which matched to those profiled by LC-MS-MS in exudates and with isolated human cells. It was clear that the 10,17S-docosatriene from exudates did not co-elute with the major 10,17S-docosatriene produced in vitro with plant enzymes suggesting it was an isomer but that this system could be used to prepare related docosatrienes via the LTA4 synthase activity of lipoxygenases to further probe the bioactions of 10, 17S-docosatriene123,124 while complete matching and total synthesis were in progress. These US patent applications123,124,158 filed in 2000–2003, in addition to the novel structures disclosed for clinical development, also described novel micellar suspensions of substrates used with plant lipoxygenases and procedures to prepare and HPLC isolate the small quantities of novel compounds needed given their potent and selective activity to assess their bioactions.
The microglial cells biosynthesized both 17S series resolvins and the 10,17S-docosatriene. Importantly, evidence for a novel omega-22-hydroxy-16,17S-trihydroxydocosatriene was obtained from these cells and human PMN that suggested that the 10,17S-docosatriene was biosynthesis via a 16(17S)-epoxide containing intermediate because the identified vicinal diol could be a product of this epoxide intermediate. Thus, a series of acid-alcohol trapping experiments were undertaken to address the potential role of epoxide-containing intermediates in the biosynthesis of the bioactive compounds, in particular the 10,17S-docosatriene. Indeed evidence for epoxide-containing intermediates in the biosynthesis of docosatrienes and 17S series resolvins was obtained from human PMN.79 In these incubations with isolated human cells, two 16-OCH3 and two 10-OCH3 methoxy-trapping products likely all-trans in their triene conjugations were obtained. Their mass spectral identification implicated a 16(17S)-epoxide intermediate is produced and was thus proposed in the biosynthetic pathway for the bioactive 10,17S-docosatriene.79 Also, this 10,17S-docosatriene proved to display potent actions at 1nM to 50 nM with human glial cells and produced 10,17S-docosatriene that reduced IL-1B production and evoked ligand-operated extracellular acidification with glial cells in a microphysiometer.79 These results indicated that DHA is precursor to novel potent protective mediators and that 10,17-docosatriene carried potent anti-inflammatory activity in mice in vivo and with isolated human cell types in vitro as well as activated surface receptors present on human glial cells to regulate their function. Thus, the novel 10,17S-docosatriene later coined NPD1/PD180 (Figure 9), given its potent bioactions in vivo, was identified first in Refs. 4,79 and found to display potent anti-inflammatory actions, i.e., reducing PMN numbers in vivo and reducing the production of inflammatory cytokines by glial cells in these initial structural elucidation studies.4,79 Moreover, during the resolution phase of peritonitis, unesterified DHA levels increase in resolving exudates, where it appears to promote catabasis or the return to homeostasis following tissue insult, via conversion to D-series resolvins and 10,17S-docosatrienes20 by shortening the resolution interval of an inflammation-resolution time course in vivo.21
Next, collaborations were initiated for this pathway, which demonstrated that 10,17S-docosatriene was produced during strokes in the ipsilateral hemisphere of murine brain following focal ischemia. In these collaborations we also demonstrated potent bioactions in this system, where the 10,17S-docosatriene limits the entry of leukocytes and sharply reduced the size of the brain injury.80 Also, the 10,17S-docosatriene is formed in retinal pigmented epithelium (RPE) with ARE-19 cells where it is also organ protective. Hence, we introduced the term neuroprotectin D1 given its potent protective actions.81. These findings in the ARPE cells,81 in inflammatory murine exudates, human PMN and glial cells4,79 underscored the need to establish the complete stereochemistry of endogenous biologically generated active 10,17S-docosatriene, namely the chirality of its carbon 10 position alcohol and its triene double geometry (see Figs. 12 and 13). In recognition of its wide scope of formation and bioactions, protectin D1 (PD1) was introduced and used to denote the structure of this chemical mediator in the immune system. The prefix Neuro before protectin D1 (NPD1) was proposed to denote the location of the biosynthesis and potent neuroprotective actions.155 It was also apparent that NPD1/PD1 was a member of a larger family of 17-hydroxy–containing docosatrienes termed protectins (Fig. 11).
Figure 12.
Synthetic scheme for PD1/NPD1 synthesis and related isomers.
Figure 13.
Potent stereoselective actions of PD1/NPD1 stereoisomers in murine peritonitis.
Biosynthesis and function studies were undertaken with human TH2-skewed peripheral blood mononuclear cells (PBMC) that specifically express 15-LO type 1 and converts DHA to the 10,17S-docosatrienes by serving as a 17-lipoxygenase with DHA as substrate. When produced by these cells, PD1 promotes T cell apoptosis via the formation of lipid raft-encoded signaling complexes and reduced T-cell traffic in vivo. These results were consistent with the physical properties of NPD1.
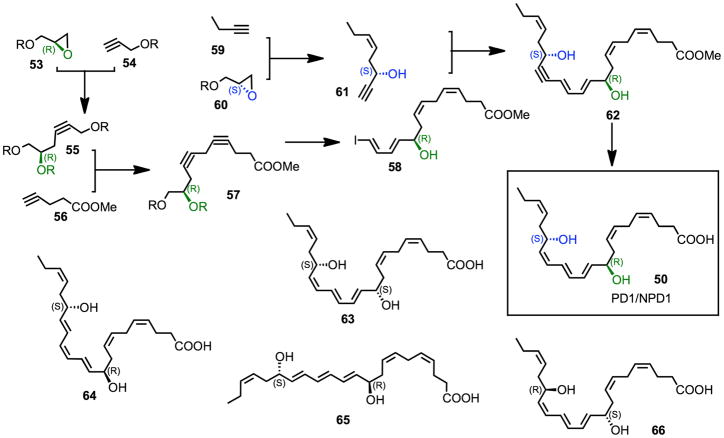
Stereochemically pure PD1/NPD1 (50) and selected stereoisomers (52, 63–66) were prepared by total organic synthesis (Fig. 12). The 10R and 17S hydroxyl groups were introduced from enantiomerically pure glycidol derivatives (53, 60), and the Z/E geometry of the various double bonds were secured via stereocontrolled processes, leading to the acetylenic precursor 62, which was converted to PD1/NPD1 (50) via selective reduction and hydrolysis. The isomerically pure synthetic materials were used to determine the complete stereochemistry of the PBMC DHA-derived product (Figs. 11, 12). NPDl/PDl generated by human PBMC carried the complete stereochemistry of (10R,17S)-dihydroxydocosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid (50) and was matched to the most potent bioactive product using several dihydroxytriene-containing DHA-derived products isolated from human PBMC, human PMN and murine exudates.51,155
During these investigations, it was reported that, as a novel anti-inflammatory compound, NPD1 had the complete structure of 10S,17S-diHDHA (52).152 This assumption was based on results from isolated lipoxygenase with DHA without any mammalian cell/tissue biosynthetic analyses and/or authentic neuroprotectin D1, as defined earlier.4,79,81 Importantly, neither bioactivity of the product nor appropriate comparisons with authentic NPD1 were presented to support the conclusions regarding the complete structure of NPD1.152 The reader might note that compound 52 is an isomer of NPD1/PD1 (see Fig. 11, compound 50)) and is biosynthesized via sequential lipoxygenation reactions in vivo and thus has a different double bond geometry and chirality at carbon 10 position, namely 10S.
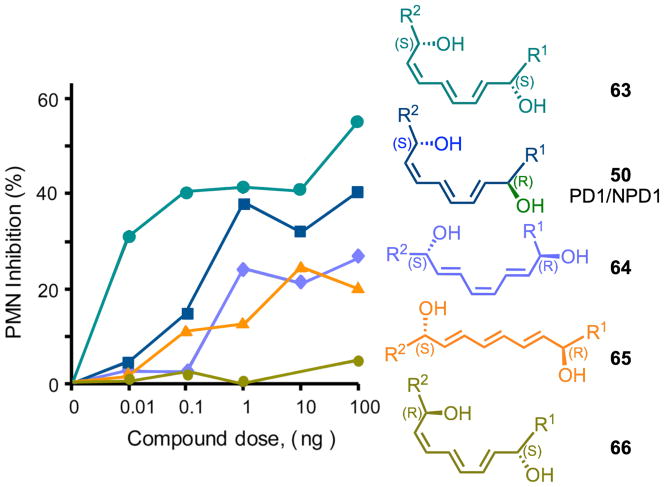
With the preparation of six stereochemically defined 10,17-dihydroxy-containing geometric isomers by total organic synthesis, it was possible to match the stereochemistry and biological actions of the endogenous-produced materials.51 In addition to PD1 formed from human leukocytes, additional isomers were identified in inflammatory exudates including Δ15-trans-PD1 (isomer III), 10S,17S-dihydroxy-docosa-4Z, 7Z, 11E, 13Z, 15Z, 19Z-hexaenoic acid (65) and the expected double dioxygenation product 10S,17S-dihydroxy-docosa-4Z, 7Z, 11E, 13Z, 15E, 19Z-hexaenoic acid (52), which are present in inflammatory exudates obtained from mice. 18O-labeling results provide evidence that this isomer was a double dioxygenation product and that the 10 position of NPD1/PD1 originated from enzymatic conversion. Also, the rank order of activities was established between these isomers, and NPD1/PD1 proved to be most potent (see Fig. 13), with doses as low as 1–10 ng reducing murine peritonitis, followed by Δ15-trans-PD1 > 10S,17S-diHDHA. Hence, although the double dioxygenation product 10S,17S-diHDHA was generated in murine exudates, it was far less active than PD1/NPD1 both in vitro and in vivo (Fig. 13).
4.3 Biological Actions of PD1/NPD1
With the stereochemistry of NPD1/PD1 established,51 its identification in human material was sought and found to be present in breath condensates from human asthmatics.94 In addition, PD1 was found to be a major product in bone marrow of female rats fed EPA and DHA,159 and PD1 was generated in vivo during ischemia-reperfusion of renal tissues, where it has profound actions, reversing the deleterious consequences of ischemia-reperfusion in renal tissues.97 Moreover, with the complete stereochemistry and quantities of the syntheticNPD1/PD1, it was possible to demonstrate that PD1 activates resolution programs and shortens the resolution time of experimental inflammation in vivo.21
From the total organic synthesis route of NPD1/PD1 outlined in Fig. 12, it was possible to radiolabel and purify 3H-NPD1/PD1 made from the synthetic intermediate. With this radiolabel, it was possible to define for the specific binding sites present with ARPE-19 cells (Kd ~ 31 pM/mg cell protein) for 3H-NPD1/PD1 as well as specific binding to human neutrophils that gave a Kd of ~25 nM. Most importantly, critical information on NPD1 biosynthesis with ARPE-19 cells was obtained, namely identification of alcohol trapping products, indicating the formation of a 16S,17S-epoxide-containing intermediate (49) from DHA in the biosynthesis of neuroprotectin D1.64 These results as well as rank order of potency established for NPD1/PD1 of defined stereochemical analysis indicated that the most potent of the isomers prepared was indeed NPD1/PD1. Also in the ARPE cells, NPD1/PD1 was more potent than either RvD1 or RvE1. In other systems, the resolvins proved more potent than PD1.55.
Along with the complete stereochemical identification of NPD1/PD1, recent studies using an unbiased LC-MS-MS profiling approach demonstrated that PD1 is made during the resolution of Lyme disease infections in mice160 and in stem cells under oxidative stress.161 NPD1 prevents retinal ganglion cell death,162 is renal protective,108 as well as regulates adiponectin in ob/ob obese mice.100 The double dioxygenation 10S,17S-diHDHA isomer of NPD1/PD1, was also recently shown to have actions on platelets, reducing platelet aggregation at 0.3 μM, 1 μM and higher concentrations.163 In peritonitis, this isomer also showed biological activity but was less potent than NPD1/PD1.51,163
NPD1/PD1 and the resolvins are produced in fish tissues where omega-3 fatty acids are enriched including trout brain and the head kidney gland, which is the hematopoietic compartment of the trout. These results suggest that the resolvin and protectin structures are highly conserved from fish to humans,164 and were recently found to stimulate stem cells at low nanomolar concentrations.161
5. Future Directions and Conclusions
The resolvins and protectins are uniquely positioned to have a role in many human diseases associated with inflammation because of their primary actions on effector cells in the innate immune system including both neutrophils and macrophages. These two main cell types play an important role in host defense as well as in clearing tissues and expediting resolution from infection and tissue injury.1 Given that the resolvins and protectins are temporally generated in the pivot from initiation to the resolution phase,2,20 they are strategically positioned to impact cell trafficking of neutrophils, stopping their excessive accumulation and activating specific receptors on both neutrophils and macrophages to enhance clearance of cellular debris, apoptotic neutrophils and microbes (reviewed in Ref. 12). These are fundamental responses to host defense and the return to tissue homeostasis.6 The unexpected finding that resolvins and protectins are produced from the omega-3 essential fatty acids also links the large body of literature that suggests that omega-3 fatty acids are beneficial to health84,85,165 and have separate therapeutic potential in supplementation of diets deficient in these natural omega-3 building blocks. The structural elucidation and stereochemical analyses reviewed herein provide ligands and receptors in a novel terrain of resolution of acute inflammation that can offer targeted therapeutics with wide-ranging potential for new treatments in many diseases in humans associated with uncontrolled inflammation (Tables 1 and 2). A key recent observation that circulating levels of EPA and DHA are transported via edema-carried proteins such as albumin and A109 proteins identified by two-dimensional TLC and proteomics20,23 demonstrates for the first time an important role of edema in providing nutrients such as EPA and DHA to the inflammatory site that are rapidly converted by inflammatory exudate cells to produce the resolvins and protectins, which can in turn expedite the resolution response and protect the surrounding tissues from collateral damage of the war with invading microbes129 by dampening PMN responses and enhancing phagocytosis and clearance.21,116 Hence, circulating levels of EPA and DHA and potentially other omega-3 essential fatty acids166 in humans play an important role in regulating the time course of resolution and production of resolvins and protectins. These mechanisms of exudate resolution and utilization of edema-supplied EPA and DHA imply a rapid utilization of dietary EPA and DHA once these substrates have reached circulation. This can put vascular insults rapidly in check if circulating substances are available to permit resolution.
This is a different scenario from arachidonic acid, where arachidonic acid is esterified into membrane phospholipids and, upon cell activation, cPLA2 cleaves phospholipids to release arachidonic acid for its transformation to prostaglandins, leukotrienes and lipoxins. This level of control by receptor-ligand interactions appears to be different from the inflammatory exudate direct utilization of EPA and DHA, which do not appear to require tight regulation at the level of membrane esterification and release in the inflammatory exudate to produce resolvins and protectins. In the case of DHA, this is quite different. DHA is well appreciated to be enriched in neural systems,151 where it is esterified into membrane phospholipids and then, on activation, is released for production of neuroprotectin D1 (Fig. 11). Again, in these studies, it should be emphasized that the precursor fatty acids EPA and DHA do not carry the potent stereoselective actions of the resolvins and protectins within the same concentration dose range. For example, see Kasuga et al.57 and direct functional comparison between native DHA and RvD1 with single human leukocytes and Marcheselli et al.64 for direct evidence of the specific binding area of PD1 to human leukocytes and retinal epithelial cells. This physiological utilization of edema-derived EPA and DHA by inflammatory exudates to produce resolvins and protectins opens a number of possibilities for investigating the mechanisms and potential physiologic role of resolvins and protectins, for example the impact of diet, gender and age in this system. Hence, our findings suggest that resolvins and their regulation by diet and infection can directly modulate multiple systems in the human exposomes.167 The structures of resolvins and protectins themselves are primordial and highly conserved, because they are identified in fish (rainbow trout), neural tissues and hematopoietic tissues.164 Thus, it is highly likely that, in addition to the roles of the resolvins and protectins in regulating the acute inflammatory response, additional roles in mammalian and human physiology will be uncovered in the years ahead. In this regard, the production of novel ω-3-derived mediators such as the maresins by specific cell types involved in resolution of inflammation and homeostasis82 opens many exciting new chemical signaling pathways that should pave an understanding of molecular nutrition and the precise structure-activity relationships that the body uses to achieve homeostasis. Importantly, introducing resolution indices20 has pinpointed the actions of SPM in shortening resolution times for inflammation and identified current drugs that are resolution toxic and those that are resolution friendly, enhancing the resolution pathways and mechanisms (Table 3).
Table 3.
What Impacts the Resolution Interval in vivo?
| Delay Ri | Shorten Ri | ||
|---|---|---|---|
| NSAIDs | Transgenic mice overexpressors | ||
| COX-2 inhibitors | Gilroy et al.168 | Human ALX/FPR2, GPR-32 | Devchand et al.a |
| Vong et al.b | Recchiuti et al.c | ||
| Schwab et al.21 | |||
|
| |||
| LOX inhibitors (resolution-toxic) | Schwab et al.21 | fat-1 (DHA, EPA) | Xia et al.d |
| Chiang et al.23 | Hudert et al.e | ||
| Resolution-friendly | |||
| Aspirin | Schwab et al.21 | ||
| Morris et al.f,g | |||
| Statins | Birnbaum et al.h | ||
| Planagumà et al.i | |||
| Glucocorticoids | Rossi and Sawatzkyj | ||
| Maderna et al.k | |||
Devchand, P. R.; Arita, M.; Hong, S.; Bannenberg, G.; Moussignac, R.-L.; Gronert, K.; Serhan, C. N. FASEB J. 2003, 17, 652.
Vong, L.; Ferraz, J. G.; Panaccione, R.; Beck, P. L.; Wallace, J. L. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 12023.
Recchiuti, A.; Krishnamoorthy, S.; Fredman, G.; Chiang, N.; Serhan, C. N. FASEB J. 2011, 25, 544.
Xia, S.; Lu, Y.; Wang, J.; He, C.; Hong, S.; Serhan, C. N.; Kang, J. X. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 12499.
Hudert, C. A.; Weylandt, K. H.; Wang, J.; Lu, Y.; Hong, S.; Dignass, A.; Serhan, C. N.; Kang, J. X. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 11276.
Morris, T.; Stables, M.; Colville-Nash, P.; Newson, J.; Bellingan, G.; de Souza, P. M.; Gilroy, D. W. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 8842.
Morris, T.; Stables, M.; Hobbs, A.; de Souza, P.; Colville-Nash, P.; Warner, T.; Newson, J.; Bellingan, G.; Gilroy, D. W. J. Immunol. 2009, 183, 2089.
Birnbaum, Y.; Ye, Y.; Lin, Y.; Freeberg, S. Y.; Huang, M.-H.; Perez-Polo, J. R.; Uretsky, B. F. Prostaglandins Other Lipid Mediat. 2007, 83, 89.
Planagumà, A.; Pfeffer, M. A.; Rubin, G.; Croze, R.; Uddin, M.; Serhan, C. N.; Levy, B. D. Mucosal Immunol. 2010, 3, 270.
The Resolution of Inflammation; Rossi, A. G.; Sawatzky, D. A., Eds.; Birkhäuser Verlag AG: Basel, 2008.
Maderna, P.; Yona, S.; Perretti, M.; Godson, C. J. Immunol. 2005, 174, 3727.
Aspirin jump-starts resolution because it triggers the biosynthesis of SPM, in particular RvE1 and AT-lipoxins and AT-D series resolvins. Indomethacin reduces 18R-HEPE production but still permits its formation.2,10 COX-2 inhibitors both block resolvin biosynthesis and relay resolution while reducing the magnitude of inflammation, as do many other nonsteroidal anti-inflammatory drugs.22,168 The direct impact of NSAID on resolvin and SPM biosynthesis remains to be established, yet lipoxygenase inhibitors block SPM biosynthesis and are resolution-toxic.21
With the structures, biosynthetic pathways, receptors and their receptor signaling studies underway, it is possible that therapeutics targeted around the resolvins, protectins and related endogenous pathways will emerge as a unique means to stimulate return to homeostasis in inflammatory diseases rather than the traditional approach of blocking enzyme pathways and receptors using antagonists and enzyme inhibitors.169 This new approach may add to the pharmacopeia for treating human diseases where uncontrolled inflammation and potentially defects in resolution pathways may become apparent.
Acknowledgments
We thank Mary H. Small for skillful manuscript preparation. This study was supported in part by the National Institutes of Health Grants DK074448, GM38765, P50-DE016191, and RC2AT005909.
C.N.S. is inventor on patents assigned to Brigham and Women’s Hospital and Partners HealthCare on the composition of matter, uses, and clinical development of anti-inflammatory and pro-resolving lipid mediators. These are licensed for clinical development. C.N.S. retains founder stock in Resolvyx Pharmaceuticals. N.A.P is inventor on patents on anti-inflammatory and pro-resolving lipid mediators assigned to the University of Southern California, which were licensed for clinical development. N.A.P. retains stock in Resolvyx Pharmaceuticals.
Biographies
 Charles N. Serhan
Charles N. Serhan
Charles N. Serhan is the Simon Gelman Professor of Anaesthesia (Biochemistry and Molecular Pharmacology) at Harvard Medical School and Professor of Oral Medicine, Infection and Immunity at HSDM, Harvard University. Since 1995, he serves as the Director of the Center for Experimental Therapeutics and Reperfusion Injury at Brigham and Women’s Hospital in Boston. Professor Serhan received his Bachelor’s degree in biochemistry from Stony Brook University, New York, and went on to receive the doctorate in experimental pathology and medical sciences from New York University School of Medicine. From 1981–86, he was a visiting scientist at the Karolinska Institutet and post-doctoral fellow with Professor Bengt Samuelsson. In 1996, he received an honorary degree from Harvard University.
Dr. Serhan was awarded an NIH MERIT Award (2000), the MacArthur Research Service Award in 2003, and the Outstanding Scientist Award in Inflammation Research at BioDefense, 2004. He delivered the 2005 Kreshover Lecture at NIH and in 2007 the Dart/New York University Biotechnology Outstanding Achievement Award. In 2008, he delivered the Sir John Vane Memorial Lecture and received the William Harvey Outstanding Scientist Medal. In 2010, Dr. Serhan delivered the Kern Lecture “in recognition of outstanding research on lipids” at the Kern Lipid Conference. He also received the Society for Leukocyte Biology 2010 Bonazinga Award for “excellence in leukocyte biology research, the highest honor that the society can bestow”. He was elected to the rank of AAAS fellow 2010, “for distinguished contributions to medical sciences with the identification of novel mechanisms in resolution of inflammation and structural elucidation of pro-resolving chemical signals”.
In 2011 he receives the ‘Oh-Dang’ Distinguished Lectureship, a prestigious award that recognizes internationally renowned scholars who have had a major impact on pharmaceutical research and related areas of life sciences. This award is given at the annual meeting of the Pharmaceutical Society of Korea “In recognition of outstanding achievement for the study of resolution of inflammation”.
He is a longtime friend and collaborator of co-author Dr. Nicos Petasis.
Professor Serhan has authored 403 publications, 5 books, and over 300 US patents.
 Nicos A. Petasis is the Harold E. and Lillian M. Moulton Chair and Professor of Chemistry at the University of Southern California (USC) in Los Angeles. He was born in Cyprus and received his undergraduate education at the Aristotle University of Thessaloniki in Greece. He earned his Ph.D. from the University of Pennsylvania (with Professor K. C. Nicolaou) and after a postdoctoral appointment at the same university he joined the USC faculty in 1987, where he is also a member of the USC Loker Hydrocarbon Research Institute and the USC Norris Comprehensive Cancer Center. His research interests span from synthetic organic chemistry, medicinal chemistry and chemical biology, to the development of new therapeutics for inflammation, cancer, and neurodegenerative diseases. He has discovered several novel and widely used synthetic methods, including a titanium-based alkenation, an aluminum-induced rearrangement, and a boron-mediated multicomponent process for the synthesis of amine derivatives. For nearly two decades he has also been collaborating with Professor Charles N.Serhan and others on the chemistry, biology, and medicinal applications of new polyunsaturated lipid mediators involved in the resolution of inflammation, including lipoxins, resolvins, protectins, and maresins. He has been the recipient of several awards, including the USC Associates Award for Creativity in Research and Scholarship, the Novartis Chemistry Lectureship, and the 2009 Arthur C. Cope Scholar Award from the American Chemical Society.
Nicos A. Petasis is the Harold E. and Lillian M. Moulton Chair and Professor of Chemistry at the University of Southern California (USC) in Los Angeles. He was born in Cyprus and received his undergraduate education at the Aristotle University of Thessaloniki in Greece. He earned his Ph.D. from the University of Pennsylvania (with Professor K. C. Nicolaou) and after a postdoctoral appointment at the same university he joined the USC faculty in 1987, where he is also a member of the USC Loker Hydrocarbon Research Institute and the USC Norris Comprehensive Cancer Center. His research interests span from synthetic organic chemistry, medicinal chemistry and chemical biology, to the development of new therapeutics for inflammation, cancer, and neurodegenerative diseases. He has discovered several novel and widely used synthetic methods, including a titanium-based alkenation, an aluminum-induced rearrangement, and a boron-mediated multicomponent process for the synthesis of amine derivatives. For nearly two decades he has also been collaborating with Professor Charles N.Serhan and others on the chemistry, biology, and medicinal applications of new polyunsaturated lipid mediators involved in the resolution of inflammation, including lipoxins, resolvins, protectins, and maresins. He has been the recipient of several awards, including the USC Associates Award for Creativity in Research and Scholarship, the Novartis Chemistry Lectureship, and the 2009 Arthur C. Cope Scholar Award from the American Chemical Society.
References
- 1.Cotran RS, Kumar V, Collins T, editors. Robbins Pathologic Basis of Disease. 6. W.B. Saunders Co; Philadelphia: 1999. [Google Scholar]
- 2.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. J Exp Med. 2000;192:1197. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Nat Immunol. 2001;2:612. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L. J Exp Med. 2002;196:1025. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;73:141. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Savill J. Nat Immunol. 2005;6:1191. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 7.Serhan CN, Chiang N, Van Dyke TE. Nat Rev Immunol. 2008;8:349. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN. Am J Pathol. 2010;177:1576. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bannenberg G, Serhan CN. Biochim Biophys Acta. 2010;1801:1260. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN, Chiang N. Br J Pharmacol. 2008;153(Suppl 1):S200. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petasis NA, Akritopoulou-Zanze I, Fokin VV, Bernasconi G, Keledjian R, Yang R, Uddin J, Nagulapalli KC, Serhan CN. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;73:301. doi: 10.1016/j.plefa.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN. Annu Rev Immunol. 2007;25:101. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 13.Libby P. Sci Am. 2002;286:46. doi: 10.1038/scientificamerican0502-46. [DOI] [PubMed] [Google Scholar]
- 14.Hansson GK, Libby P. Nat Rev Immunol. 2006;6:508. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence T, Willoughby DA, Gilroy DW. Nat Rev Immunol. 2002;2:787. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 16.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Science. 1987;237:1171. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 17.Samuelsson B. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Almqvist & Wiksell; Stockholm: 1982. [Google Scholar]
- 18.Vane JR. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Almqvist & Wiksell; Stockholm: 1982. [Google Scholar]
- 19.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LAJ, Perretti M, Rossi AG, Wallace JL. FASEB J. 2007;21:325. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. J Immunol. 2005;174:4345. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 21.Schwab JM, Chiang N, Arita M, Serhan CN. Nature. 2007;447:869. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro-Xavier RA, Newson J, Silveira VLF, Farrow SN, Gilroy DW, Bystrom J. J Immunol. 2010;184:1516. doi: 10.4049/jimmunol.0902866. [DOI] [PubMed] [Google Scholar]
- 23.Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. PLoS ONE. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Nat Rev Drug Discov. 2004;3:401. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Biochemistry. 1995;34:14609. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 26.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. J Biol Chem. 1997;272:6972. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 27.Scalia R, Gefen J, Petasis NA, Serhan CN, Lefer AM. Proc Natl Acad Sci USA. 1997;94:9967. doi: 10.1073/pnas.94.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy BD, Petasis NA, Serhan CN. Nature. 1997;389:985. doi: 10.1038/40180. [DOI] [PubMed] [Google Scholar]
- 29.Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. J Exp Med. 1997;185:1693. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. J Clin Invest. 1998;101:819. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL. J Clin Invest. 1998;101:1860. doi: 10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gewirtz AT, Fokin VV, Petasis NA, Serhan CN, Madara JL. Am J Physiol Cell Physiol. 1999;276:C988. doi: 10.1152/ajpcell.1999.276.4.C988. [DOI] [PubMed] [Google Scholar]
- 33.Clish CB, O’Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Proc Natl Acad Sci USA. 1999;96:8247. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hachicha M, Pouliot M, Petasis NA, Serhan CN. J Exp Med. 1999;189:1923. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy BD, Fokin VV, Clark JM, Wakelam MJO, Petasis NA, Serhan CN. FASEB J. 1999;13:903. doi: 10.1096/fasebj.13.8.903. [DOI] [PubMed] [Google Scholar]
- 36.Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Biochemistry. 2000;39:4761. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- 37.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. J Immunol. 2000;164:1663. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR, Godson C. J Am Soc Nephrol. 2002;13:2497. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 39.Jozsef L, Zouki C, Petasis NA, Serhan CN, Filep JG. Proc Natl Acad Sci USA. 2002;99:13266. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fierro IM, Colgan SP, Bernasconi G, Petasis NA, Clish CB, Arita M, Serhan CN. J Immunol. 2003;170:2688. doi: 10.4049/jimmunol.170.5.2688. [DOI] [PubMed] [Google Scholar]
- 41.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. J Immunol. 2003;170:6266. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 42.Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. J Immunol. 2003;171:6856. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 43.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, Petasis NA. Nat Immunol. 2004;5:388. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 44.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. J Exp Med. 2005;201:713. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Proc Natl Acad Sci USA. 2005;102:7671. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karp CL, Flick LM, Yang R, Uddin J, Petasis NA. Prostaglandins Leukot Essent Fatty Acids. 2005;73:263. doi: 10.1016/j.plefa.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Levy BD, Hickey L, Morris AJ, Larvie M, Keledjian R, Petasis NA, Bannenberg G, Serhan CN. Br J Pharmacol. 2005;146:344. doi: 10.1038/sj.bjp.0706338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petasis NA, Akritopoulou-Zanze I, Fokin VV, Bernasconi G, Keledjian R, Yang R, Uddin J, Nagulapalli KC, Serhan CN. Prostaglandins Leukot Essent Fatty Acids. 2005;73:301. doi: 10.1016/j.plefa.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 49.Arita M, Oh SF, Chonan T, Hong S, Elangovan S, Sun Y-P, Uddin J, Petasis NA, Serhan CN. J Biol Chem. 2006;281:22847. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 50.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. FASEB J. 2006;20:401. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 51.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. J Immunol. 2006;176:1848. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 52.El Kebir D, Jozsef L, Khreiss T, Pan W, Petasis NA, Serhan CN, Filep JG. J Immunol. 2007;179:616. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 53.Hong S, Lu Y, Yang R, Gotlinger KH, Petasis NA, Serhan CN. J Am Soc Mass Spectrom. 2007;18:128. doi: 10.1016/j.jasms.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Y, Hong S, Yang R, Uddin J, Gotlinger KH, Petasis NA, Serhan CN. Rapid Commun Mass Spectrom. 2007;21:7. doi: 10.1002/rcm.2798. [DOI] [PubMed] [Google Scholar]
- 55.Sun Y-P, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. J Biol Chem. 2007;282:9323. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 56.Masoodi M, Mir AA, Petasis NA, Serhan CN, Nicolaou A. Rapid Commun Mass Spectrom. 2008;22:75. doi: 10.1002/rcm.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. J Immunol. 2008;181:8677. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petasis NA, Keledjian R, Sun Y-P, Nagulapalli KC, Tjonahen E, Yang R, Serhan CN. Bioorg Med Chem Lett. 2008;18:1382. doi: 10.1016/j.bmcl.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 59.El Kebir D, Jozsef L, Pan W, Wang L, Petasis NA, Serhan CN, Filep JG. Am J Respir Crit Care Med. 2009;180:311. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Nature. 2009;461:1287. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Y-P, Tjonahen E, Keledjian R, Zhu M, Yang R, Recchiuti A, Pillai PS, Petasis NA, Serhan CN. Prostaglandins Leukot Essent Fatty Acids. 2009;81:357. doi: 10.1016/j.plefa.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calandria JM, Marcheselli VL, Mukherjee PK, Uddin J, Winkler JW, Petasis NA, Bazan NG. J Biol Chem. 2009;284:17877. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee C-H, Yang R, Petasis NA, Serhan CN. Proc Natl Acad Sci USA. 2010;107:1660. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, Sheets K, Winkler JW, Petasis NA, Serhan CN, Bazan NG. Prostaglandins Leukot Essent Fatty Acids. 2010;82:27. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radmark O, Werz O, Steinhilber D, Samuelsson B. Trends in Biochemical Sciences. 2007;32:332. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Werz O, Steinhilber D. Pharmacology & Therapeutics. 2006;112:701. doi: 10.1016/j.pharmthera.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Marnett LJ. Annual Review of Pharmacology and Toxicology. 2009;49:265. doi: 10.1146/annurev.pharmtox.011008.145638. [DOI] [PubMed] [Google Scholar]
- 68.Wang D, DuBois RN. Nat Rev Cancer. 2010;10:181. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rajakariar R, Yaqoob MM, Gilroy DW. Mol Interv. 2006;6:199. doi: 10.1124/mi.6.4.6. [DOI] [PubMed] [Google Scholar]
- 70.FitzGerald GA. Nature Reviews Drug Discovery. 2003;2:879. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- 71.Norel X, Brink C. Pharmacology & Therapeutics. 2004;103:81. doi: 10.1016/j.pharmthera.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 72.Serhan CN. Biochim Biophys Acta. 1994;1212:1. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 73.Ryan A, Godson C. Curr Opin Pharmacol. 2010;10:166. doi: 10.1016/j.coph.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Tobin DM, Vary JC, Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King M-C, Hawn TR, Moens CB, Ramakrishnan L. Cell. 2010;140:717. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwab JM, Serhan CN. Current Opinion in Pharmacology. 2006;6:414. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 76.Guilford WJ, Parkinson JF. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;73:245. doi: 10.1016/j.plefa.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 77.Dufton N, Perretti M. Pharmacol Ther. 2010;127:175. doi: 10.1016/j.pharmthera.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 78.Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Proc Natl Acad Sci USA. 2007;104:20979. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN. J Biol Chem. 2003;278:14677. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 80.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. J Biol Chem. 2003;278:43807. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 81.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Proc Natl Acad Sci USA. 2004;101:8491. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. J Exp Med. 2009;206:15. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fetterman JW, Jr, Zdanowicz MM. Am J Health Syst Pharm. 2009;66:1169. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 84.Simopoulos AP. J Am Coll Nutr. 2002;21:495. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 85.Calder PC. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2007;77:327. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 86.Cleland LG, James MJ, Proudman SM. Drugs. 2003;63:845. doi: 10.2165/00003495-200363090-00001. [DOI] [PubMed] [Google Scholar]
- 87.Harper CR, Jacobson TA. Arch Intern Med. 2001;161:2185. doi: 10.1001/archinte.161.18.2185. [DOI] [PubMed] [Google Scholar]
- 88.Breslow JL. Am J Clin Nutr. 2006;83:S1477. doi: 10.1093/ajcn/83.6.1477S. [DOI] [PubMed] [Google Scholar]
- 89.Nettleton JA, Katz R. J Am Diet Assoc. 2005;105:428. doi: 10.1016/j.jada.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 90.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Am J Clin Nutr. 2004;79:935. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 91.Soehnlein O, Lindbom L. Nat Rev Immunol. 2010;10:427. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 92.Tabas I. Nat Rev Immunol. 2010;10:36. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Nat Med. 2002;8:1018. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 94.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. J Immunol. 2007;178:496. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, Petasis NA. Nature Immunology. 2004;5:388. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 96.Karp CL, Flick LM, Yang R, Uddin J, Petasis NA. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;73:263. doi: 10.1016/j.plefa.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 97.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. J Immunol. 2006;177:5902. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 98.Wu S-H, Liao P-Y, Yin P-L, Zhang Y-M, Dong L. Am J Pathol. 2009;174:115. doi: 10.2353/ajpath.2009.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lukiw WJ, Cui J-G, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. J Clin Invest. 2005;115:2774. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, Clària J. FASEB J. 2009;23:1946. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. FASEB J. :2011. doi: 10.1096/fj.10–178657. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li N, He J, Schwartz CE, Gjorstrup P, Bazan HEP. J Ocul Pharmacol Ther. 2010;26:431. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem NN, Jr, Serhan CN, Smith LEH. Nat Med. 2007;13:868. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, Serhan CN, Dana MR. Invest Ophthalmol Vis Sci. 2009;50:4743. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang F, Yang H, Pan Z, Wang Z, Wolosin JM, Gjorstrup P, Reinach PS. Invest Ophthalmol Vis Sci. 2010;51:5601. doi: 10.1167/iovs.09-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Nat Immunol. 2008;9:873. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, Mizuno S, Takagawa T, Morita Y, Kutsumi H, Inokuchi H, Serhan CN, Blumberg RS, Azuma T. Inflamm Bowel Dis. 2010;16:87. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hassan IR, Gronert K. J Immunol. 2009;182:3223. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- 109.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. J Immunol. 2010;184:836. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Br J Pharmacol. 2010;161:707. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu Z-Z, Zhang L, Liu T, Park J-Y, Berta T, Yang R, Serhan CN, Ji R-R. Nat Med. 2010;16:592. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Svensson CI, Zattoni M, Serhan CN. J Exp Med. 2007;204:245. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ren K, Dubner R. Nat Med. 2010;16:1267. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Calder PC. Am J Clin Nutr. 2006;83:S1505. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 115.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. J Immunol. 2007;178:3912. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 116.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. J Biol Chem. 2009;285:3451. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. J Clin Invest. 2011;121:569. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Petasis NA. 6,949,664. US Patent. 2005
- 119.Ogawa N, Kobayashi Y. Tetrahedron Lett. 2009;50:6079. [Google Scholar]
- 120.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Blood. 2008;112:848. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bäck M, Dahlén S-E, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE. Pharmacol Rev. 2011 doi: 10.1124/pr.110.004184. In Press. [DOI] [PubMed] [Google Scholar]
- 122.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. J Immunol. 2008;180:3512. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 123.Serhan CN. 7,585,856. US Patent. 2004:A1. and Application Publication 2004/0116408.
- 124.Goodman D, Hanley M, Bursten S, Serhan C. 7,378,444. US Patent. 2008
- 125.Clish CB, Levy BD, Chiang N, Tai H-H, Serhan CN. J Biol Chem. 2000;275:25372. doi: 10.1074/jbc.M002863200. [DOI] [PubMed] [Google Scholar]
- 126.Serhan CN, Fiore S, Brezinski DA, Lynch S. Biochemistry. 1993;32:6313. doi: 10.1021/bi00076a002. [DOI] [PubMed] [Google Scholar]
- 127.Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandins Oth Lipid Mediat. 2002:68–69. 483. doi: 10.1016/s0090-6980(02)00050-3. [DOI] [PubMed] [Google Scholar]
- 128.Serhan CN, Ward PA, Gilroy DW, editors. Fundamentals of Inflammation. Cambridge University Press; New York: 2010. [Google Scholar]
- 129.Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. 2. Oxford University Press; New York: 2004. [Google Scholar]
- 130.Spite M, Summers L, Porter T, Srivastava S, Bhatnagar A, Serhan C. Br J Pharmacol. 2009;158:1062. doi: 10.1111/j.1476-5381.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shimizu T, Rådmark O, Samuelsson B. Proc Natl Acad Sci USA. 1984;81:689. doi: 10.1073/pnas.81.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Annu Rev Biochem. 2000;69:419. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 133.Cooper D, Norling LVMP. J Leukoc Biol. 2008;83:1450. doi: 10.1189/jlb.1207831. [DOI] [PubMed] [Google Scholar]
- 134.Kubes P, Suzuki M, Granger DN. Proc Natl Acad Sci USA. 1991;88:4651. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moncada S, Higgs EA. Br J Pharmacol. 2006;147(Suppl 1):S193. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shimizu T. Annu Rev Pharmacol Toxicol. 2009;49:123. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 137.Rittersch D, Huber-Lang MS, Flierl MA, Ward PA. Nat Protoc. 2009;4:31. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buras JA, Holzmann B, Sitkovsky M. Nat Rev Drug Discov. 2005;4:854. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 139.Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Intensive Care Med. 2008;34:1580. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- 140.Farolan LR, Goto M, Myers TF, Anderson CL, Zeller WP. Shock. 1996;6:263. doi: 10.1097/00024382-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 141.Pluess TT, Hayoz D, Berger MM, Tappy L, Revelly JP, Michaeli B, Carpentier YA, Chioléro RL. Intensive Care Med. 2007;33:789. doi: 10.1007/s00134-007-0591-5. [DOI] [PubMed] [Google Scholar]
- 142.Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, Kurihara Y, Kodama T, Gordon S. J Exp Med. 1997;186:1431. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, Nykter M, Shmulevich I, Aderem A. Nat Immunol. 2009;10:437. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mosser DM, Edwards JP. Nat Rev Immunol. 2008;8:958. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JL, Ward PA. FASEB J. 2008;22:2198. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 146.Souza DG, Fagundes CT, Amaral FA, Cisalpino D, Sousa LP, Vieira AT, Pinho V, Nicoli JR, Vieira LQ, Fierro IM, Teixeira MM. J Immunol. 2007;179:8533. doi: 10.4049/jimmunol.179.12.8533. [DOI] [PubMed] [Google Scholar]
- 147.Osuchowski MF, Welch K, Siddiqui J, Remick DG. J Immunol. 2006;177:1967. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 148.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. Proc Natl Acad Sci USA. 2009;106:6303. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hotchkiss RS, Karl IE. N Engl J Med. 2003;348:138. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 150.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Crit Care Med. 2007;35:1244. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 151.Bazan NG, Calandria JM, Serhan CN. J Lipid Res. 2010;51:2018. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Butovich IA. J Lipid Res. 2005;46:2311. doi: 10.1194/jlr.C500015-JLR200. [DOI] [PubMed] [Google Scholar]
- 153.Serhan CN. Prostaglandins. 1997;53:107. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- 154.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Proc Natl Acad Sci USA. 2004;101:8491. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ariel A, Li P-L, Wang W, Tang W-X, Fredman G, Hong S, Gotlinger KH, Serhan CN. J Biol Chem. 2005;280:43079. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 156.Bazan NG, Birkle DL, Reddy TS. Biochem Biophys Res Commun. 1984;125:741. doi: 10.1016/0006-291x(84)90601-6. [DOI] [PubMed] [Google Scholar]
- 157.Serhan CN, Lundberg U, Weissmann G, Samuelsson B. Prostaglandins. 1984;27:563. doi: 10.1016/0090-6980(84)90092-3. [DOI] [PubMed] [Google Scholar]
- 158.Serhan CN, Clish CB. 6,670,396 and Application No. 09/785866. US Patent. 2003
- 159.Poulsen RC, Gotlinger KH, Serhan CN, Kruger MC. Am J Hematol. 2008;83:437. doi: 10.1002/ajh.21170. [DOI] [PubMed] [Google Scholar]
- 160.Blaho VA, Buczynski MW, Brown CR, Dennis EA. J Biol Chem. 2009;284:21599. doi: 10.1074/jbc.M109.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yanes O, Clark J, Wong DM, Patti GG, Sánchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G. Nat Chem Biol. 2010;6:411. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qin Q, Patil KA, Gronert K, Sharma SC. Prostaglandins Leukot Essent Fatty Acids. 2008;79:201. doi: 10.1016/j.plefa.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 163.Chen P, Fenet B, Michaud S, Tomczyk N, Véricel E, Lagarde M, Guichardant M. FEBS Lett. 2009;583:3478. doi: 10.1016/j.febslet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 164.Hong S, Tjonahen E, Morgan EL, Yu L, Serhan CN, Rowley AF. Prostaglandins Other Lipid Mediat. 2005;78:107. doi: 10.1016/j.prostaglandins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 165.Salem N, Jr, Litman B, Kim H-Y, Gawrisch K. Lipids. 2001;36:945. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 166.Dangi B, Obeng M, Nauroth JM, Teymourlouei M, Needham M, Raman K, Arterburn LM. J Biol Chem. 2009;284:14744. doi: 10.1074/jbc.M809014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rappaport SM, Smith MT. Science. 2010;330:460. doi: 10.1126/science.1192603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Nat Med. 1999;5:698. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 169.Filep J. Br J Pharmacol. 2009;158:1059. doi: 10.1111/j.1476-5381.2009.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]