Abstract
Rationale
Rats raised in isolation self-administer more amphetamine than rats raised in enrichment.
Objective
This study examined whether differential rearing alters basal and amphetamine-stimulated corticosterone and whether blocking glucocorticoid receptors alters amphetamine self-administration in differentially reared rats.
Methods
Rats were raised from 21–51 days of age in either an enriched condition (EC), social condition (SC) or isolated condition (IC). Following repeated collection of basal blood samples, rats were administered amphetamine (0.5 or 2.0 mg/kg, i.p.) or saline, and blood samples were collected again. In another experiment, EC and IC rats were trained to self-administer i.v. amphetamine (0.003 or 0.03 mg/kg/infusion) and then were pretreated with the glucocorticoid receptor antagonist RU-486 (5, 10 or 20 mg/kg; i.p.) or vehicle prior to the session.
Results
Basal free corticosterone levels were ~4 times higher in IC rats than in either EC or SC rats with the first blood collection, but not with repeated collections. IC rats showed a more rapid amphetamine-induced increase in corticosterone levels than EC and SC rats. RU-486 pretreatment decreased amphetamine self-administration dose-dependently in both EC and IC rats; however, using an amphetamine unit dose of 0.03 mg/kg/infusion, the effect of RU-486 was blunted in IC rats (maximal decrease of ~40% in IC and ~90% in EC), suggesting an environment-induced difference in the role of glucocorticoid receptors in stimulant reinforcement.
Conclusion
The increase in stimulant self-administration produced by social isolation may involve enhanced reactivity of the hypothalamo-pituitary-adrenal stress axis.
Keywords: Stress, Environmental Enrichment, Social Isolation, Amphetamine, Self-administration, Rats
While many individuals experiment with drugs, the majority do not go on to abuse drugs (Adams et al. 1999). The increased vulnerability to drug abuse is due to a number of individual differences such as genetics, personality traits and environmental experiences. One animal model that has been developed to investigate the role of differential environments in drug abuse vulnerability is the rodent environmental enrichment paradigm (Bardo et al. 2001; Green et al. 2002; Zakharova et al. 2009). In the typical environmental enrichment paradigm, rats are housed for several weeks post weaning (~ 21 days of age) in either an enriched condition (EC) with novel objects and social cohorts or an isolated condition (IC) without objects or cohorts. A social condition (SC), in which rats are housed with a social cohort without the novel objects, is also often used for comparison. These three conditions allow researchers to determine the role of novelty exposure and social interaction in differential drug effects.
Differential rearing alters a number of behavioral and neurochemical effects of stimulant drugs. For example, although EC rats have a lower baseline level of locomotor behavior in an open field relative to their IC counterparts (Bardo et al. 1995; Bowling and Bardo 1994; Bowling et al. 1993), when an amphetamine challenge is administered, EC rats have a greater increase in locomotor behavior relative to IC rats (Bardo et al. 1995; Bowling and Bardo 1994; Bowling et al. 1993). While EC rats are more sensitive to acute amphetamine-induced locomotor activity than IC rats, EC rats show less sensitization following repeated injections of amphetamine and cocaine (Bardo et al. 1995; Smith et al. 1997). Thus, the effects of differential rearing on the sensitivity to amphetamine vary based on whether the drug is given acutely or repeatedly.
In addition to the enrichment-induced decrease in amphetamine locomotor sensitization, EC rats self-administer less amphetamine compared to IC rats (Bardo et al. 2001; Green et al. 2002). Bardo et al. (2001) found no group differences using an amphetamine unit dose of 0.1 mg/kg/infusion, while a lower unit dose (0.03 mg/kg/infusion) produced less self-administration in both EC and SC rats compared to IC rats (Bardo et al. 2001). Green et al. (2002) extended these findings by investigating the entire dose response curve of amphetamine self-administration and found that low unit doses of amphetamine maintained significantly lower levels of fixed ratio and progressive ratio responding in EC rats compared to IC rats. These studies indicate that environmental enrichment blunts the reinforcing effect of low unit doses of amphetamine, thus leaving IC rats more addiction prone.
A potential neural mechanism that may explain, at least in part, the alteration in amphetamine self-administration produced by differential rearing is suggested from previous results using the high responder (HR) and low responder (LR) rodent model (Piazza et al. 1989; Piazza et al. 1991). Rats are categorized as HR or LR based on their level of activity in an inescapable novel open field. Similar to IC rats, HR rats show greater baseline activity and greater amphetamine self-administration compared to LR rats, particularly at low unit doses (Klebaur et al. 2001; Piazza et al. 1989). A critical mechanism thought to underlie the difference in amphetamine self-administration between HR and LR rats is the hypothalamo-pituitary-adrenal (HPA) stress axis. In rats, the most common glucocorticoid is corticosterone, which follows a circadian rhythm, with peak levels occurring just prior to the dark phase of the animals light/dark cycle (Butte et al. 1976; Cheifetz et al. 1968; Hiroshige and Sakakura 1971). HR rats have higher levels of corticosterone in response to novelty than LR rats (Piazza et al. 1991). Also, when plasma corticosterone levels are increased in LR rats, amphetamine self-administration increases to levels similar to HR rats (Piazza et al. 1991).
Regardless of any individual or environmental differences, numerous studies have found that acute stressors or pharmacologically-induced elevations in corticosterone increase stimulant self-administration (Deroche et al. 1997; Deroche-Gamonet et al. 2003; Goeders and Guerin 1994; 1996). Exposure to footshock prior to cocaine self-administration sessions increases cocaine intake, as well as increasing the probability that cocaine will maintain reliable self-administration (Goeders and Guerin 1994; 1996). In contrast, cocaine self-administration under a progressive ratio schedule is decreased by pretreatment with the glucocorticoid receptor (GR) antagonist RU-486 (Deroche-Gamonet et al. 2003). These results indicate a role for glucocorticoids and GR receptors in altering stimulant reinforcement.
Given the involvement of glucocorticoids in the differential rates of stimulant self-administration between HR and LR rats, the present study examined the role of corticosterone and glucocorticoid receptors in environmentally-induced differences in response to amphetamine. Experiment 1 determined if basal and/or amphetamine-stimulated levels of free plasma corticosterone differed among EC, SC and IC rats. Experiment 2 determined if GR receptor blockade altered amphetamine self-administration differentially in EC and IC rats.
Materials and methods
Experiment 1: Basal Levels and Effect of Amphetamine on Free Corticosterone in EC, SC and IC Rats
Animals
Thirty-six male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN, USA) arrived at approximately 21 days of age. Animals had unlimited access to food and water in their home cage, and were maintained on a 12/12 hr light/dark cycle with lights on from 6:00–18:00 hr. All procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee and conformed to the 1996 NIH Guide for the Care and Use of Laboratory Animals.
Environmental Conditions
Upon arrival to the colony, rats were placed randomly into one of three conditions; EC (n= 12), IC (n= 12) or SC (n = 12) conditions for the duration of the experiment. EC rats were housed in stainless steel cages (60 × 120 × 45 cm) with social cohorts (12 per cage) and 14 hard plastic objects (commercial toys, plastic containers, etc.) placed randomly throughout the cage. Seven objects were replaced daily with new objects and all objects were rearranged to create a novel object arrangement every day. EC rats were removed and handled briefly during the daily object change. On the days of plasma collection, objects were not changed until after plasma collections. SC rats were housed 2 per cage in standard clear plastic cages (26 × 48 × 20 cm) with a wire top, bedding. SC rats were also removed and handled briefly everyday. The SC condition was chosen for comparison because it allowed for social interaction without any novel objects and because it represents a standard housing condition used across different laboratories (Institute of Laboratory Animal Resources (U.S.) 1996). IC rats were housed in individual hanging stainless steel cages with wire mesh floor and front panel, and solid metal side walls and top (17 × 24 × 20 cm). IC rats were handled minimally from 21–51 days of age. All animals remained in these conditions during postnatal days 21–51 and throughout the duration of the experiment.
Procedures
Catheterization Surgery
On post natal day 51, all rats were implanted with an indwelling catheter in the jugular vein, which allowed for intravenous blood collection. Rats were anesthetized with an injection of ketamine (80 mg/kg; i.p.) and diazepam (5 mg/kg; i.p.). The silastic catheter (0.2 mm i.d.; Fisher Scientific) was threaded subcutaneously to exit from a piece of stainless-steel hypodermic tubing (22 ga) embedded in a dental acrylic head cap mounted to the top of the skull with four stainless-steel jeweler’s screws. For seven days following surgery, the catheters were flushed daily at approximately 09:00 hr with 0.1 mg/ml-heparinized saline (0.25 ml/day) to maintain patency and habituate the animals to handling.
Basal Corticosterone Collection
To habituate rats to the blood collection procedure and to determine basal levels of corticosterone, a blood draw was conducted at 09:00 hr (early light cycle) and then rats were returned to their respective housing conditions. This procedure was repeated every 4 hr for a total of 6 blood collections.
Amphetamine Treatment
Two days following the basal corticosterone blood collections, EC, SC and IC rats (n = 5–6/group) were randomly treated once with each dose of amphetamine (0, 0.5 or 2.0 mg/kg) given on three different occasions, separated by two days each. Injections were at 09:00 hr and plasma was collected at 15, 60, and 180 min after injection as described below. Following each blood draw all animals were placed back in their respective housing conditions. The amphetamine injection time of 09:00 hr was chosen to mimic the time that amphetamine self-administration sessions were conducted in Experiment 2 (see below).
Blood Collections
For blood draws, each animal was removed from their home cage. A 0.2-ml sample was collected and transferred to a 1.5-ml centrifuge tube and placed on ice. The catheter was then flushed with 0.3 ml of heparinized saline and the rat was transferred back to its home cage. Following collection, samples were centrifuged at 2,000 g for 7 min at 4°C. The plasma supernatant was then collected and stored at −70° C until assay.
Separation of Free From Bound Plasma Corticosterone
Protein-bound corticosterone was removed from the samples by centrifugation with MPS-1 Centrifree Micropartition Devices (Amicon, Inc., Beverly, MA). Plasma samples were thawed and 100–170 μl were transferred to the barrel of the MPS-1 device, and incubated in a water bath at 37°C for 1 hr. Following incubation, samples were centrifuged at 1,500 g for 18 min at 37°C. The ultrafiltrate was then stored at −70° C until performing the assay.
Competitive Enzymeimmunoassay (EIA)
Free corticosterone levels were determined by competitive EIA using the OCTEIA Corticosterone kit (IDS Limited, Fountain Hills, AZ). Samples (30 μl) were diluted with 270 μl of sample buffer, vortexed, and 100 μl of sample was added to each well of an antibody coated plate. One hundred μl of enzyme conjugate was then added to each well and the plate was incubated at 2–8 °C for 24 hr. Following incubation, wells were washed three times with wash solution and 200 μl of tetramethylbenzidine substrate was added to each well. The plate was then incubated at 18–25 °C for 30 min and 100 μl of stop solution (HCL; 0.5M) was added to each well. The absorbance of each well was measured at 450 nm using a microplate reader.
Data Analysis
The mean absorbance for each sample was used to calculate the percent binding (B/B0% = [(mean absorbance)/(mean absorbance for “0” calibrator)] × 100). Based on the calibration curve from external standards, reliable corticosterone detection was in the range of 0.01–190 ng/ml. Basal corticosterone concentrations were analyzed at each sample interval using a one way analysis of variance (ANOVA). Concentrations following amphetamine were converted to percent change from saline control and analyzed using a mixed factor analysis of variance (ANOVA). Post hoc comparisons were conducted using a LSD post hoc test. For tests of significance, an alpha of p < 0.05 was used.
Drug
D-amphetamine was purchased from Sigma/RBI (Natick, MA), dissolved in 0.9% NaCl (saline) and injected in a volume of 1 ml/kg body weight. Doses represent salt weights.
Experiment 2: Effect of RU 486 on Amphetamine Self-Administration in EC and IC Rats
Animals
A separate group of 68 male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN, USA) arrived in the laboratory at 21 days of age. Animals were cared for and treated as described previously in Experiment 1, with the exception that animals were food deprived to 85% of their free-feeding body weight prior to food pre-training (described below).
Environmental Conditions
The same housing conditions were used as described in Experiment 1.
Behavioral Apparatus
Rats were tested in a standard 2-lever operant conditioning chamber (ENV-001; MED Associates, St. Albans, VT) as described previously (Bardo et al. 2001). Drug infusions were delivered via a MED Associates infusion pump (PHM-100; MED Associates). All responses and scheduled consequences were recorded and controlled by a computer interface.
Procedure
Food Pre-training
Since EC rats do not readily acquire amphetamine self-administration, all rats were pre-trained initially to respond for food reinforcement. At approximately 51 days of age, food access was restricted for 4 days to decrease body weights to approximately 85% of free feeding weights. EC rats were housed individually during restricted feeding (60 min). Rats were exposed to food pellets (45 mg pellets; BioServ, Frenchtown, NJ) on one day to alleviate neophobia during training. On the following day, rats were placed into the operant conditioning chamber with one lever extended into the chamber (active lever) and were allowed to complete a 60-min session using a fixed ratio (FR1) schedule. The position of the active lever was counterbalanced within each environmental condition. On the next day, rats were again placed into the operant conditioning chamber for a 60-min session, although the lever (inactive) was also extended, but pressing it had no programmed consequence. The FR value was increased to a FR5 schedule over 3 daily 60-min sessions. Following food reinforced pre-training; rats were maintained on ad libitum food access for the remainder of the experiment.
Catheterization Surgery
Following return to free-feeding body weights, rats underwent the same surgery procedure as in Experiment 1 and were allowed to recover for 7 days.
Amphetamine Self-Administration
Rats were placed in the operant conditioning chamber and allowed to acquire amphetamine self-administration during daily 60-min sessions using a unit dose of 0.1 mg/kg/infusion, which engenders reliable self-administration in both EC and IC rats (Green et al. 2002). Amphetamine self-administration was established under an FR1 schedule of reinforcement, with a 20-sec signaled timeout (TO). During the TO, the white cue lights above each lever were illuminated and lever presses were recorded but had no programmed consequence. The active and inactive levers for amphetamine self-administration were the same as those used in food pre-training. Each infusion was delivered in a volume of 0.06 ml over 3.33 sec, which coincided with the beginning of the 20-sec signaled TO.
Rats were allowed to self-administer amphetamine at the 0.1 mg/kg/infusion dose for a minimum of 7 sessions in order to establish reliable responding. EC and IC rats then were separated into one of two amphetamine dose groups; one group had the dose of amphetamine switched to 0.03 mg/kg/infusion and the other group was switched to 0.003 mg/kg/infusion. Rats were given a minimum of 5 daily sessions for behavior to stabilize, defined as less than 20% variability in the number of infusions earned across 3 consecutive sessions and a minimum of 2:1 (active: inactive lever) response ratio. Once behavior was stable, RU-486 pretreatments began. Forty-five min prior to the session, rats received either RU-486 (5, 10 or 20 mg/kg, i.p.) or vehicle. Each rat received each dose of RU-486 in random order. Following each pretreatment session, rats were given at least 2 intervening sessions of self-administration maintenance (no pretreatment) in order to maintain stable responding.
Data Analysis
The number of amphetamine injections earned was analyzed as a percent of control values (control values were determined by the number of infusions on maintenance days prior to pretreatment). The effect of RU-486 on the number of amphetamine infusions was analyzed using a mixed-factor ANOVA. Post hoc comparisons were conducted using a LSD post hoc test. For tests of significance, an alpha of p < 0.05 was used.
Drugs
D-amphetamine and RU-486 were purchased from Sigma/RBI (Natick, MA). RU-486 was dissolved in polyethylene glycol (mol wt. 400; PEG). Amphetamine for i.v. self-administration was dissolved in 0.9% w/v NaCl (saline) and injected in a volume of 0.06 ml over 3.33 sec. D-amphetamine and RU-486 doses are expressed as salt weights.
Results
Experiment 1: Basal Levels and Effect of Amphetamine on Free Corticosterone in EC, SC and IC Rats
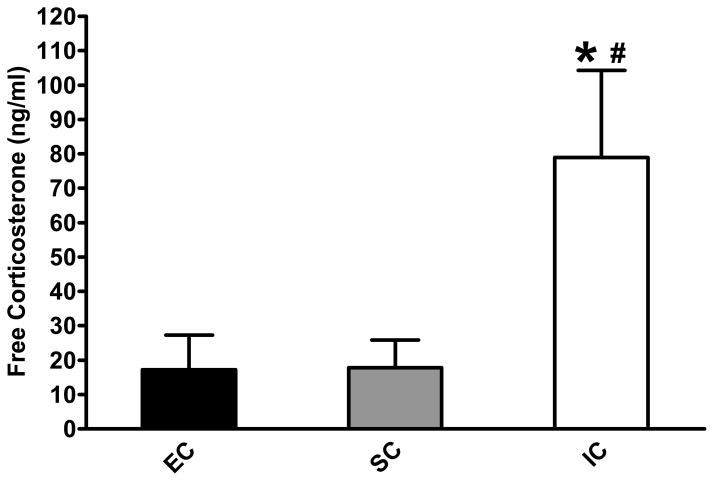
The environmental treatment groups displayed different basal levels of free corticosterone with the first blood collection (Fig. 1). A one way ANOVA demonstrated a significant effect of environment, F(2, 28) = 3.559, p < 0.05. Post hoc analyses revealed that IC rats had significantly higher levels of free corticosterone compared to both EC and SC rats (p < 0.05). Across the 6 repeated blood collections, however, this difference dissipated completely (results not shown). IC rats did not differ significantly from either EC or SC rats on the last (sixth) basal collection; mean (± SEM) free corticosterone for IC rats was 2.68 (±1.21) for SC rats was 4.91 (±1.01) and for EC rats was 1.62 (±0.57)
Fig 1.
Mean (± SEM) basal levels of free corticosterone (ng/ml) in plasma from EC, SC, and IC rats (N = 10/group). *indicates a significant difference compared to EC rats; and # indicates a significant difference compared to SC rats; p < 0.05.
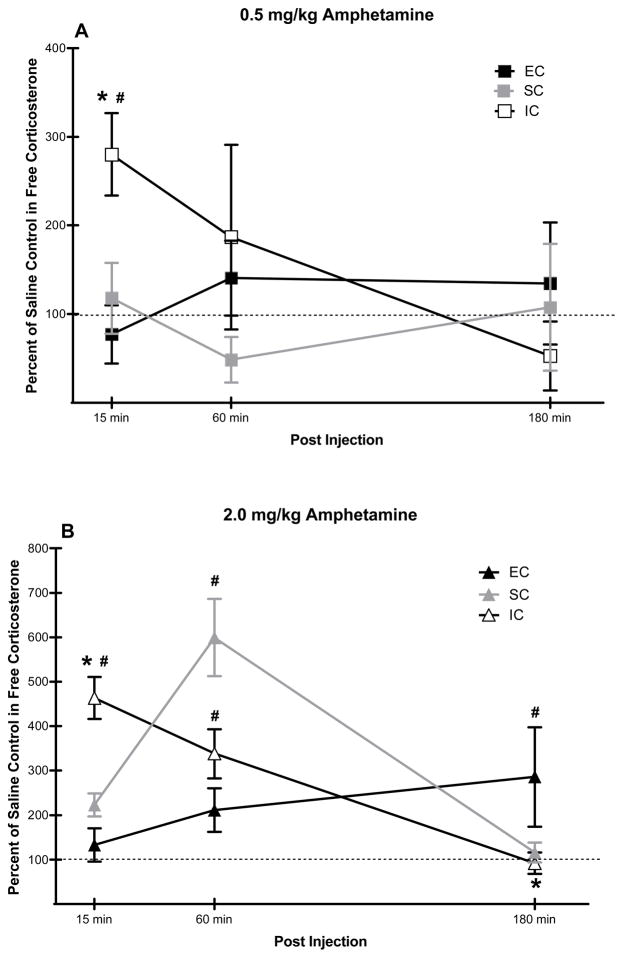
Differential rearing also altered the effect of amphetamine on free corticosterone. Expressed as a percent change from saline control levels, a mixed-factor ANOVA revealed a significant environmental condition × amphetamine dose × time interaction, F(8, 44) = 4.76, p < 0.01. With 0.5 mg/kg of amphetamine (Fig. 2A), post hoc comparisons revealed that IC rats had a significantly greater percent increase in free corticosterone at the 15 min interval compared to both EC and SC rats, p < 0.05; no group differences were found at either the 60 or 180 min intervals. With 2.0 mg/kg of amphetamine (Fig. 2B), post hoc comparisons also revealed that IC rats had a significantly greater percent increase in free corticosterone at the 15 min interval compared to both EC and SC rats (p < 0.05). At the 60 min interval, SC rats had significantly higher levels of corticosterone compared to both EC and IC rats (p < 0.05). At the 180 min interval, EC rats had significantly higher levels of corticosterone than both SC and IC rats (p < 0.05).
Fig 2.
A–B. Mean (± SEM) percent difference from saline control in free corticosterone for EC, SC, and IC rats (N = 5–6/group) at 15, 60, and 180 min following 0.5 mg/kg of amphetamine (Panel A) or 2.0 mg/kg of amphetamine (Panel B). *indicates a significant difference compared to EC rats; and # indicates a significant difference compared to SC rats; p < 0.05. Mean (±SEM) free corticosterone levels (ng/ml) at the 3 time intervals following saline for IC rats were 2.8±1.3, 2.1±0.8 and 3.5±0.6; for SC rats were 6.2±1.9, 2.6±0.6 and 2.6±0.8; and for EC rats were 9.1±1.5, 4.2±1.4 and 2.2±0.7.
Experiment 2: Effect of RU 486 on Amphetamine Self-Administration in EC and IC Rats
Table 1. shows the baseline number of amphetamine infusions for the training dose (0.1 mg/kg/infusion) and the two lower doses (0.03 and 0.003 mg/kg/infusion) at which RU-486 was tested. At the initial training dose (0.1 mg/kg/infusion), EC and IC rats did not differ in the number of amphetamine infusions earned. When unit doses of amphetamine were changed to 0.003 or 0.03 mg/kg/infusion, there were main effects of environment, F(1, 75) = 61.59, p <0.001, and amphetamine dose, F(1, 75) = 52.31, p <0.001, but no significant interaction of amphetamine dose and environment in the baseline number of amphetamine infusions earned prior to RU-486 pretreatments. Post hoc comparisons indicated that IC rats earned significantly more infusions compared to EC rats at both the 0.03 and 0.003 mg/kg/infusions doses.
Table 1.
Baseline number (mean±SEM) of amphetamine infusions at the training dose (0.1 mg/kg/infusion) and the two test doses (0.003 and 0.03 mg/kg/infusion).
| Unit Dose of Amphetamine | |||
|---|---|---|---|
| Rearing Condition | 0.003 mg/kg/infusion | 0.03 mg/kg/infusion | 0.1 mg/kg/infusion (Training Dose) |
| IC | 22.3±2.1* | 45.9±3.1* | 19±1.6 |
| EC | 5.1±0.8 | 24.0±4.1 | 19±2.3 |
Significant difference from EC; p < 0.05
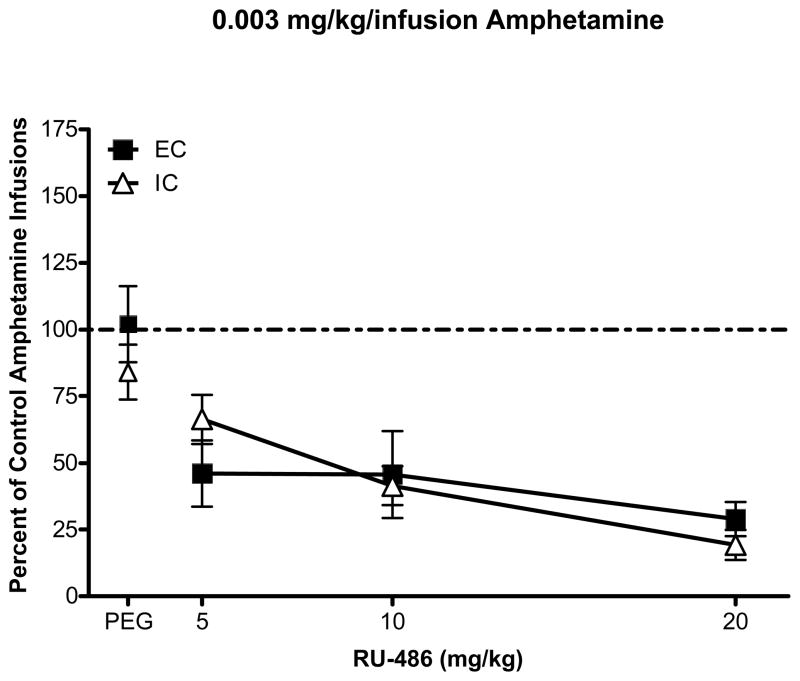
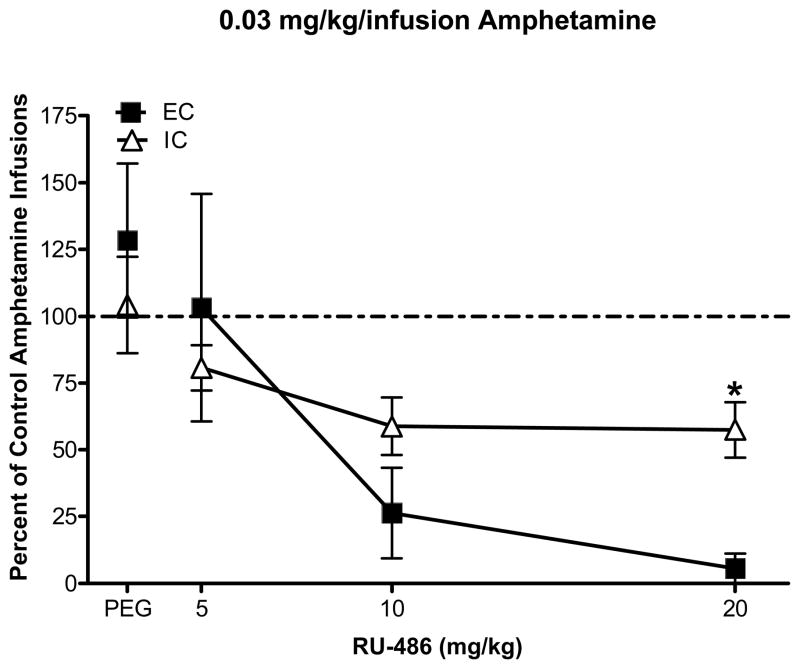
When the number of infusions was expressed as a percent change from control (session prior to pretreatment injections), ANOVA revealed a main effect of RU-486 pretreatment dose, F(3, 30) = 15.64, p < 0.001 and a significant environment × pretreatment dose interaction, F(3, 30) = 3.71, p < 0.05. While RU-486 decreased responding in both EC and IC rats, post hoc comparisons revealed that IC rats showed a blunted effect of RU-486 compared to EC rats at the high unit dose, but not at the low unit dose (Figs 3 and 4). RU-486 had no significant effect on the number of inactive lever responses or timeout responses in either IC or EC rats (results not shown).
Fig 3.
Mean (± SEM) percent change in the number of amphetamine infusions compared to the baseline session prior to pretreatment (designated by broken line) in EC and IC rats self-administering the 0.003 mg/kg/infusion dose of amphetamine.
Fig 4.
Mean (± SEM) percent change in the number of amphetamine infusions compared to the baseline session prior to pretreatment (designated by broken line) in EC and IC rats self-administering the 0.03 mg/kg/infusion dose of amphetamine. * indicates a significant difference compared to EC rats; p < 0.05.
Discussion
The results from Experiment 1 indicate that basal free corticosterone levels are elevated in IC rats compared to EC and SC rats following the first blood collection in the early portion of the light cycle. These results are congruent with previous literature showing that social isolation (Heidbreder et al. 2000) and wire grid floors (Belz et al. 2003) increase glucocorticoid levels. These results also indicate that the isolated condition used in the current experiment, as well as in previous experiments (Bardo et al. 2001; Bowling and Bardo 1994; Bowling et al. 1993; Green et al. 2002), increases free corticosterone and thus may be viewed as a chronically stressful environment. Importantly, however, across 6 repeated blood collections, this isolation-induced increase in corticosterone dissipated completely. Thus, while isolation rearing may have sensitized rats to the stress associated with the first blood collection, habituation occurred across repeated collections.
The effects of amphetamine pretreatment also are generally congruent with previous literature showing that amphetamine increases corticosterone levels in rats (Cador et al. 1993; Spangler et al. 1997). However, an important finding observed here is that isolation rearing produced an enhanced corticosterone response to amphetamine, with IC rats showing both the greatest dose-dependent sensitivity and most rapid response. Specifically, with 0.5 mg/kg of amphetamine, IC rats were the only group to show a drug-induced increase in free corticosterone at 15 min. With 2.0 mg/kg of amphetamine, IC rats again were the only group to show a drug-induced increase in free corticosterone at 15 min. However, both SC and EC showed a delayed increase in corticosterone following the higher dose of amphetamine (2.0 mg/kg), with SC rats showing an increase at 60 min and EC showing an increase at 180 min. Thus, the most rapid amphetamine-induced rise in corticosterone levels occurred in IC rats, with SC rats showing a delayed response, and EC rats showing the most delayed response, indicating that both enrichment per se (novel objects) and social interaction alone contribute to the altered reactivity of the HPA axis in response to amphetamine.
The enhanced reactivity to amphetamine in IC rats could be due to a sensitized HPA axis. Previous literature has shown that elevated levels of corticosterone due to restraint stress result in a sensitized locomotor response to amphetamine in rats (Deroche et al. 1992) and with experimentally altered corticosterone levels in mice (Pauly et al. 1993). Thus, it is possible that the isolation condition represents a stressful manipulation that resulted in a sensitized physiological (corticosterone) response to amphetamine.
Results from Experiment 2 replicate previous work showing that baseline rates of amphetamine self-administration are higher in IC rats than EC rats, but only at lower unit doses (Bardo et al. 2001; Green et al. 2002). In addition, regardless of differential rearing, RU-486 resulted in a dose-dependent decrease in amphetamine self-administration at both test doses of amphetamine (0.003 and 0.03 mg/kg/infusion). The effect of RU-486 on low-dose amphetamine self-administration is congruent with previous literature which found that the GR antagonist ketoconazole specifically decreases low-dose cocaine self-administration in standard housed rats (Goeders et al. 1998). While Goeders, et al (1998) found that ketoconazole did not affect high-dose cocaine self-administration, RU-486 in the current study decreased the 0.03 mg/kg dose of amphetamine self-administration. This discrepancy between studies may be due to differences in selectivity of the two GR receptor antagonists used (Svec 1988) and/or the drug self-administered (cocaine vs. amphetamine).
The RU-486-induced decrease in amphetamine self-administration was blunted in IC rats compared to EC rats. The decrease in amphetamine self-administration seen in both IC and EC rats is not likely due to a general decrease in overall activity since previous studies have found that RU-486 in the dose range used in the current report does not alter baseline levels of locomotor behavior or oral water intake (De Vries et al. 1996; Koenig and Olive 2004). Instead, the blunted response in IC rats may reflect an enhancement in amphetamine-induced corticosterone levels, which presumably would remove competition for RU-486 to bind to GR receptors, thus diminishing its ability to decrease amphetamine self-administration. Alternatively, isolation rearing may have decreased the sensitivity of GR receptors directly. In any case, numerous studies have revealed a role of the HPA axis in stimulant self-administration (Goeders 1997; Goeders and Guerin 1996; Piazza and Le Moal 1998; Sarnyai 1998; Sarnyai et al. 2001) and the current results extend these findings by demonstrating a role of the HPA axis even among rats with differential rearing histories.
Over activity of the HPA axis may also explain why IC rats displayed higher rates of baseline amphetamine self-administration compared to EC rats at low unit doses (0.003 and 0.03 mg/kg/infusion). This interpretation is congruent with previous literature showing that rats given 24-hr access to cocaine have the highest level of drug intake during the dark cycle, when corticosterone levels are typically the highest (Fitch and Roberts 1993; Roberts and Andrews 1997; Roberts et al. 2002). While we did not test corticosterone levels during amphetamine self-administration in the current report, which would have allowed for a more direct comparison to those previous reports, the finding that IC rats show an enhanced corticosterone response to amphetamine compared to EC rats suggest that elevated levels of corticosterone in IC rats is most likely playing a role in the environmentally-induced differences in amphetamine self-administration seen in previous studies (Bardo et al. 2001; Green et al. 2002).
One potential explanation for how glucocorticoids play a role in environmentally-induced differences in amphetamine self-administration is that repeated exposure to daily novelty during development is a form of mild stress that activates the HPA axis, thus promoting normal development. Given that previous research has shown that exposure to novelty increases corticosterone (Misslin et al. 1982; Ostrander et al. 2006), the daily object change experienced by EC rats may have released corticosterone to levels essential for normal development of the HPA axis, including the expression of GR receptors. In contrast, the lack of novel stimulation during development in IC rats may have led to a supersensitive HPA axis when challenged with a stressor (handling or novelty) or drug. Consistent with this explanation, previous research indicates that enrichment increases GR receptors in hippocampus (Meaney et al. 1985b; Olsson et al. 1994; Zou et al. 2001). The increase in GR receptors with environmental enrichment may result in a more efficient HPA negative feedback system, thus resulting in lower levels of corticosterone (Meaney and Aitken 1985; Meaney et al. 1985a; Meaney et al. 1985c). In contrast, a dysfunctional negative feedback control of the HPA axis may explain why IC rats have higher levels of free corticosterone in response to amphetamine compared to EC rats. Further, a decrease in GR receptor density in IC rats compared to EC rats could explain the blunted sensitivity of IC rats to the RU-486-induced decrease in amphetamine self-administration.
A second potential explanation for how glucocorticoids play a role in environmentally-induced differences in amphetamine self-administration is that chronic activation of the HPA axis during development in IC rats could lead to a sensitized mesolimbic dopamine pathway. Glucocorticoids exert effects by binding to GR receptors in nucleus accumbens shell (sNAcc; Ahima and Harlan 1990; Cho and Little 1999; Piazza et al. 1996). Elevated corticosterone levels in IC rats may activate GR receptors in sNAcc, thus resulting in a sensitized dopamine response that may explain the greater sensitivity to the reinforcing effect of low doses of amphetamine (Bardo et al. 2001; Green et al. 2002; Stairs et al. 2006). Sensitization of the mesolimbic dopamine pathway by stress in IC rats could also occur through increased activation of noradrenergic neurons in the locus coeruleus. Noradrenergic neurons within the locus coeruleus have excitatory effects on the mesocorticolimbic system (Morilak et al. 2005), which could also result in greater levels of extracellular dopamine in reward-relevant terminals.
While the current results indicate that social isolation enhances reactivity of the HPA axis, which could explain the enhanced self-administration of amphetamine in IC rats, more research is needed to fully understand the differences between EC and IC rats in the function of the HPA axis. For instance, it will be important to determine if EC and IC rats differ in GR receptor levels and binding affinity in important mesolimbic structures. Also, it would be interesting to determine if EC and IC rats differ in levels of corticosterone-releasing hormone or adrenocorticotropic hormone, which could determine if the negative feedback loop for the HPA axis is altered by differential rearing. Such information may be important not only for understanding the role of stress in environmentally-induced differences in stimulant use, but also in the development of novel treatments for relapse (Anker and Carroll 2010; Solinas et al. 2008; Thiel et al. 2009)
Acknowledgments
Acknowledgements of Funding:
Supported by NIH grants P50 DA 05312 and R01 DA 12964.
References
- Adams J, Bowman K, Burke B, Casson L, Caviness L, Coffey LE, Devore J, Durham J, Ellis C, Hewitt D, Hinsdale M, Johnson I, Myers S, Penne M, Zelon H. National Household Survey on Drug Abuse Data Collection. Final Report 1999 [Google Scholar]
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 2010;208:211–22. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–6. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–64. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–93. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Butte JC, Kakihana R, Noble EP. Circadian rhythm of corticosterone levels in rat brain. J Endocrinol. 1976;68:235–9. doi: 10.1677/joe.0.0680235. [DOI] [PubMed] [Google Scholar]
- Cador M, Dulluc J, Mormede P. Modulation of the locomotor response to amphetamine by corticosterone. Neuroscience. 1993;56:981–8. doi: 10.1016/0306-4522(93)90144-5. [DOI] [PubMed] [Google Scholar]
- Cheifetz P, Gaffud N, Dingman JF. Effects of bilateral adrenalectomy and continuous light on the circadian rhythm of corticotropin in female rats. Endocrinology. 1968;82:1117–24. doi: 10.1210/endo-82-6-1117. [DOI] [PubMed] [Google Scholar]
- Cho K, Little HJ. Effects of corticosterone on excitatory amino acid responses in dopamine-sensitive neurons in the ventral tegmental area. Neuroscience. 1999;88:837–45. doi: 10.1016/s0306-4522(98)00264-4. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Tjon GH, Nestby P, Mulder AH, Vanderschuren LJ. Mifepristone prevents the expression of long-term behavioural sensitization to amphetamine. Eur J Pharmacol. 1996;307:R3–4. doi: 10.1016/0014-2999(96)00308-1. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants. II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther. 1997;281:1401–7. [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Maccari S, Le Moal M, Simon H. Repeated corticosterone administration sensitizes the locomotor response to amphetamine. Brain Res. 1992;584:309–13. doi: 10.1016/0006-8993(92)90911-r. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, Kellendonk C, Le Moal M, Spanagel R, Schutz G, Tronche F, Piazza PV. The glucocorticoid receptor as a potential target to reduce cocaine abuse. J Neurosci. 2003;23:4785–90. doi: 10.1523/JNEUROSCI.23-11-04785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch TE, Roberts DC. The effects of dose and access restrictions on the periodicity of cocaine self-administration in the rat. Drug Alcohol Depend. 1993;33:119–28. doi: 10.1016/0376-8716(93)90053-s. [DOI] [PubMed] [Google Scholar]
- Goeders NE. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology. 1997;22:237–59. doi: 10.1016/s0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996;64:337–48. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Peltier RL, Guerin GF. Ketoconazole reduces low dose cocaine self-administration in rats. Drug Alcohol Depend. 1998;53:67–77. doi: 10.1016/s0376-8716(98)00108-2. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–8. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Hiroshige T, Sakakura M. Circadian rhythm of corticotropin-releasing activity in the hypothalamus of normal and adrenalectomized rats. Neuroendocrinology. 1971;7:25–36. doi: 10.1159/000121952. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (U.S.) Guide for the care and use of laboratory animals. National Academy Press, National Academy Press; 1996. [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–75. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology. 2004;29:999–1003. doi: 10.1016/j.psyneuen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 1985;354:301–4. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Sapolsky RM. The effects of postnatal handling on the development of the glucocorticoid receptor systems and stress recovery in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1985a;9:731–4. doi: 10.1016/0278-5846(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Tatarewicz JE, Sapolsky RM. Early postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. Behav Neurosci. 1985b;99:765–70. doi: 10.1037//0735-7044.99.4.765. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. I. Ontogeny and autoregulation. Brain Res. 1985c;350:159–64. doi: 10.1016/0165-3806(85)90259-7. [DOI] [PubMed] [Google Scholar]
- Misslin R, Herzog F, Koch B, Ropartz P. Effects of isolation, handling and novelty on the pituitary--adrenal response in the mouse. Psychoneuroendocrinology. 1982;7:217–21. doi: 10.1016/0306-4530(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–24. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Olsson T, Mohammed AH, Donaldson LF, Henriksson BG, Seckl JR. Glucocorticoid receptor and NGFI-A gene expression are induced in the hippocampus after environmental enrichment in adult rats. Brain Res Mol Brain Res. 1994;23:349–53. doi: 10.1016/0169-328x(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–17. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Robinson SF, Collins AC. Chronic corticosterone administration enhances behavioral sensitization to amphetamine in mice. Brain Res. 1993;620:195–202. doi: 10.1016/0006-8993(93)90156-h. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Barrot M, Rouge-Pont F, Marinelli M, Maccari S, Abrous DN, Simon H, Le Moal M. Suppression of glucocorticoid secretion and antipsychotic drugs have similar effects on the mesolimbic dopaminergic transmission. Proc Natl Acad Sci U S A. 1996;93:15445–50. doi: 10.1073/pnas.93.26.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A. 1991;88:2088–92. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Andrews MM. Baclofen suppression of cocaine self-administration: demonstration using a discrete trials procedure. Psychopharmacology (Berl) 1997;131:271–7. doi: 10.1007/s002130050293. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–9. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z. Neurobiology of stress and cocaine addiction. Studies on corticotropin-releasing factor in rats, monkeys, and humans. Ann N Y Acad Sci. 1998;851:371–87. doi: 10.1111/j.1749-6632.1998.tb09011.x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–43. [PubMed] [Google Scholar]
- Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology (Berl) 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A. 2008;105:17145–50. doi: 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Zhou Y, Schlussman SD, Ho A, Kreek MJ. Behavioral stereotypies induced by “binge’ cocaine administration are independent of drug-induced increases in corticosterone levels. Behav Brain Res. 1997;86:201–4. doi: 10.1016/s0166-4328(96)02257-7. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol. 2006;17:597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- Svec F. Differences in the interaction of RU 486 and ketoconazole with the second binding site of the glucocorticoid receptor. Endocrinology. 1988;123:1902–6. doi: 10.1210/endo-123-4-1902. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12:1151–6. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163(3):890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou B, Golarai G, Connor JA, Tang AC. Neonatal exposure to a novel environment enhances the effects of corticosterone on neuronal excitability and plasticity in adult hippocampus. Brain Res Dev Brain Res. 2001;130:1–7. doi: 10.1016/s0165-3806(01)00173-0. [DOI] [PubMed] [Google Scholar]