Abstract
Dendritic spines are dynamic structures that accommodate the majority of excitatory synapses in the brain and are influenced by extracellular signals from presynaptic neurons, glial cells and the extracellular matrix (ECM). The ECM surrounds dendritic spines and extends into the synaptic cleft, maintaining synapse integrity as well as mediating trans-synaptic communications between neurons. Several scaffolding proteins and glycans that compose the ECM form a lattice-like network, which serves as an attractive ground for various secreted glycoproteins, lectins, growth factors and enzymes. ECM components can control dendritic spines through the interactions with their specific receptors or by influencing the functions of other synaptic proteins. In this review, we focus on ECM components and their receptors that regulate dendritic spine development and plasticity in the normal and diseased brain.
Keywords: dendritic spines, synapse, extracellular matrix, brain, neurological disorders
DENDRITIC SPINES
Over a century ago, Santiago Ramón y Cajal first discovered the existence of dendritic spines upon analysis of the surface of Purkinje cell dendrites, and proposed that electrochemical signals may be transduced through those spines (Ramon y Cajal, 1888; Ramon y Cajal, 1899). Since then, it has been well established that dendritic spines are small extensions from the surface of a dendrite that accommodate the postsynaptic sites of most excitatory synapses in the brain and our knowledge of their structure and function has significantly progressed in recent years (reviewed in Sorra and Harris, 2000; Hering and Sheng, 2001; Yuste and Bonhoeffer, 2004; Ethell and Pasquale, 2005). While aspiny interneurons receive both excitatory and inhibitory pre-synaptic inputs directly onto the dendritic shaft, spiny neurons preferentially form excitatory synapses on dendritic spines, which come in a variety of shapes and sizes, ranging from long thin spines with small heads to short stubby- and mushroom-shaped spines with large heads (Fig. 1A–D). Excitatory postsynaptic sites are usually located on spine heads, which are bulbous, biochemical chambers that are separated from the rest of the dendrite by a thin neck (Fig. 1E), giving it the ability to function as a relatively independent synaptic response element. The postsynaptic site is identified as an electron-dense region known as the postsynaptic density (PSD in Fig. 1E), which populates about 10% of the spine head area and is juxtaposed with the presynaptic terminal containing neurotransmitter-packaged synaptic vesicles (SV in Fig. 1E; Harris and Stevens, 1989; Knott et al., 2006; Zhang et al., 2010). Glutamate, the most common excitatory neurotransmitter in the brain, is released from presynaptic terminals during synaptic transmission to activate specific glutamate receptors located at the postsynaptic site. In addition to glutamate receptors, the PSD contains cell adhesion molecules and various cell surface receptors that act together with scaffolding proteins to link the PSD to filamentous actin (F-actin). Dendritic spines are enriched in F-actin that plays an important role in shaping dendritic spines as well as the trafficking of functional glutamate receptors in and out of the PSD. Some dendritic spines also contain a spine apparatus, which consists of smooth endoplasmic reticulum (SER)-like membrane compartments that serve as Ca2+ stores and as a harbor for additional glutamate receptors.
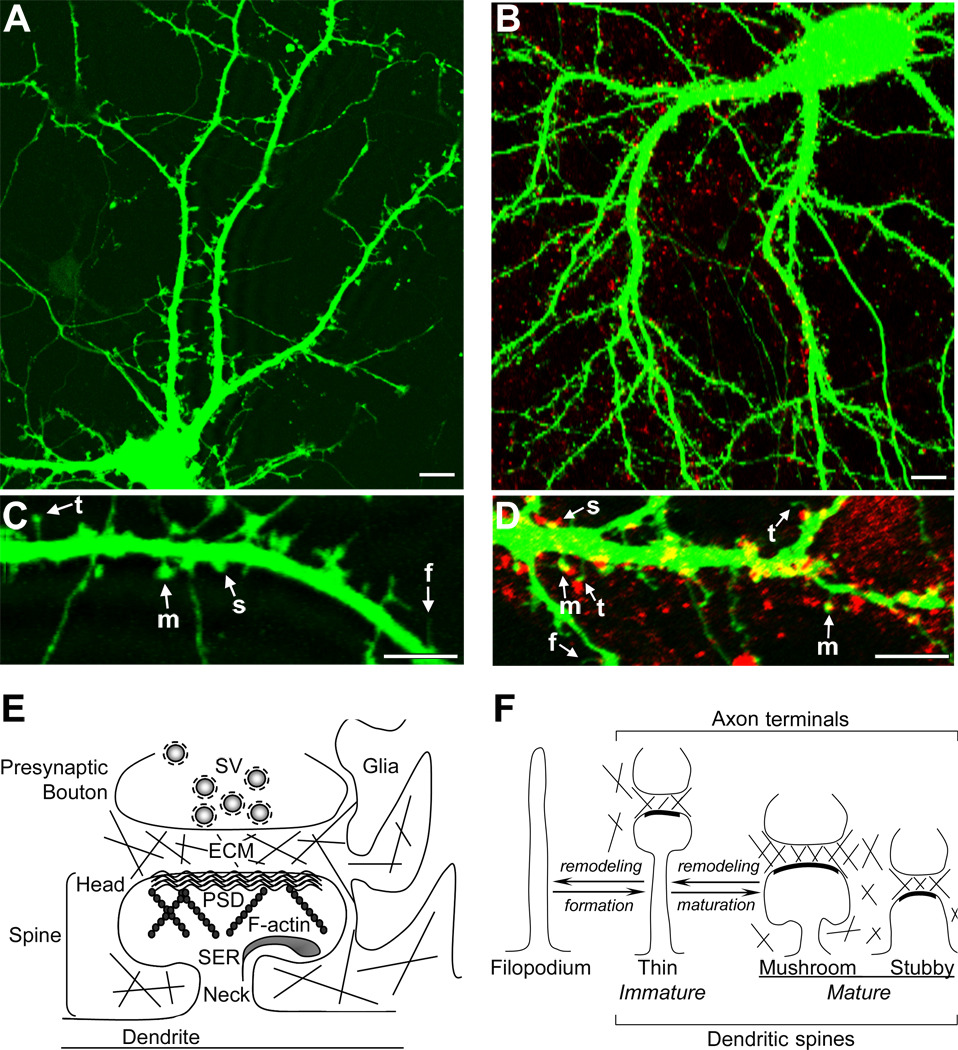
FIGURE 1.
Dendritic spine ultrastructure and morphology. (A) GFP-expressing hippocampal neuron from 14DIV hippocampal cultures. (B) GFP-expressing hippocampal neuron (green) with presynaptic boutons labeled by immunostaining against synaptophysin (red). (C) High magnification view of the dendrite shows the typical thin (t), mushroom (m) and stubby (s) spines and dendritic filopodia (f). (D) High magnification view of the dendrite shows GFP-positive spines (green) with presynaptic synaptophysin-positive boutons (red). (E) Schematic illustration showing ultrastructure of dendritic spine with adjacent presynaptic terminal and surrounding glial processes. (F) Schematic illustration of a filopodium and main categories of dendritic spines: thin, mushroom and stubby spines. ECM, extracellular matrix; F-actin, filamentous actin; PSD, postsynaptic density; SER, smooth endoplasmic reticulum; SV, synaptic vesicles. Scale bar in (A) and (C), 10µm; scale bar in (B) and (D), 5µm.
Dendritic spine formation directly coincides with the formation of postsynaptic sites. Before the appearance of dendritic spines, dendrites exhibit long, thin filopodia-like protrusions. As the brain matures, the number of dendritic filopodia declines and the number of stable dendritic spines with mushroom-like and stubby shapes increases (see examples of different spines in Fig. 1C). Although dendritic spines can arise directly from the dendritic shaft (Fiala et al., 1998; Marrs et al., 2001; Kwon and Sabatini, 2011), it is widely accepted that spines can also form from filopodia, which are perceived as dendritic spine precursors both in vitro and in vivo (Dailey and Smith, 1996; Ziv and Smith, 1996; Maletic-Savatic et al., 1999; Lendvai et al., 2000; Marrs et al., 2001; Okabe et al., 2001; Trachtenberg et al., 2002; Portera-Cailliau et al., 2003; Ziv and Garner, 2004; Knott et al., 2006). Filopodia are long, headless and transient extensions, which can transform into immature thin spines upon contact with axon terminals (Fig. 1F). It has also been suggested that filopodia may have additional functions, such as involvement in dendritic growth and branching (Niell et al., 2004; Yuste and Bonhoeffer, 2004; Zhang et al., 2010). Filopodia-derived spines typically first appear as immature thin spines with long, thin necks and small head areas (Fig. 1F), which can directly transform into mature mushroom spines (Vaughn, 1989; Ziv and Smith, 1996; Marrs et al., 2001; Knott et al., 2006; Knott and Holtmaat, 2008), or can shrink and completely collapse followed by the formation of mushroom spines from the dendritic shaft (Dailey and Smith, 1996; Fiala et al., 1998; Knott and Holtmaat, 2008). Immature dendritic spines and filopodia are very dynamic and plastic structures that are highly susceptible to changes in synaptic activity (Lang et al., 2004; Matsuzaki et al., 2004; Nimchinsky et al., 2004; Ashby et al., 2006; Zhang et al., 2010).
Dendritic spine morphology undergoes developmental changes leading to spine maturation, which reflects the transformation of immature thin spines into mature spines and coincides with postsynaptic differentiation. Mature spines are primarily characterized as two types: mushroom spines with short thin necks and large head areas, and stubby spines with thick necks and head areas that are not clearly distinguishable (Fig. 1F). Mature spines are shown to be more stable and highly sensitive to glutamate than immature spines due to a larger PSD and sometimes contain perforated synapses that exhibit discontinuous PSD profiles (Matsuzaki et al., 2001; Murthy et al., 2001; Smith et al., 2003; Nicholson et al., 2006). Perforated synapses are typically much larger, contain a higher number of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) and N-methyl-D-aspartate receptors (NMDARs) and are capable of generating large synaptic conductances (Nicholson et al., 2006). Several studies demonstrated a direct correlation between the size of the PSD and the number of glutamate receptors that the spine accommodates (Matsuzaki et al., 2001; Shinohara and Hirase, 2009). Although, there is a large variation in the expression of functional AMPARs among different types of dendritic spines, the size of PSD was shown to be proportional to the spine head size, and mushroom spines with a larger head volume contained a higher proportion of functional AMPARs in hippocampal neurons (Matsuzaki et al., 2001).
The majority of mature spines are highly stable structures that can last from months to a lifetime, implicating mature spines as the physical manifestation of long-term information storage (Grutzendler et al., 2002; Holtmaat et al., 2005; Zuo et al., 2005; Zuo et al., 2005; Yang et al., 2009; Zhang et al., 2010). However, mature spines remain plastic in the adult brain and can also undergo structural remodeling, such as enlargement, reduction or complete elimination, in response to various stimuli (Segal, 2005; Yasumatsu et al., 2008; Kasai et al., 2010; Zhang et al., 2010). Mature mushroom-shaped spines can transform back into immature thin spines, revert into filopodia lacking synaptic contact or completely collapse. This structural plasticity of dendritic spines has been shown to correlate with functional plasticity at the level of the individual synapse (Matsuzaki et al., 2004; Okamoto et al., 2004; Zhou et al., 2004; Yasumatsu et al., 2008; Kasai et al., 2010). The induction of long-term potentiation (LTP) or long-term depression (LTD), which may enhance or decrease synapse strength has been shown to trigger changes in dendritic spine morphology. For example, high-frequency stimulation (HFS) typically results in high calcium influx, LTP and the insertion of additional AMPARs into the postsynaptic membrane, which also coincides with the enlargement of existing dendritic spines and formation of new spines (Lang et al., 2004; Matsuzaki et al., 2004; Nagerl et al., 2004; Okamoto et al., 2004; Otmakhov et al., 2004). Induction of LTP in mouse CA1 hippocampal neurons by HFS demonstrated a transient expansion of dendritic spines that was further enhanced with subsequent stimulations (Lang et al., 2004). LTP-induced spine enlargement was dependent on the activation of NMDA- and AMPA-type glutamate receptors, as well as actin reorganization that shifted the equilibrium toward polymerized F-actin (Okamoto et al., 2004). In addition, Gu and colleagues (2010) have recently shown that LTP-induced spine enlargement temporally lags behind AMPAR insertion, suggesting that changes in spine shape may accommodate maintenance of newly inserted AMPARs rather than induce their insertion into the postsynaptic site (Gu et al., 2010). In contrast, LTD that is usually triggered by persistent, low frequency stimulation (LFS) and low levels of calcium influx, results in AMPAR endocytosis, spine shrinkage and elimination (Nagerl et al., 2004; Okamoto et al., 2004; Zhou et al., 2004; Bastrikova et al., 2008). LFS stimulation of mouse CA1 hippocampal neurons induced synapse loss as well as selective spine retraction that could be reversed with subsequent HFS stimulation (Nagerl et al., 2004; Zhou et al., 2004; Bastrikova et al., 2008). LFS-induced spine shrinkage and retraction were dependent on NMDAR activation (Zhou et al., 2004) and actin depolymerization, which led to changes in dendritic spine morphology and number (Okamoto et al., 2004).
Synaptic activity plays a major role in regulating dendritic spine formation, maturation and plasticity by molecular mechanisms involving the activation of postsynaptic glutamate receptors, as well as other cell surface receptors that are not directly engaged in synaptic transmission, but can modulate synaptic activity and trigger changes in dendritic spine number and morphology. Some of the postsynaptic receptors mediate trans-synaptic communications, whereas others are directly influenced by components of the ECM. Both physiological and pathological stimuli can trigger the activation of these cell surface receptors that serve as a link between extracellular signals and intracellular events leading to formation, maturation or remodeling of postsynaptic spines. In this review, we focus on the receptors that respond to changes in ECM composition mediating the effects of the ECM on dendritic spines, including integrins, proteoglycans and some receptor tyrosine kinases.
ECM COMPONENTS SURROUNDING DENDRITIC SPINES
What is the ECM?
Extracellular space (ECS) occupies around 20% of the brain (Ruoslahti, 1996; Nicholson and Syková, 1998), but its volume and composition varies depending on the brain region (Brückner et al., 1996; Hausen et al., 1996; Matthews et al., 2002). ECS volume is higher in the hippocampus, striatum and cerebellum as compared to the cerebral cortex (Zhang and Verkman, 2010). ECS is filled with ECM that surrounds neurons and forms a perineuronal net (PNN), which is prominent around inhibitory interneurons, but also found around excitatory neurons. PNNs envelope neuronal cell bodies and proximal dendrites, where they control the three-dimensional organization, growth, movement, and shape of neurons that is important for maintaining structural integrity (Celio et al., 1998) as well as functioning in repair of the central nervous system (CNS), which is covered by Kwok and colleagues within this special edition (Kwok et al., 2011). Although synapses in the CNS do not contain basal lamina, ECM components also extend into the synaptic clefts as well as extrasynaptic space surrounding dendritic spines in the brain (Pappas et al., 2002; Chen et al., 2003; Indyk et al., 2003; Lucic et al., 2005).
Each component of the ECM is specialized for different functions such as repulsion or adhesion, and can have varying properties from rigidity to elasticity (Mecham, 1998; Kleinman et al., 2003). ECM composition ranges from scaffolding proteins such as laminin, fibronectin and tenascin that link various ECM components into a net, to heavily glycosylated proteins called proteoglycans, to matrix metalloproteinases (MMPs) that cleave and remodel the ECM (Table 1). In this review, we focus on the properties of ECM components in CNS synapses and their roles in dendritic spine formation, maturation and plasticity.
TABLE 1.
ECM Components Regulating Dendritic Spines and Synapses
| ECM Component | Size kD | Glycosylation1 | Effects on Spines/Synapses | Assay | Factors regulating levels and activity |
ECM receptors/binding partners/targets |
References |
|---|---|---|---|---|---|---|---|
| Scaffolding proteins | |||||||
| Laminin chains α1, α2, α3, α4, α5, β2, γ3 |
850–900 | N-linked oligosaccharides |
Promotes dendritic spine proliferation in Purkinje cells Controls LTP maintenance in hippocampus Regulates synaptic density in hippocampus and controls the size of synaptic cleft and PSD length |
Astrocyte-derived laminin in primary cultures Proteolytic degradation of laminin Laminin β2 KO mice |
Secreted primarily by astrocytes Cleaved by MMPs |
integrins, agrin, heparin, heparan sulfate |
Liesi et al., 1983; Beck et al., 1990; Ruoslahti, 1991; Represa et al., 1995; Jucker et al., 1996; Seil, 1998; Nakagami et al., 2000; Egles et al., 2007 |
| Fibronectin | 440 | N- and O-linked Oligosaccharides within type III repeats |
Potentiates NMDAR currents via integrin signaling |
Recombinant fibronectin in acute hippocampal slices |
Secreted primarily by astrocytes Cleaved by MMPs |
collagen, fibrin, thrombospondin, heparin, chondroitin sulfate, integrins, tenascin-C, syndecans |
Liesi et al., 1983; Hynes, 1990; Yoshida and Takeuchi, 1991; Chung et al., 1995; Represa et al., 1995; Romberger, 1997; Pankov and Yamada, 2002; Bernard-Trifilo et al., 2005; Mao and Schwarzbauer, 2005 |
| Tenascins tenascin-C, tenascin-R |
160–250 | N-linked sulfated oligosaccharides, chondroitin sulfate O-linked sialylated glycans |
Control distribution of stubby spines in cerebral cortex and LTD maintenance in hippocampus Regulate density and length of dendrites; and basal levels of excitatory transmission and LTP |
Tenascin-C KO mice Tenascin-R KO mice |
Cleaved by MMPs Expressed by oligodendrocytes and small interneurons |
integrins, lecticans, syndecans, Na+ and K+ channels |
Weber et al., 1999; Zamze et al., 1999; Saghatelyan et al., 2000; Bukalo et al., 2001; Saghatelyan et al., 2001; Strekalova et al., 2002; Woodworth et al., 2002; Woodworth et al., 2004; Irintchev et al., 2005; Syková et al., 2005; Bukalo et al., 2007 |
| Proteoglycans | |||||||
| Agrin NtA-Agrin (secreted) TM-Agrin (transmembrane) |
400–600 | N- and O-linked Heparan and chondroitin sulfate |
Regulates dendritic spine density and number of excitatory synapses in cerebral cortex Regulates dendritic filopodia and synapse density Promotes dendritic filopodia formation Promotes axonal filopodia formation |
Agrin KO mice (Tg/agrn−/−) RNAi knockdown of TM-Agrin in cultured mouse hippocampal neurons 22 kDa agrin fragmen t in acute hippocampal slices Deletion mutants of N- terminus in cultured rat hippocampal neurons |
Cleaved by Neurotrypsin |
laminin, α-dystroglycan, heparin, NCAM,, LRP4 |
Denzer et al., 1995; Denzer et al., 1997; Gesemann et al., 1996; Neumann et al., 2001; Bezakova and Ruegg, 2003; Annies et al., 2006; McCroskery et al., 2006; Ksiazek et al., 2007; Ngo et al., 2007; Reif et al., 2007; Zhang et al., 2008; McCroskery et al., 2009; Matsumoto-Miyai et al., 2009; Lin et al., 2010 |
| Growth factors | |||||||
| Thrombospondins thrombospondin-1 thrombospondin-2 |
180 | O-linked fucosylation C-linked mannosylation |
Promotes synaptogenesis | Primary cultures of rat retinal ganglion neurons from Thrombospondin-1 and -2 double KO mice |
Secreted by astrocytes |
laminin, fibronectin, integrins, ApoE2, LRP, VLDLR, neuroligin-1, α2δ-1 |
Prater et al., 1991; Kosfeld and Frazier, 1992; Godyna et al., 1995; Tucker, 2004; Christopherson et al., 2005; Blake et al., 2008; Eroglu et al., 2009; Xu et al., 2009; Garcia et al., 2010; Leonhard-Melief and Haltiwanger, 2010 |
| Reelin | 400 | N- and O-linked glycans |
Regulates dendritic spine density and size within cortex Promotes LTP |
Heterozygous reeler mice Recombinant reelin in acute hippocampal slices |
Secreted by neurons |
integrins, ApoE2, VLDLR |
D’Arcangelo et al., 1997; Rodriguez et al., 2000; Costa et al., 2001; Liu et al., 2001; Pappas et al., 2002; Quattrocchi et al., 2002; Weeber et al., 2002; Ridley et al., 2003; Strasser et al., 2004; Beffert et al., 2006 |
| Lectins | |||||||
| Neuronal Pentraxins NP-1 NP-2 (Narp) |
100 | ND | Promote AMPAR clustering at PSD Regulates activity dependent synapse formation and elimination |
Cultured hippocampal neurons Narp and NP-1 KO mice |
ND | proteoglycans and NPR |
Dodds et al., 1997; O’Brien et al., 2002; Xu et al., 2003; Bjartmar et al., 2006; Sia et al., 2007; Cho et al., 2008; Chang et al., 2010 |
| Proteases | |||||||
| MMPs MT-MMP-5 MMP-3 MMP-2 MMP-9 |
72–92 | ND | Enhances LTP Mediates LTP-induced enlargement of dendritic spines Promotes fiber sprouting and reactive synaptogenesis Induces dendritic spine elongation and immature spine morphology |
Recombinant active MMP-9 in acute hippocampal slices MMP inhibition in acute hippocampal slices Kainate-evoked epileptogenesis in rat model and rat hippocampal slice cultures Recombinant active MMP-9 in cultured hippocampal neurons |
Secreted by neurons and glia Regulated by ischemic brain injuries and epileptogenesis Regulated by learning and LTP |
Degrade laminin, fibronectin and collagen Promote cleavage of NCAM and ICAM, Eph receptors and ephrins, and cadherins |
Backstrom et al., 1996; Zuo et al., 1998; Rosenberg et al., 2001; Kotra et al., 2002; Szklarczyk et al., 2002; Falo et al., 2006; Meighan et al., 2006; Monea et al., 2006; Rosenblum et al., 2007; Tian et al., 2007; Wang et al., 2008; Bilousova et al., 2009; Dwivedi et al., 2009; Hernández-Guillamon et al., 2009 |
| tPA | 63 | N-linked oligosaccharides O-linked fucosylation |
Promotes synaptic growth during L-LTP Regulates dendritic spine motility Promotes stress-induced pruning of dendritic spines |
Acute hippocampal slices Acute slices of P28 visual cortex Stress mouse model |
Secreted by astrocytes and neurons |
LRP | Vehar et al., 1986; Gualandris et al., 1996; Baranes et al., 1998; Zhuo et al., 2000; Mataga et al., 2004; Oray et al., 2004; Pawlak et al., 2005; Leonhard-Melief and Haltiwanger, 2010 |
Table includes information about glycosylation within the CNS
ND = not determined
Laminin is involved in the formation of spine synapses
Laminin, fibronectin and tenascins, which provide a structural scaffold for the ECM and are known to be involved in cell migration and axon guidance during brain development, are also implicated in the regulation of dendritic spine formation (Table 1; Powell and Kleinman, 1997; Tian et al., 1997; Strekalova et al., 2002; Bernard-Trifilo et al., 2005; Irintchev et al., 2005; Egles et al., 2007). Laminin is a multi-domain protein that exists in several isoforms and is comprised of three non-covalently associated chains (α, β and γ), which form a cross-like structure inter-linking other ECM molecules with each other and with cell surface receptors (Beck et al., 1990; Mecham, 1998; Garwood et al., 2003). Laminin is secreted by various cell types within the CNS, but its release from astrocytes has been best characterized (Liesi et al., 1983; Represa et al., 1995; Jucker et al., 1996; Seil, 1998). Laminin isolated from astrocyte-conditioned media induces dendritic spine proliferation in Purkinje cells (Seil, 1998), whereas the proteolytic degradation of laminin in the hippocampus impairs LTP maintenance (Nakagami et al., 2000). Multiple laminin isoforms were also detected in neurons (Yin et al., 2003; Sharif et al., 2004). The laminin α2 chain is the most abundant in the brain and is found to be most highly immunoreactive in association with dendritic spines (Tian et al., 1997). Other laminin chains, including α1, α3, α4, α5, β2, and γ3 were also found in the CNS (Table 1; Libby et al., 2000; Yagi et al., 2003). Laminin β2 was shown to play a role in synapse formation in the hippocampus, as reduced density of spine synapses was noted in the hippocampus of laminin β2 knockout (KO) mice and the formation of axo-dendritic synapses on the dendrites of laminin β2 KO neurons was impaired in mixed hippocampal cultures (Egles et al., 2007). Moreover, electron microscopy demonstrated structural changes in hippocampal synapses of laminin β2 KO mice with a 31% increase in the average distance between the pre and postsynaptic membrane and a 39% increase in the length of the PSD as compared to wild type mice. Although synaptic targeting of the laminin β2 chain, its interacting partners and the molecular mechanisms that mediate its effects on CNS synapses are still not clear, this study presents the first evidence that laminin is involved in the formation of spine synapses and postsynaptic differentiation in the CNS. The large number of laminin isoforms identified within CNS synapses increases the complexity of laminin organization, functions and targets in dendritic spines, which remains to be an interesting topic of future research.
Like laminin, fibronectin is also expressed by glial cells in the brain such as astrocytes (Yoshida and Takeuchi, 1991; Represa et al., 1995). Fibronectin forms rod-like structures that are composed of three types of repeating units, types I, II and III, which demonstrate selective affinities to collagen, fibrin and heparin (Table 1; Hynes, 1990; Romberger, 1997; Pankov and Yamada, 2002; Garwood et al., 2003). While fibronectin type-I binds to all three, fibronectin type-II and -III are specific to collagen and heparin, respectively. Although the role of fibronectin in dendritic spines is still not clear, fibronectin is a prominent component of the ECM and is known to interact with syndecans and tenascin-C (Chung et al., 1995; Mao and Schwarzbauer, 2005), both of which can regulate dendritic spine morphology and distribution and are discussed later in this review.
Tenascins are implicated in synaptic plasticity and dendritic spine distribution
Like laminin and fibronectin, tenascins are also scaffolding ECM proteins that assemble large ECM complexes together with lecticans and consist of five distinct members, with tenascin-R and tenascin-C being the most notable for their role in synaptic plasticity (Table 1). The primary structure of tenascins is characterized by a cysteine-rich N-terminus followed by epidermal growth factor (EGF) domains and fibronectin type-III domains (Garwood et al., 2003). Tenascins crosslink with proteoglycans such as lecticans, interact with Na+ and Ca2+ channels, and compete with fibronectin for binding to syndecans (reviewed in Ruoslahti, 1996; Saghatelyan et al., 2000; Yamaguchi, 2000; Strekalova et al., 2002). Tenascin-R is found exclusively within the CNS and is expressed by oligodendrocytes and small interneurons within the hippocampus and cerebellum (Zamze et al., 1999; Woodworth et al., 2002; Woodworth et al., 2004). Mice deficient in tenascin-R exhibit increased basal levels of excitatory transmission and impaired LTP (Weber et al., 1999; Bukalo et al., 2001; Saghatelyan et al., 2001; Syková et al., 2005; Bukalo et al., 2007). Tenascin-R KO mice show a reduction in ECS (Syková et al., 2005) and develop fewer and shorter dendrites within the cortex and hippocampus (Weber et al., 1999). While tenascin-R KO mice do not display any apparent changes to dendritic spines, they do demonstrate significant remodeling of ECM components, as shown by reduced immunoreactivity of hyaluronan, as well as brevican and neurocan (Aspberg et al., 1995; Aspberg et al., 1997; Brückner et al., 2000). Brevican and neurocan are members of the lectican family of secreted chondroitin sulfate proteoglycans (CSPGs) which contain hyaluronic acid binding sites and recruit lectins to their carbohydrate-rich central domain (Yamaguchi, 2000; Garwood et al., 2003). The lecticans are involved in creating the extracellular lattice-like ECM structure around dendritic spines and have been implicated in affecting synaptic plasticity (Zhou et al., 2001; Brakebusch et al., 2002) as well as lateral surface diffusion of AMPARs (Frischknecht et al., 2009). Future studies evaluating the effects of the disruption of lectican-hyaluronan-tenascin-R aggregates by genetic approach or via digestion of chondroitin sulfate and hyaluronic acid chains may contribute to our understanding of whether these secreted proteoglycans play a role in dendritic spine formation, remodeling and maintenance.
In contrast to the studies regarding tenascin-R, studies focusing on mice that are deficient in tenascin-C showed normal basal excitatory transmission, but impaired LTD (Strekalova et al., 2002). Although no changes in total spine density were found in the cerebral cortex of tenascin-C KO mice, deletion of tenascin-C shifted the distribution of stubby spines in motor and somatosensory cortices, such that fewer stubby spines were found on first order dendrites while third order basal and apical dendrites contained more stubby spines (Irintchev et al., 2005). These results demonstrate a clear role for the tenascins in the formation of the ECM surrounding dendritic spines, and suggest that tenascins may regulate dendritic spine distribution within different dendritic domains. The scaffolding proteins associate with the proteoglycans creating a lattice-like network that recruits sugar-binding ptroteeins lectins and secreted growth factors, such as reelin and thrombospondin, which are shown to influence dendritic spine/synapse formation and maintenance (Liu et al., 2001; Christopherson et al., 2005).
Synaptogenic activity of secreted growth factor thrombospondin
Thrombospondins belong to a family of astrocyte-secreted growth factors that are involved in cell-cell and cell-ECM interactions primarily through their association with heparan sulfate chains. Thrombospondin-1 has been shown to interact with ECM scaffolding proteins such as fibronectin, laminin and collagen, as well as ECM receptors such as integrins, apoliproprotein E2 (ApoE2) receptor, low density lipoprotein receptor (LRP), very low density lipoprotein receptor (VLDLR), and neuroligin-1 (Prater et al., 1991; Kosfeld and Frazier, 1992; Godyna et al., 1995; Christopherson et al., 2005; Blake et al., 2008; Xu et al., 2009). Among five thrombospondin members, thrombospondin-1 and trombospondin-2 were shown to mimic the effects of astrocyte-conditioned media and promote synaptogenesis in neuronal cultures (Christopherson et al., 2005; Xu et al., 2009; Garcia et al., 2010). Rat retinal ganglion neurons formed synaptic connections in the presence of thrombospondin-1 or thrombospondin-2, which were ultrastructurally identical to the synapses induced by a feeding layer of astrocytes (Christopherson et al., 2005). The thrombospondin-induced synapses were presynaptically active but postsynaptically silent, containing fewer functional AMPARs and no detectable AMPA component to the evoked response. The thrombospondin-mediated formation of synapses in cultured retinal ganglion neurons was shown to occur through gabapentin α2δ-1 receptor (Eroglu et al., 2009), since competitive inhibition of thrombospondin-binding to α2δ-1 with the high-affinity α2δ-1 ligand was shown to block thrombospondin-mediated synapse formation. In addition, cortical neurons deficient in thrombospondin-1 and thrombospondin-2 formed fewer synapses, showing significant decrease in the number of presynaptic boutons in P8 and P21 brains of thrombospondin-1 and -2 double KO mice (Christopherson et al., 2005). Moreover, astrocytes derived from human subjects with Down’s syndrome (DS) were less effective in promoting the formation of mature spines in rat hippocampal neurons than normal astrocytes due to reduced levels of thrombospondin-1 (Garcia et al., 2010). Altogether these studies indicate a role of thrombospondin in the formation of synapses (Table 1) and encourage future work concerning the mechanisms of thrombospondin signaling in dendritic spines under both normal and pathological conditions. Thrombospondin may signal through ECM receptors such as ApoE2 receptor, VLDLR, and LRP, which can interact with thrombospondins and are also shown to mediate the synaptogenic effects of reelin, which are discussed later in this review. Recent studies suggested that the synapse-promoting effects of thrombospondin-1 may be also mediated through its interaction with cell adhesion molecule, neuroligin-1 (Xu et al., 2009). Thrombospondin-1-induced formation of synapses in developing hippocampal neurons was blocked by competitive inhibition of the association of thrombospondins with neuroligin-1, suggesting that the regulation of the synaptic cell adhesion molecules may contribute to thrombospondin effects in synapses.
Lectins regulate AMPAR trafficking and activity-dependent synaptic plasticity
The lectins belong to a class of ECM proteins that are known for their ability to interact with sugar-containing proteins, both proteoglycans and glycoproteins (Garwood et al., 2003). The lectin family of neuronal pentraxins (NP) have been implicated in AMPAR trafficking. Neuronal activity-regulated pentraxin, Narp (also known as NP-2), is a calcium-dependent pentraxin that is localized to both pre- and postsynaptic sites of excitatory synapses (Xu et al., 2003). Narp and another member of the pentraxin family, NP-1, can co-assemble with the neuronal pentraxin receptor (NPR) into a large complex at excitatory synapses in cultured hippocampal neurons (Dodds et al., 1997; Xu et al., 2003; Sia et al., 2007). NPR was first discovered by its binding to the snake venom neurotoxin taipoxin, is an integral membrane pentraxin that shares 50% homology with Narp and NP-1, and is thought to provide a membrane anchor for Narp and NP-1 (Dodds et al., 1997; Kirkpatrick et al., 2000; Bjartmar et al., 2006). It has been demonstrated that the complex of NP-1, Narp and NPR co-localizes with and triggers clustering of AMPARs at postsynaptic sites through their pentraxin domains as well as mediate the synaptic recruitment of the AMPAR subunits, GluR1 and GluR4 (O'Brien et al., 2002; Xu et al., 2003; Sia et al., 2007; Cho et al., 2008). RNAi knockdown of either NP-1 or NPR reduced the number of AMPAR clusters in cultured hippocampal neurons (Cho et al., 2008), with a similar reduction noted in triple Narp/NP-1/NPR KO neurons (Sia et al., 2007). Narp and NP-1 were also implicated in AMPAR recruitment during homeostatic scaling of excitatory synapses in cultured hippocampal neurons, suggesting a role of NPs in synaptic plasticity (Chang et al., 2010). In addition, analysis of Narp and NP-1 KO mice demonstrated that these lectins are also involved in activity-dependent synapse formation and elimination via segregation of retinal ganglion cell projections to the dorsal lateral geniculate nucleus (Bjartmar et al., 2006). Involvement of NPs in AMPAR trafficking, homeostatic scaling of excitatory synapses and activity-dependent synapse formation suggest that NPs play a role in postsynaptic differentiation and plasticity in interneurons. In spiny neurons AMPAR distribution has been linked to dendritic spine geometry as functional AMPARs are abundant in mature mushroom-shaped spines and mature spine morphology is shown to be important in the regulation of glutamatergic synaptic transmission (Craig et al., 1993; Shi et al., 1999; Matsuzaki et al., 2001; Hering et al., 2003). Therefore, it is possible that NPs may also contribute to dendritic spine maturation by regulating AMPAR trafficking and clustering at the postsynaptic dendritic spine. Future studies will determine how these and/or other lectins are recruited to spine synapses and their role in the formation and plasticity of dendritic spines.
CELL SURFACE RECEPTORS THAT MEDIATE EFFECTS OF THE ECM ON DENDRITIC SPINES AND SYNAPSES
All of the aforementioned effects of the ECM on dendritic spine development and remodeling primarily occur through cell surface receptors that are localized on dendritic spines. This section focuses on the receptors that respond to changes in ECM composition and mediate the effects of the ECM on dendritic spines.
Integrins are ECM receptors that regulate dendritic spine formation and remodeling
Integrins comprise a family of cell surface proteins that are found across many cell types and mediate various cell processes, including cell proliferation, survival, differentiation and migration (reviewed in Ruoslahti, 1991; Giancotti and Ruoslahti, 1999; Hynes, 2002; Milner and Campbell, 2002; Miranti and Brugge, 2002; Parsons, 2003; Ridley et al., 2003; Rolli et al., 2003; Arnaout et al., 2005). Integrins are composed of non-covalently linked α and β subunits, and each αβ pair has its own binding and signaling specificities. Integrins signal through their associations with intracellular signaling proteins, such as focal adhesion kinase (FAK), the non-receptor tyrosine kinase Src, and scaffolding proteins such as Grb2 and paxillin, to mediate their effects on the actin cytoskeleton. Integrins are well known for their role in promoting cell migration by triggering the assembly of these focal adhesion complexes in migrating cells. Within the brain, numerous α and β integrin subunits were found in several regions including the hippocampus, cerebellum, thalamus and cortex (Chan et al., 2003), and a subset of these were shown to be localized within the PSD of dendritic spines (Chavis and Westbrook, 2001; Bernard-Trifilo et al., 2005; Bourgin et al., 2007).
Integrins have also been implicated in brain development and synaptogenesis (reviewed in Milner and Campbell, 2002), potentiation of NMDAR-dependent LTP (Chun et al., 2001; Chan et al., 2003; Kramar et al., 2003; Lin et al., 2003) as well as dendritic spine remodeling (Shi and Ethell, 2006; Bourgin et al., 2007; Webb et al., 2007). Many integrins are activated by their interaction with the Arg-Gly-Asp (RGD) motif, which is found in many ECM components including laminin, fibronectin, tenascin and thrombospondins (reviewed in Hynes, 1987; Ruoslahti and Pierschbacher, 1987). Activation of integrins with RGD-containing peptide was shown to induce the transformation of mature mushroom-shaped spines with large heads into immature thin spines and filopodia in primary hippocampal cultures (Shi and Ethell, 2006). These effects were blocked by function-blocking antibodies against β1 and β3 integrin subunits and by the NMDAR antagonist MK801, suggesting the involvement of NMDARs in integrin induced dendritic spine remodeling. Fibronectin was also shown to potentiate NMDAR-mediated synaptic responses in acute hippocampal slices through β1 integrin signaling (Bernard-Trifilo et al., 2005). In addition, Webb and colleagues have shown that depletion of integrin α5 using an siRNA knockdown approach decreased the number of dendritic spines and synapses in hippocampal neurons by a mechanism involving Src, Rac and adaptor protein GIT1 (Webb et al., 2007). In contrast, EphA4 receptor tyrosine kinase was demonstrated to have opposing effects on dendritic spine morphology within cultured hippocampal neurons by inhibiting integrin β1 signaling (Bourgin et al., 2007), which suggests that EphA4 activation by ephrin-A3 promotes spine shortening at least partially by inhibiting integrin β1 induced spine elongation.
Together, these studies suggest that integrins mediate the effects of ECM on dendritic spines and synapses by influencing NMDAR activity and cytoskeletal organization. However, most of the studies rely on the activation of integrins in synapses by exogenous substrates, whereas origin and identity of endogenous integrin-activating ECM proteins are yet to be identified in CNS synapses.
Agrin promotes extension of dendritic filopodia
Beside integrins, some membrane-bound heparin sulfate proteoglycans (HSPGs), such as agrin and syndecan, are also shown to mediate ECM effects on postsynaptic differentiation through their sugar chains as well as protein-protein interactions. Agrin is a single-chain, multidomain HSPG with several splice variants, and has been well characterized for its role in clustering acetylcholine receptors at the neuromuscular junction (NMJ), which is reviewed by Singhal and Martin within this special edition (Singhal and Martin, 2011). Agrin contains nine follistatin-like domains in its N-terminal portion, followed by a serine/threonine-rich region, four EGF-like domains that alternate with three laminin γ-like domains at the C terminus, as well as binding sites for both heparan and chondroitin sulfate GAG side-chains (Bowe and Fallon, 1995; Winzen et al., 2003). In addition to the acetylcholine receptors, agrin associates with other cell-surface receptors such as α-dystroglycan and the low density lipoprotein receptor (LRP)-4, through its C-terminal region (Bowe and Fallon, 1995; Gesemann et al., 1996; Kim et al., 2008; Zhang et al., 2008). Agrin can exist in several isoforms, which are generated as a result of alternative splicing of its N- and C- terminal domains to create secreted and transmembrane forms of agrin (reviewed in Bezakova and Ruegg, 2003). In the CNS, the transmembrane form of agrin (TM-agrin) was found to associate with both axons and dendrites of neurons and induce formation of filopodia-like protrusions from primary neurites (Neumann et al., 2001; Annies et al., 2006; McCroskery et al., 2006; Kroger and Pfister, 2009; McCroskery et al., 2009). Although agrin immunoreactivity in the brain was mostly associated with blood vessels, agrin-like immunoreactivity was also detected in synapses of the CA3 hippocampus and the dentate gyrus, suggesting its role in CNS synapses (Ksiazek et al., 2007). Analysis of agrin-deficient neurons, however, showed no detectable defects in the formation of glutamatergic or GABAergic synapses in cultured hippocampal and cortical neurons derived from agrin KO mice (Li et al., 1999; Serpinskaya et al., 1999). In contrast, synaptic differentiation was shown to be affected in cultured hippocampal neurons by acute suppression of agrin signaling with anti-agrin antibodies and an antisense oligonucleotide approach (Ferreira, 1999; Bose et al., 2000). Recent studies also demonstrated a reduced number of excitatory synapses within the cerebral cortex of agrin KO mice (Tg/agrn−/−), which were engineered to express agrin in motor neurons to prevent perinatal death (Ksiazek et al., 2007). In this study, both the number of presynaptic boutons and the density of dendritic spines were ~30% lower in the layer II/III of the motor cortex of 7-week old Tg/agrn−/− as compared to wild type mice. Consistent with a decreased number of glutamatergic synapses, a significant decrease in the miniature excitatory postysynaptic current frequency was also noted in agrin deficient neurons, suggesting a role of agrin in excitatory synapse formation in the brain. Several studies have also implicated TM-agrin in the formation of filopodia-like protrusions from the surface of neurites (Annies et al., 2006; McCroskery et al., 2009; Ramseger et al., 2009; Lin et al., 2010). Annies and colleagues first demonstrated a role for agrin in the induction of filopodia-like processes on axons and dendrites of both peripheral nervous system and CNS neurons. Clustering of TM-agrin with its antibodies induced formation of filopodia-like processes on the axons of retinal ganglion neurons, tectobulbar neurons, sympathetic neurons and somatosensory neurons from dorsal root ganglia, as well as dendrites of cultured mouse hippocampal neurons (Annies et al., 2006). The possibility of the involvement of TM-agrin in the formation of spine synapses through the modulation of dendritic filopodia has also been suggested in most recent studies showing that RNAi knockdown of TM-agrin in primary rat hippocampal cultures decreased the density of both dendritic filopodia and axo-dendritic synapses primarily due to the absence of postsynaptically localized TM-agrin in dendrites (McCroskery et al., 2009). Several groups have also gained insights into the mechanism by which TM-agrin regulates filopodia formation suggesting the involvement of both the N-terminal and C-terminal portions of agrin (McCroskery et al., 2006; Matsumoto-Miyai et al., 2009). Matsumoto-Miyai and colleagues have shown that a C-terminal 22kDa fragment of agrin was able to restore LTP-dependent formation of dendritic filopodia in the hippocampus of 4–6 week old neurotrypsin-deficient mice (Matsumoto-Miyai et al., 2009). This fragment of agrin was primarily produced in the presence of neurotrypsin (Reif et al., 2007) as its levels were reduced in neurotrypsin-deficient mice and increased in hippocampal slices derived from neurotrypsin-expressing mice (Matsumoto-Miyai et al., 2009). Neurotrypsin is a serine protease that is expressed and secreted by neurons specifically in the cerebral cortex, hippocampus and amygdala (Gschwend et al., 1997). The release of neurotrypsin in the brain was shown to be activity-dependent and primarily localized to axons and presynaptic boutons (Frischknecht et al., 2008). The studies by Matsumoto-Miyai and colleagues suggest that TM-agrin may play a role in new spine formation in response to LTP through neurotrypsin-mediated cleavage of its C-terminal domain (Matsumoto-Miyai et al., 2009). In contrast, other studies have demonstrated that the N-terminal half of agrin was sufficient to trigger the growth of new filopodia within cultured rat hippocampal neuron, whereas deletion mutants of agrin that lack the N-terminal half failed to induce axonal filopodia (McCroskery et al., 2006; Lin et al., 2010). These studies also suggested the importance of the heparan sulfate chains in the filopodia-promoting effects of agrin as the agrin mutant lacking the heparan sulfate attachment sites lost its ability to induce filopodia in cultured rat hippocampal neurons (Lin et al., 2010). Although the mechanisms mediating the effects of agrin on filopodia are not completely clear, the activation of Cdc42 and Rac1 may contribute to this process, possibly through agrin interaction with heparan sulfate binding proteins (McCroskery et al., 2006; Lin et al., 2010). Future studies will determine whether agrin interacting partners such as neuronal cell adhesion molecule (NCAM) or integrins mediate its filopodia-promoting effect in neurons and also investigate the effects of agrin on spine formation from dendritic filopodia. In addition, it would be interesting to delineate the role of the GAG chains of agrin in the formation and stability of the lattice-like ECM network and its contribution to the maintenance and plasticity of spine synapses in the mature brain.
Involvement of the transmembrane HSPG syndecan in synapse and spine formation
The syndecans belong to a family of transmembrane HSPGs that are comprised of four members, which contain short cytoplasmic tails that can directly signal to the cytoskeleton. Syndecans are known to act as co-receptors with integrins and through these associations link to many ECM components, including laminin, fibronectin, tenascin, collagen, thrombospondin and heparin binding growth associated molecule (Raulo et al., 1994; Carey, 1997; Woods, 2001; Kaksonen et al., 2002; Garwood et al., 2003). Syndecan-2 and -3 are both found in the brain where they are implicated in neural development and dendritic spine formation (Ethell et al., 2001; Kaksonen et al., 2002). Interactions of the heparan sulfate chains of syndecan-3 with the heparin binding growth associated molecule HB-GAM has been implicated in the regulation of LTP, neurite outgrowth and synaptogenesis through mediation of GABAergic inhibition (Raulo et al., 1994; Kaksonen et al., 2002). Syndecan-2, on the other hand, was shown to directly affect excitatory synapses and dendritic spines (Hsueh et al., 1998; Ethell and Yamaguchi, 1999). Our previous studies demonstrated that syndecan-2 overexpression in primary hippocampal neurons accelerated the transformation of filopodia into mature mushroom-shaped spines (Ethell and Yamaguchi, 1999; Ethell et al., 2001). The spinogenic activity of syndecan-2 required the presence of its cytoplasmic domain and was shown to be regulated by EphB2 receptors. Furthermore, depletion of syndecan-2 using an shRNA approach significantly reduced the number of dendritic spines in cultured hippocampal neurons, supporting a role of syndecan-2 in spinogenesis (Lin et al., 2007). Syndecans, like other ECM proteoglycans, are clearly involved in synapse and spine formation, and regulate both excitatory and inhibitory synapses. Additional work is needed to further dissect the downstream mechanisms through which the different syndecans are able to mediate their excitatory versus inhibitory responses. Syndecans can recruit several ECM molecules to synaptic sites through their heparan sulfate chains, including matrix metalloproteinases (MMPs), lectins and some growth factors and it would be interesting to determine how these interactions may affect dendritic spines.
LPRs mediate effects of reelin and tPA on dendritic spines
Reelin is a 400-kDa glycoprotein that is secreted into the extracellular space primarily by various neuronal populations within the brain and has been shown to co-localize with integrins within the PSDs of dendritic spines (Rodriguez et al., 2000; Pappas et al., 2002; Quattrocchi et al., 2002). Reelin is primarily characterized for its role in neuronal migration during the development of laminar organization within the mammalian brain and has been suggested to degrade laminin and fibronectin within the ECM (Quattrocchi et al., 2002), however its ability to cleave laminin and fibronectin has recently been challenged (Kohno and Hattori, 2010). Reelin has been implicated in both LTP and dendritic spine morphogenesis within the hippocampus and cerebral cortex through the activation of LPRs (Table 1; Costa et al., 2001; Liu et al., 2001; Weeber et al., 2002). Heterozygous reeler mice, which express half the normal levels of reelin, demonstrated a reduction in dendritic spine density and size in cortical layer III (Liu et al., 2001). In contrast, the treatment of mouse hippocampal slices with recombinant reelin promoted induction of LTP, most likely through the activation of ApoE2 and VLDLR, which are known receptors for reelin (Costa et al., 2001; Rice and Curran, 2001; Strasser et al., 2004; Beffert et al., 2006), as there was impairment in the ability of reelin to induce LTP in ApoE or VLDLR KO mice (Weeber et al., 2002). Reelin application to acute hippocampal slices also enhanced NMDAR- and AMPAR-dependent currents through increased surface expression of AMPARs (Qiu et al., 2006). Enhanced AMPAR clustering was also noted in cultured hippocampal neurons treated with reelin. Reelin-induced LTP, NMDAR phosphorylation, and surface expression of AMPARs were impaired by genetic depletion or pharmacological inhibition of ApoE2 receptor and VLDLR. Moreover, reelin-induced clustering of ApoE2 receptor and VLDLR were similar to the effects of ligands specific for ApoE2 receptor and VLDLR (Strasser et al., 2004). It would be interesting to determine whether the AMPAR recruitment to the postsynaptic site in reelin treated neurons also coincides with spine maturation, such as formation of mushroom or stubby spines, and whether its effects on dendritic spines are also mediated through ApoE2 receptor and VLDLR signaling.
VLDLR, ApoE2 receptor and another low density lipoprotein receptor, LRP, are comprised of a cysteine-rich ligand binding domain, EGF precursor homology domain, a region rich in O-linked oligosaccharides, a membrane-spanning domain, and an intracellular sequence required for receptor internalization (Innerarity et al., 1984; Takahashi et al., 1995; Zhuo et al., 2000). LRP was shown to respond to tissue plasminogen activator (tPA), and inhibition of LRP blocked the ability of tPA to induce L-LTP in acute hippocampal slices (Zhuo et al., 2000). The protease tPA is known for its ability to convert plasminogen into the enzymatically-active form plasmin, which is important for blood clot breakdown (Hoylaerts et al., 1982), and has also been implicated in synaptic growth during L-LTP (Table 1; Baranes et al., 1998). Recent studies demonstrated that tPA is also involved in dendritic spine dynamics in the visual cortex (Oray et al., 2004). Significant enhancement of dendritic spine motility was noted in acute slices derived from P28 visual cortex after treatment with either plasmin or tPA, and later also induced dendritic spine loss. Increase in tPA proteolytic activity was also observed during the critical period in visual cortex development (Mataga et al., 2004). In addition, tPA treatment was shown to promote monocular deprivation-induced dendritic spine loss while targeted disruption of tPA release prevented that spine loss. These results demonstrate a role of tPA in the experience-dependent pruning of spines that is required for the formation of visual maps. Similar to monocular deprivation-induced dendritic spine pruning within the visual cortex, stress can induce dendritic spine pruning in the hippocampus in a tPA-dependent manner (Pawlak et al., 2005). A significant reduction in spine density was observed in CA1 pyramidal neurons of stressed wild-type mice, but not in tPA KO or plasminogen KO mice. Stress-induced reduction in the number of NMDARs also coincided with dendritic spine loss at the sites of tPA activation in the hippocampus, suggesting that tPA may play a role in this form of stress-induced plasticity. These studies implicate tPA and its potential substrates in experience-dependent pruning of dendritic spines. Although the next group of proteins discussed in this review mediates cell-cell interactions and are called cell adhesion molecules, they are also sensitive to changes in the ECM composition, in particular MMPs and thrombospondins.
Cell adhesion molecules respond to changes in the ECM to affect dendritic spine remodeling
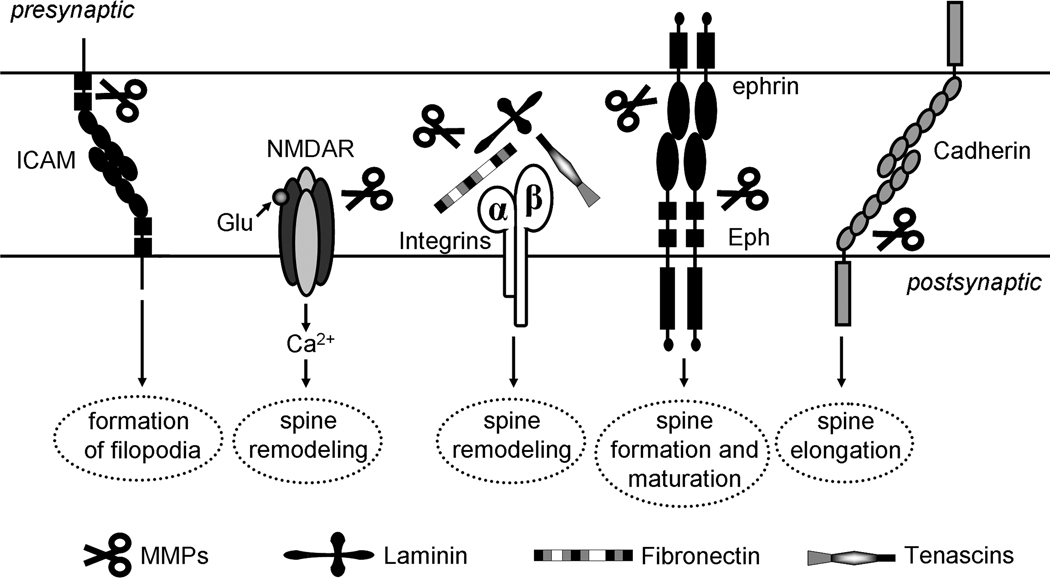
Cell adhesion molecules can be indirectly involved in ECM signaling by responding to changes in ECM composition through their sugar chains and sensitivity to MMPs (Fig. 2; Takeichi, 1990; Aplin et al., 1998; Ranscht, 2000; Dwivedi et al., 2009). Cadherins form a superfamily of transmembrane glycoproteins that contain extracellular Ca2+-binding domains and promote cell adhesion in a calcium-dependent manner (Ranscht, 2000; Angst et al., 2001). E-cadherin and N-cadherin are widely expressed within the CNS and are also found at excitatory synapses (Benson and Tanaka, 1998; Ranscht, 2000). Both E- and N-cadherin can be cleaved by secreted MMPs such as MMP-9 and MMP-12 (Dwivedi et al., 2009), and membrane-bound MT-MMP-5 to produce a 35kDa C-terminal fragment (Monea et al., 2006), suggesting the possibility that some of the MMP effects on dendritic spines, which are discussed later in this review, may be mediated through cadherins.
FIGURE 2.
MMP cleavage of ECM substrates and cell surface receptors regulates dendritic spines.
In addition to cadherins, MMPs can also cleave other synaptic cell adhesion molecules such as Neuronal Cell Adhesion Molecules (NCAMs) and Intracellular Cell Adhesion Molecules (ICAMs, Fig. 2). NCAMs and ICAMs belong to an immunoglobulin family of Ca2+-independent cell adhesion molecules that contain immunoglobulin and fibronectin type-III domains in their extracellular portion and are involved in cell-cell adhesion, neurite outgrowth and synaptic plasticity, as well as learning and memory (Doherty et al., 1995; Dityatev and Schachner, 2003; Dityatev et al., 2010). Both NCAM and ICAM have been implicated in the regulation of dendritic spine morphology through their sugar chains (Meighan et al., 2007; Tian et al., 2007). There are three NCAM isoforms identified, including the GPI-anchored 120 kDa form and two transmembrane 140 kDa and 180 kDa forms. NCAM is glycosylated through its immunoglobulin domains and can contain polysialic acid (PSA) chains. PSA-containing NCAM has been shown to be highly expressed during development and plays a role in synapse formation and plasticity (Muller et al., 1996; Uryu et al., 1999; Cremer et al., 2000; Dityatev et al., 2004). Transfection of cultured NCAM deficient hippocampal neurons with PSA-containing NCAM revealed enhanced synaptophysin immunoreactivity on the dendrites of NCAM expressing neurons (Dityatev et al., 2004). Enzymatic removal of PSA or heparan sulfate significantly diminished synaptogenic activity of NCAM and also blocked the increase in the number of perforated spine synapses associated with NMDAR-dependent LTP. Since most excitatory synapses in hippocampal neurons are formed on dendritic spines, it would be interesting to determine whether PSA-NCAM is also involved in the maturation of postsynaptic spines and/or enlargement of spine heads induced by NMDAR-dependent LTP. Future studies will identify the heparan sulfate proteoglycan that mediates synaptogenic effects of NCAM in the developing and mature brain.
ICAM-5, known as telecephalin, can be cleaved by MMP-2/9 and was implicated in NMDA-induced dendritic spine remodeling in hippocampal neurons (Tian et al., 2007). While the soluble fragment of ICAM5, a product of MMP-9 cleavage, induced formation and elongation of dendritic filopodia (Tian et al., 2007), ICAM-5 deficient neurons exhibited decreased density of dendritic filopodia and accelerated maturation of dendritic spines (Matsuno et al., 2006). These studies suggest that ICAM-5 may mediate dendritic spine formation by promoting dendritic filopodia through MMP-mediated cleavage of ICAM-5 and its release into the ECS. Although homophilic interactions of the soluble fragment of ICAM-5 with membrane bound ICAM-5 was suggested to mediate its filopodia promoting effects, the mechanisms that regulate MMP-9 cleavage of ICAM-5 require further investigation.
Neuroligins belong to another class of cell adhesion molecules, which are shown to interact with ECM molecules and are well known for their role in synapse formation. Neuroligins are postsynaptic membrane proteins that mediate the formation of synapses between neurons through their interaction with the presynaptically-located neurexins (Chih et al., 2005; Fabrichny et al., 2007), and are dynamically regulated at postsynaptic sites by LTP and LTD (Chih et al., 2005; Xu et al., 2009; Schapitz et al., 2010). Furthermore, neuroligin-1 was also shown to control synapse formation through interactions with thrombospondin-1 (Xu et al., 2009). The inhibition of thrombospondin/neuroligin-1 interactions with the neuroligin-1 soluble extracellular domain or by neuroligin-1 knockdown using an shRNA approach blocked thrombospondin-induced synapse formation in cultured hippocampal neurons, suggesting that synaptogenic activity of neuroligin-1 can be regulated by ECM components as well. In addition to neuroligins, neurexins are known to regulate synapse formation through associations with other transmembrane, postsynaptic receptors such as the leucine-rich repeat transmembrane protein 2, GluRδ2 and cerebellin 1 (de Wit et al., 2009; Woo et al., 2009; Uemura et al., 2010; Joo et al., 2011). Neurexins have also been suggested to interact with integrins, Eph receptors, ephrins, cadherins and dystroglycans (Wilczynski and Kaczmarek, 2008; Benson and Huntley, 2010), all of which are sensitive to the ECM and are implicated in mediating dendritic spine remodeling. While it is apparent that the ECM can influence synaptogenic effects of neuroligins and possibly neurexins, more studies are necessary to determine the specific mechanisms and players involved. It would be interesting to see if thrombospondin may also affect the synaptogenic effects of neurexin either in conjunction with neuroligins, or via the interactions of neurexin with leucine-rich repeat transmembrane protein 2, GluRδ2 or cerebellin 1. Additionally, the interactions of neurexins with integrins and Eph receptors in dendritic spines could prove to be interesting since both receptors are known to have opposing functions on dendritic spines.
REMODELING PROTEINS OF THE ECM
In addition to scaffolding proteins and large proteoglycans with various glycosaminoglycan chains, the ECM also contains several classes of enzymes that are involved in proteolytic cleavage of the ECM components and their cell surface receptors. Among the enzymes that are found within the synaptic cleft are MMPs, which are shown to play roles in dendritic spine plasticity and remodeling.
MMPs induce dendritic spine remodeling
MMPs belong to a family of metzincin endopeptidases and were named for their ability to cleave the ECM. 24 mammalian MMPs have been identified so far, which share a highly conserved catalytic domain (reviewed in Mott and Werb, 2004; Ethell and Ethell, 2007; Wilczynski and Kaczmarek, 2008). While most MMPs are secreted proteins, there are six membrane-type MMPs (MT-MMPs) that were also identified. In addition to the catalytic domain, all MMPs contain a pro-domain, which occupies the catalytic domain and prevents MMP activation. Therefore, removal of the pro-domain by proteolytic cleavage or displacement is required for MMP activation. Within the brain, expression of several MMPs, including MT-MMP-5, MMP-3 and the gelatinases, MMP-2 and MMP-9 (named for their ability to cleave gelatin), was shown to be regulated during development and in response to learning (Table 1; Ayoub et al., 2005; Ulrich et al., 2005; Meighan et al., 2006; Monea et al., 2006). Both MMP-3 and MMP-9 were increased in the hippocampus of mice following successful learning in the Morris water maze, which was impaired in the presence of MMP inhibitors (Meighan et al., 2006). With regards to developmental expression, mRNA transcripts of MMP-2, -9, -11, -13, -14, -15, -24 and the tissue inhibitors of metalloproteinases (TIMP)-1, -2, and -3 were all found to be upregulated within the proencephalon and rhombencephalon of 1 week old mice (Ulrich et al., 2005). Within the cerebellar cortex, measures of the mRNA transcript levels of MMP-2 and MMP-9 revealed an initial decrease between postnatal day (PD) 3 to PD6, with a relatively constant expression thereafter (Ayoub et al., 2005). Zymography analysis demonstrated that the expression and gelatinase activity of both MMP-2 and MMP-9 persisted longer than their transcripts with downregulation occurring around PD9 and subsequent undetectable immunoreactivity after PD21. Specific inhibition of MMP-2 and MMP-9 within organotypic cerebellar slices significantly increased the thickness of the external granule layer and decreased the number of migrating granule neurons in the molecular layer, indicating a role for MMP-2 and MMP-9 in postnatal cerebellar morphogenesis. Purification of the synaptosomal fraction as well as fluorescence immunolabeling of cultured rat cortical neurons demonstrated synaptic localization of MT-MMP-5 at 2 and 5 days in vitro (DIV), as well as within the growth cones of both axons and dendrites indicating a role for MT-MMP-5 in promoting the extension of processes and synaptogenesis (Monea et al., 2006).
The regulation of MMPs during development and in response to learning may impact the formation and maintenance of dendritic spines. Our studies showed that active MMP-9 can induce remodeling of dendritic spines in cultured hippocampal neurons by transforming mature mushroom-shaped spines with large heads into immature thin spines with small heads and promote growth of new filopodia (Bilousova et al., 2009). Similar to our findings, analysis of organotypic hippocampal slice cultures and dissociated hippocampal neurons from transgenic rats overexpressing an autoactivating MMP-9 demonstrated that enzymatic activity of MMP-9 promotes longer and thinner dendritic spines in a manner dependent on integrin β1 signaling (Michaluk et al., 2011). On the other hand, brief exposure of CA1 pyramidal neurons to active MMP-9 enhanced synaptic potentiation in acute rat hippocampal slices, leading to expansion of dendritic spines and more mature dendritic spine morphology (Wang et al., 2008). Incubation of these slices with a broad-spectrum MMP inhibitor or with integrin-blocking antibody impaired LTP as well as dendritic spine expansion induced by theta burst stimulation. Several other studies have also shown that MMP-9 can enhance LTP through integrin-dependent regulation of NMDAR activity (Fig. 2; Nagy et al., 2006; Michaluk et al., 2009). The studies on the effects of MMPs as well as other secreted proteases in the regulation of LTP and other forms of synaptic plasticity are reviewed by Wlodarczyk and colleagues within this special edition (Wlodarczyk et al., 2011).
These effects of MMPs on the synaptic plasticity and morphology of dendritic spines are suggested to be mediated through the ability of MMP-9 to cleave ECM proteins and to trigger integrin signaling (Fig. 2). Specifically, cleavage of laminin and fibronectin by MMP-9 has been proposed to be a mechanism by which MMP-9 mediates its effects on dendritic spine morphology and LTP via integrin-dependent regulation of NMDAR activity (Nagy et al., 2006; Wang et al., 2008). In addition to integrins, MMPs may also influence dendritic spines by regulating the activities of several other synaptic receptors that can be cleaved by MMPs. As mentioned previously, cadherins can be cleaved by several MMPs (Fig. 2), such as MMP-9, MMP-12 (Dwivedi et al., 2009) and MT-MMP-5 (Monea et al., 2006), to produce an extracellular fragment which has been implicated in promoting dendritic spine elongation (Togashi et al., 2002). Another cell-surface receptor, β-dystroglycan is found within the PSD of dendritic spines and has been suggested to mediate MMP-9 effects on dendritic spines (Michaluk et al., 2007; Gawlak et al., 2009). Inhibition of GABAA receptors demonstrated an increase in MMP-9 levels and MMP-9 dependent cleavage of β-dystroglycan within the rat hippocampus (Michaluk et al., 2007). Eph receptors, a family of tyrosine kinases that are implicated in dendritic spine formation and maintenance, and their ligands ephrins can also be cleaved by MMPs (Fig. 2; Lin et al., 2008; Inoue et al., 2009). MMP-9 cleavage of the EphB2 receptor enhanced EphB2 signaling and EphB2-mediated growth cone collapse (Lin et al., 2008), whereas MMP-induced cleavage of EphA4 was independent of its stimulation with ephrins-A3 ligand (Inoue et al., 2009). However, it is still unclear whether the ability of MMPs to cleave Ephs and cadherins contributes their effects on dendritic spines. Future studies will provide new insights into synaptic regulation of MMP secretion and activity in the CNS synapses, as well as the mechanisms of MMP action in dendritic spines especially as it relates to ECM remodeling and MMP effects on various signaling pathways that involve NMDARs, integrins, Ephs and cell adhesion molecules.
ECM IN NEUROLOGICAL DISEASES, COGNITIVE IMPAIRMENTS AND DENDRITIC SPINE PATHOLOGY
Dendritic spine pathology, which is usually characterized by abnormal dendritic spine morphology and/or altered spine density, is a hallmark of many neurological disorders associated with learning deficits, memory loss and other cognitive deficits, including Fragile X Syndrome, Down’s Syndrome, Williams Syndrome, schizophrenia and Alzheimer’s disease (Suetsugu and Mehraein, 1980; Rudelli et al., 1985; Ferrer and Gullotta, 1990; Takashima et al., 1994; Irwin et al., 2000; Kaufmann and Moser, 2000; Barnes and Milgram, 2002; Fiala et al., 2002). As we have discussed during the course of this review, many components of the ECM can affect dendritic spines and synapses. Therefore, it is not surprising that the changes in ECM composition as a result of gene mutations or posttranslational modifications of several ECM genes can affect cognitive functions as well. Several ECM proteins, including reelin, MMPs, elastin, thrombospondins, and some proteoglycans, have been implicated in cognitive deficits seen in several neurological disorders that are discussed in this section.
Mental Retardation and ECM
Both Williams and Down’s syndromes are genetic neurodevelopmental disorders characterized by mental retardation and abnormal physical attributes (Epstein, 2006; Dykens, 2007; Morris, 2010). Williams syndrome is caused by deletion of up to 26 consecutive genes on chromosome 7 (Morris, 2010), with the deletion of the gene encoding for elastin occurring in 90% of individuals with Williams syndrome (Nickerson et al., 1995). Elastin is an ECM protein that is primarily associated with connective tissues and allows for these tissues to return to their original shape after stretching or contracting (Kielty et al., 2002). The deletion of elastin underlies the physical abnormalities associated with Williams syndrome, whereas cognitive impairments associated with Williams syndrome may relate to the gene encoding CAP-Gly-domain-containing linker protein-2, which has been found in axon-glial fractions associated with CNS myelin (Dhaunchak et al., 2010; Pober, 2010). Another astrocyte-secreted factor, thrombospondin-1, has been shown to promote synapse formation and has been suggested to play a role in the pathology of Down’s syndrome (DS) (Garcia et al., 2010). DS is a chromosomal disorder characterized by the presence of an additional full or partial chromosome 21 which causes cognitive impairments (Epstein, 2006; Dykens, 2007). New studies by Garcia and colleagues have demonstrated a reduction in the levels of intracellular and secreted thrombospondin-1 in human DS astrocytes, which causes a significant decrease in the total number of spines and overall immature spine morphology (Garcia et al., 2010). In addition to elastin and thrombospondin, the protease neurotrypsin has been implicated in autosomal recessive nonsyndromic mental retardation (Molinari et al., 2002). A genome-wide screen of eight siblings, including individuals with cognitive impairment and low IQ, revealed that the affected siblings suffered from a four base pair deletion within the gene encoding for neurotrypsin. How this deletion affects the function of neurotrypsin and whether it impacts agrin signaling in dendritic spines would be interesting to investigate in future studies.
Role of MMPs in neurological disorders
MMPs have been previously characterized for their role in the regulation of the blood-brain barrier (Zlokovic, 2008), and were suggested to underlie the progression of the autoimmune disorder multiple sclerosis (Agrawal et al., 2008), to facilitate immune access to the CNS in experimental autoimmune encephalomyelitis (Buhler et al., 2009) and to increase inflammation associated with cerebral ischemia (Cunningham et al., 2005). In addition to the effects on the blood brain barrier, recent studies have also implicated MMPs in synapse pathologies associated with brain injuries. Elevation of MMP-3 expression and activity correlated with trauma-induced synaptogenesis following brain injury (Kim et al., 2005; Falo et al., 2006). Moreover, increased MMP-9 activity has been linked to the fiber sprouting and reactive synaptogenesis associated with kainite-evoked epilepsies (Szklarczyk et al., 2002; Wilczynski et al., 2008) and traumatic brain injury (Wang et al., 2000). In addition, changes in the levels and activity of MMP-9 are shown to correlate with dendritic spine pruning in epilepsies (Wilczynski et al., 2008), and suggested to underlie abnormal dendritic spine development associated with Fragile X Syndrome (Bilousova et al., 2009). Our studies demonstrated that active MMP-9 promoted immature dendritic spine morphology that was similar to immature spine profiles found in hippocampal neurons isolated from Fragile X mental retardation gene 1 (Fmr1) KO mice, a mouse model for Fragile X (Bilousova et al., 2009). In addition, MMP-9 expression and activity were upregulated in the hippocampus of Fmr1 KO mice and the inhibition of MMP activity induced mature dendritic spine morphology in Fmr1 KO neurons (Dansie et al., unpublished data). Furthermore, increased MMP-9 activity was reported in patients with mild cognitive impairment and Alzheimer’s disease, suggesting MMP-9 may also be responsible for dendritic spine loss seen in this disease (Bruno et al., 2009).
ECM in Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disease associated with dementia that typically occurs in older individuals (Hardy and Selkoe, 2002; Miranti and Brugge, 2002). Pathology of AD is identified as a protein misfolding disease characterized by dendritic spine loss and neuronal degeneration related to accumulation of extracellular amyloid β (Aβ)-containing senile plaques and formation of intracellular neurofibrillary tangles caused by abnormal aggregation of the tau protein (Hashimoto et al., 2003; Wenk, 2003; Ohnishi and Takano, 2004; Tiraboschi et al., 2004; Wenk, 2006; Hernandez and Avila, 2007; Bittner et al., 2010; Perez-Cruz et al., 2011). Since Aβ-containing senile plaques form within the ECS, they most likely affect ECM organization and composition. Several proteoglycans such as agrin, syndecans and glypicans are shown to be associated with senile plaques (Bonneh-Barkay and Wiley, 2009). Agrin is the most abundant proteoglycan in AD plaques and its aggregation appears to play a role in plaque formation as well as formation of neurofibrillary tangles by accelerating Aβ accumulation (Donahue et al., 1999; Verbeek et al., 1999; Cotman et al., 2000). Conversely, one of the lecticans, aggrecan was demonstrated to provide neuroprotective effects against tau pathologies of AD within many subcortical regions, including the amygdala, thalamus and basal ganglia, as well as motor cortex and basal forebrain (Morawski et al., 2010). These regions are highly susceptible to AD neurodegeneration, and neurons that are ensheathed by aggregan-containing PNNs are devoid of neurofibrillary degeneration. It appears that the ECM helps to protect against the neurodegenerative decline while age-related changes in the ECM composition may contribute to the AD pathology.
RELN in the pathophysiology of autism and schizophrenia
The reelin encoding gene, RELN, has been associated with executive functioning in healthy individuals, and mutations in this gene are suggested to underlie autism and schizophrenia (Skaar et al., 2004; Laviola et al., 2009; Baune et al., 2010; Knuesel, 2010). Healthy adults with certain single nucleotide polymorphisms of the RELN gene demonstrated deficits in perseverative error processing, suggesting a role for reelin in higher cognitive functioning (Baune et al., 2010). In addition, a significant proportion of autistic patients revealed specific single nucleotide polymorphisms within their RELN gene (Skaar et al., 2004). Genetic differences in reelin have also been shown to increase the effects of environmental factors in promoting neurological dysfunction (Laviola et al., 2009). Heterozygous reeler mice show enhanced sensitivity to early environmental challenges, which significantly impact their social behaviors. Additionally, heterozygous reeler mice demonstrate subtle yet consistent behavioral anomalies during the critical adolescence age, which coincides with the first appearance of altered behaviors in schizophrenic patients. Furthermore, abnormal spine and synapse development associated with schizophrenia correlates with reduced reelin signaling and may relate to an enhanced sensitivity to inflammatory responses (Knuesel, 2010).
CONCLUSIONS AND PERSPECTIVES
ECM composition and signaling have been studied directly in relation to dendritic spines, as well as through their ability to regulate LTP and LTD. The ECM can control spine formation, maturation and remodeling through interactions with their specific receptors or by influencing the functions of other synaptic proteins. The effects of different ECM components on dendritic spine development vary from agrin-induced formation of filopodia, the precursors to dendritic spines, to laminin-mediated spine proliferation to tPA-dependent spine/synapse elimination. In addition, astrocyte-secreted factors thrombospondins and reelin have been also implicated in synaptogenesis and the regulation of dendritic spine density. However, some ECM proteins can have opposing effects on spine/synapses. For example, lectins can play a role in both synapse formation and elimination, suggesting activation of different signaling events under different circumstances. On the other hand, different ECM components can utilize the same receptors, such as lipoprotein receptors are shown to mediate synaptic effects of reelin, tPA and thrombospondin. Integrins, which are thought to be primary ECM receptors, have been demonstrated to promote spine remodeling, including spine elongation and filopodia formation, in response to changes in ECM composition triggered by MMPs. Although laminin, fibronectin and tenascin are primary integrin substrates, the origin and identity of endogenous integrin-activating ECM proteins are yet to be identified in CNS synapses. In addition to integrins, transmembrane HSPG agrin was also shown to induce filopodia formation, whereas another HSPG syndecan-2 has been implicated in spine maturation. It is clear that more work is needed to define the complex interactions between the various scaffolding proteins, proteoglycans, growth factors, sugar-binding proteins and proteases and how these interactions regulate dendritic spine formation and remodeling.
In summary, ECM organization influences normal physiology of dendritic spines and synapses, and alterations in the ECM composition may underlie abnormal dendritic spine development and neuropathologies. Future studies will provide new insights into the regulation of ECM organization in the brain and the mechanisms of ECM action in the normal and diseased brain.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Crystal Pontrello and Dr. Douglas Ethell for helpful comments on the manuscript. The work in the author’s laboratory is supported by grants from NIH, the Department of Defense and the FRAXA Foundation.
ABBREVIATIONS
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- Aβ
amyloid β
- AD
Alzheimer’s Disease
- ApoE2
apolipoprotein E2
- CNS
central nervous system
- CSPG
chondroitin sulfate proteoglycan
- DS
Down’s syndrome
- ECM
extracellular matrix
- ECS
extracellular space
- Fmr1
Fragile X mental retardation gene 1
- GAG
glycosaminoglycan
- HSPG
heparan sulfate proteoglycan
- HFS
high frequency stimulation
- ICAM
intracellular cell adhesion molecule
- KO
knockout
- LFS
low frequency stimulation
- LRP
low density lipoprotein receptor-type protein
- LTD
long-term depression
- LTP
long-term potentiation
- MMP
matrix metalloproteinase
- MT-MMP
membrane type matrix metalloproteinase
- NCAM
neuronal cell adhesion molecule
- NMDAR
N-methyl-D-aspartate receptor
- NMJ
neuromuscular junction
- NP
neuronal pentraxin
- NPR
neuronal pentraxin receptor
- PD
postnatal day
- PNN
perineuronal net
- PSA
polysialic acid
- PSD
postsynaptic density
- RGD
Arg-Gly-Asp
- tPA
tissue plasminogen activator
- VLDLR
very low density lipoprotein receptor
BIBLIOGRAPHY
- Agrawal SM, Lau L, Yong VW. MMPs in the central nervous system: Where the good guys go bad. Sem Cell and Dev Bio. 2008;19:42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Angst B, Marcozzi C, Magee A. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- Annies M, Bittcher G, Ramseger R, Loschinger J, Woll S, Porten E, Abraham C, Ruegg MA, Kroger S. Clustering transmembrane-agrin induces filopodia-like processes on axons and dendrites. Mol Cell Neurosci. 2006;31:515–524. doi: 10.1016/j.mcn.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal Transduction and Signal Modulation by Cell Adhesion Receptors: The Role of Integrins, Cadherins, Immunoglobulin-Cell Adhesion Molecules, and Selectins. Pharmacol Rev. 1998;50:197–264. [PubMed] [Google Scholar]
- Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- Ashby MC, Maier SR, Nishimune A, Henley JM. Lateral Diffusion Drives Constitutive Exchange of AMPA Receptors at Dendritic Spines and Is Regulated by Spine Morphology. J Neurosci. 2006;26:7046–7055. doi: 10.1523/JNEUROSCI.1235-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspberg A, Binkert C, Ruoslahti E. The versican C-type lectin domain recognizes the adhesion protein tenascin-R. Proc Natl Acad Sci U S A. 1995;92:10590–10594. doi: 10.1073/pnas.92.23.10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspberg A, Miura R, Bourdoulous S, Shimonaka M, Heinegard D, Schachner M, Ruoslahti E, Yamaguchi Y. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc Natl Acad Sci U S A. 1997;94:10116–10121. doi: 10.1073/pnas.94.19.10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub AE, Cai T-Q, Kaplan RA, Luo J. Developmental expression of matrix metalloproteinases 2 and 9 and their potential role in the histogenesis of the cerebellar cortex. Wiley Subscription Services, Inc., A Wiley Company; 2005. pp. 403–415. [DOI] [PubMed] [Google Scholar]
- Baranes D, Lederfein D, Huang Y-Y, Chen M, Bailey CH, Kandel ER. Tissue Plasminogen Activator Contributes to the Late Phase of LTP and to Synaptic Growth in the Hippocampal Mossy Fiber Pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Milgram SL. Signals from the X: signal transduction and X-linked mental retardation. Int J Dev Neurosci. 2002;20:397–406. doi: 10.1016/s0736-5748(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Konrad C, Suslow T, Domschke K, Birosova E, Sehlmeyer C, Beste C. The Reelin (RELN) gene is associated with executive function in healthy individuals. Neurobiol Learn Mem. 2010;94:446–451. doi: 10.1016/j.nlm.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Beck K, Hunter I, Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990;4:148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Beffert U, Durudas A, Weeber EJ, Stolt PC, Giehl KM, Sweatt JD, Hammer RE, Herz J. Functional Dissection of Reelin Signaling by Site-Directed Disruption of Disabled-1 Adaptor Binding to Apolipoprotein E Receptor 2: Distinct Roles in Development and Synaptic Plasticity. J Neurosci. 2006;26:2041–2052. doi: 10.1523/JNEUROSCI.4566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Huntley GW. Building and remodeling synapses. Hippocampus. 2010 doi: 10.1002/hipo.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Tanaka H. N-Cadherin Redistribution during Synaptogenesis in Hippocampal Neurons. J Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Trifilo JA, Kramar EA, Torp R, Lin CY, Pineda EA, Lynch G, Gall CM. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J Neurochem. 2005;93:834–849. doi: 10.1111/j.1471-4159.2005.03062.x. [DOI] [PubMed] [Google Scholar]
- Bezakova G, Ruegg MA. New insights into the roles of agrin. Nat Rev Mol Cell Biol. 2003;4:295–308. doi: 10.1038/nrm1074. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- Bittner T, Fuhrmann M, Burgold S, Ochs SM, Hoffmann N, Mitteregger G, Kretzschmar H, LaFerla FM, Herms J. Multiple events lead to dendritic spine loss in triple transgenic Alzheimer's disease mice. PLoS One. 2010;5:e15477. doi: 10.1371/journal.pone.0015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, RenterÃa RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, Cho R, Worley P, Malenka RC, Ball S, Peachey NS, Copenhagen D, Chapman B, Nakamoto M, Barres BA, Perin MS. Neuronal Pentraxins Mediate Synaptic Refinement in the Developing Visual System. J Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake SM, Strasser V, Andrade N, Duit S, Hofbauer R, Schneider WJ, Nimpf J. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J. 2008;27:3069–3080. doi: 10.1038/emboj.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Wiley CA. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009;19:573–585. doi: 10.1111/j.1750-3639.2008.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose CM, Qiu D, Bergamaschi A, Gravante B, Bossi M, Villa A, Rupp F, Malgaroli A. Agrin Controls Synaptic Differentiation in Hippocampal Neurons. J Neurosci. 2000;20:9086–9095. doi: 10.1523/JNEUROSCI.20-24-09086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella-Lopez A, Burgaya F, Gavin R, Garcia-Ayllon MS, Gomez-Tortosa E, Pena-Casanova J, Urena JM, Del Rio JA, Blesa R, Soriano E, Saez-Valero J. Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. PNAS. 2006;103:5573–5578. doi: 10.1073/pnas.0601279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin C, Murai KK, Richter M, Pasquale EB. The EphA4 receptor regulates dendritic spine remodeling by affecting [beta]1-integrin signaling pathways. J Cell Biol. 2007;178:1295–1307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe MA, Fallon JR. The role of agrin in synapse formation. Annu Rev Neurosci. 1995;18:443–462. doi: 10.1146/annurev.ne.18.030195.002303. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Seidenbecher CI, Asztely F, Rauch U, Matthies H, Meyer H, Krug M, Bockers TM, Zhou X, Kreutz MR, Montag D, Gundelfinger ED, Fassler R. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol. 2002;22:7417–7427. doi: 10.1128/MCB.22.21.7417-7427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner G, Bringmann A, Köppe G, Härtig W, Brauer K. In vivo and in vitro labelling of perineuronal nets in rat brain. Brain Res. 1996;720:84–92. doi: 10.1016/0006-8993(96)00152-7. [DOI] [PubMed] [Google Scholar]
- Brückner G, Grosche J, Schmidt S, Härtig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol. 2000;428:616–629. doi: 10.1002/1096-9861(20001225)428:4<616::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Mufson EJ, Wuu J, Cuello AC. Increased matrix metalloproteinase 9 activity in mild cognitive impairment. J Neuropathol Exp Neurol. 2009;68:1309–1318. doi: 10.1097/NEN.0b013e3181c22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler LA, Samara R, Guzman E, Wilson CL, Krizanac-Bengez L, Janigro D, Ethell DW. Matrix metalloproteinase-7 facilitates immune access to the CNS in experimental autoimmune encephalomyelitis. BMC Neurosci. 2009;10:17. doi: 10.1186/1471-2202-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukalo O, Schachner M, Dityatev A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience. 2001;104:359–369. doi: 10.1016/s0306-4522(01)00082-3. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Schachner M, Dityatev A. Hippocampal Metaplasticity Induced by Deficiency in the Extracellular Matrix Glycoprotein Tenascin-R. J Neurosci. 2007;27:6019–6028. doi: 10.1523/JNEUROSCI.1022-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DJ. Syndecans: multifunctional cell-surface co-receptors. Biochem J. 1997;327(Pt 1):1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- Chan C-S, Weeber EJ, Kurup S, Sweatt JD, Davis RL. Integrin Requirement for Hippocampal Synaptic Plasticity and Spatial Memory. J Neurosci. 2003;23:7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci. 2010;13:1090–1097. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411:317–321. doi: 10.1038/35077101. [DOI] [PubMed] [Google Scholar]
- Chen Z-L, Indyk JA, Strickland S. The Hippocampal Laminin Matrix Is Dynamic and Critical for Neuronal Survival. Mol Biol Cell. 2003;14:2665–2676. doi: 10.1091/mbc.E02-12-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of Excitatory and Inhibitory Synapse Formation by Neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Cho RW, Park JM, Wolff SBE, Xu D, Hopf C, Kim J-a, Reddy RC, Petralia RS, Perin MS, Linden DJ, Worley PF. mGluR1/5-Dependent Long-Term Depression Requires the Regulated Ectodomain Cleavage of Neuronal Pentraxin NPR by TACE. Neuron. 2008;57:858–871. doi: 10.1016/j.neuron.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CCA, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins Are Astrocyte-Secreted Proteins that Promote CNS Synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Chun D, Gall CM, Bi X, Lynch G. Evidence that integrins contribute to multiple stages in the consolidation of long term potentiation in rat hippocampus. Neuroscience. 2001;105:815–829. doi: 10.1016/s0306-4522(01)00173-7. [DOI] [PubMed] [Google Scholar]
- Chung CY, Zardi L, Erickson HP. Binding of tenascin-C to soluble fibronectin and matrix fibrils. J Biol Chem. 1995;270:29012–29017. doi: 10.1074/jbc.270.48.29012. [DOI] [PubMed] [Google Scholar]
- Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C. Dendritic Spine Hypoplasticity and Downregulation of Reelin and GABAergic Tone in Schizophrenia Vulnerability. Neurobiol Dis. 2001;8:723–742. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]
- Cotman SL, Halfter W, Cole GJ. Agrin binds to beta-amyloid (Abeta), accelerates abeta fibril formation, and is localized to Abeta deposits in Alzheimer's disease brain. Mol Cell Neurosci. 2000;15:183–198. doi: 10.1006/mcne.1999.0816. [DOI] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir RL, Banker G. The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron. 1993;10:1055–1068. doi: 10.1016/0896-6273(93)90054-u. [DOI] [PubMed] [Google Scholar]
- Cremer H, Chazal G, Lledo PM, Rougon G, Montaron MF, Mayo W, Le Moal M, Abrous DN. PSA-NCAM: an important regulator of hippocampal plasticity. Int J Dev Neurosci. 2000;18:213–220. doi: 10.1016/s0736-5748(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O'Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaunchak AS, Huang JK, De Faria Junior O, Roth AD, Pedraza L, Antel JP, Bar-Or A, Colman DR. A proteome map of axoglial specializations isolated and purified from human central nervous system. Glia. 2010;58:1949–1960. doi: 10.1002/glia.21064. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, Muller D, Schachner M. Polysialylated Neural Cell Adhesion Molecule Promotes Remodeling and Formation of Hippocampal Synapses. J Neurosci. 2004;24:9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11:735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- Dodds DNC, Omeis IA, Cushman SJ, Helms JA, Perin MS. Neuronal Pentraxin Receptor, a Novel Putative Integral Membrane Pentraxin That Interacts with Neuronal Pentraxin 1 and 2 and Taipoxin-associated Calcium-binding Protein 49. J Biol Chem. 1997;272:21488–21494. doi: 10.1074/jbc.272.34.21488. [DOI] [PubMed] [Google Scholar]
- Doherty P, Fazeli MS, Walsh FS. The neural cell adhesion molecule and synaptic plasticity. J Neurobiol. 1995;26:437–446. doi: 10.1002/neu.480260315. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Berzin TM, Rafii MS, Glass DJ, Yancopoulos GD, Fallon JR, Stopa EG. Agrin in Alzheimer's disease: altered solubility and abnormal distribution within microvasculature and brain parenchyma. Proc Natl Acad Sci U S A. 1999;96:6468–6472. doi: 10.1073/pnas.96.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi A, Slater SC, George SJ. MMP-9 and -12 cause N-cadherin shedding and thereby [beta]-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res. 2009;81:178–186. doi: 10.1093/cvr/cvn278. [DOI] [PubMed] [Google Scholar]
- Dykens EM. Psychiatric and behavioral disorders in persons with Down syndrome. Ment Retard Dev Disabilities Res Rev. 2007;13:272–278. doi: 10.1002/mrdd.20159. [DOI] [PubMed] [Google Scholar]
- Egles C, Claudepierre T, Manglapus MK, Champliaud M-F, Brunken WJ, Hunter DD. Laminins containing the [beta]2 chain modulate the precise organization of CNS synapses. Mol Cell Neurosci. 2007;34:288–298. doi: 10.1016/j.mcn.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Epstein CJ. Down's syndrome: critical genes in a critical region. Nature. 2006;441:582–583. doi: 10.1038/441582a. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Irie F, Kalo MS, Couchman JR, Pasquale EB, Yamaguchi Y. EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron. 2001;31:1001–1013. doi: 10.1016/s0896-6273(01)00440-8. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Yamaguchi Y. Cell surface heparan sulfate proteoglycan syndecan-2 induces the maturation of dendritic spines in rat hippocampal neurons. J Cell Biol. 1999;144:575–586. doi: 10.1083/jcb.144.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrichny IP, Leone P, Sulzenbacher G, Comoletti D, Miller MT, Taylor P, Bourne Y, Marchot P. Structural analysis of the synaptic protein neuroligin and its beta-neurexin complex: determinants for folding and cell adhesion. Neuron. 2007;56:979–991. doi: 10.1016/j.neuron.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falo MC, Fillmore HL, Reeves TM, Phillips LL. Matrix metalloproteinase-3 expression profile differentiates adaptive and maladaptive synaptic plasticity induced by traumatic brain injury. J Neurosci Res. 2006;84:768–781. doi: 10.1002/jnr.20986. [DOI] [PubMed] [Google Scholar]
- Ferreira A. Abnormal synapse formation in agrin-depleted hippocampal neurons. J Cell Sci. 1999;112:4729–4738. doi: 10.1242/jcs.112.24.4729. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Gullotta F. Down's dyndrome and Alzheimer's disease: dendritic spine counts in the hippocampus. Acta Neuropathol. 1990;79:680–685. doi: 10.1007/BF00294247. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Fejtova A, Viesti M, Stephan A, Sonderegger P. Activity-Induced Synaptic Capture and Exocytosis of the Neuronal Serine Protease Neurotrypsin. J Neurosci. 2008;28:1568–1579. doi: 10.1523/JNEUROSCI.3398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- Garcia O, Torres M, Helguera P, Coskun P, Busciglio J. A role for thrombospondin-1 deficits in astrocyte-mediated spine and synaptic pathology in Down's syndrome. PLoS One. 2010;5:e14200. doi: 10.1371/journal.pone.0014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood J, Heck N, Rigato F, Faissner A. The Extracellular Matrix in Neural Development, Plasticity, and Regeneration. In: Walz W, editor. The Neuronal Environment: Brain Homeostasis in Health and Disease. Totowa, NJ: Humana Press; 2003. pp. 109–158. [Google Scholar]
- Gawlak M, Górkiewicz T, Gorlewicz A, Konopacki FA, Kaczmarek L, Wilczynski GM. High resolution in situ zymography reveals matrix metalloproteinase activity at glutamatergic synapses. Neuroscience. 2009;158:167–176. doi: 10.1016/j.neuroscience.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Cavalli V, Denzer AJ, Brancaccio A, Schumacher B, Ruegg MA. Alternative splicing of agrin alters its binding to heparin, dystroglycan, and the putative agrin receptor. Neuron. 1996;16:755–767. doi: 10.1016/s0896-6273(00)80096-3. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Godyna S, Liau G, Popa I, Stefansson S, Argraves WS. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J Cell Biol. 1995;129:1403–1410. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan W-B. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Gschwend TP, Krueger SR, Kozlov SV, Wolfer DP, Sonderegger P. Neurotrypsin, a Novel Multidomain Serine Protease Expressed in the Nervous System. Mol Cell Neurosci. 1997;9:207–219. doi: 10.1006/mcne.1997.0616. [DOI] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The Amyloid Hypothesis of Alzheimer's Disease: Progress and Problems on the Road to Therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases. Neuromolecular Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- Hausen D, Bruckner G, Drlicek M, Hartig W, Brauer K, Bigl V. Pyramidal cells ensheathed by perineuronal nets in human motor and somatosensory cortex. Neuroreport. 1996;7:1725–1729. doi: 10.1097/00001756-199607290-00006. [DOI] [PubMed] [Google Scholar]
- Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Avila J. Tauopathies. Cell Mol Life Sci. 2007;64:2219–2233. doi: 10.1007/s00018-007-7220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat AJGD, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and Persistent Dendritic Spines in the Neocortex In Vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257:2912–2919. [PubMed] [Google Scholar]
- Hsueh Y-P, Yang F-C, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M. Direct Interaction of CASK/LIN-2 and Syndecan Heparan Sulfate Proteoglycan and Their Overlapping Distribution in Neuronal Synapses. J Cell Biol. 1998;142:139–151. doi: 10.1083/jcb.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO, editor. Fibronectins. New York: Springer-Verlag; 1990. [Google Scholar]
- Hynes RO. Integrins: Bidirectional, Allosteric Signaling Machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Indyk JA, Chen ZL, Tsirka SE, Strickland S. Laminin chain expression suggests that laminin-10 is a major isoform in the mouse hippocampus and is degraded by the tissue plasminogen activator/plasmin protease cascade during excitotoxic injury. Neuroscience. 2003;116:359–371. doi: 10.1016/s0306-4522(02)00704-2. [DOI] [PubMed] [Google Scholar]
- Innerarity TL, Weisgraber KH, Arnold KS, Rall SC, Jr, Mahley RW. Normalization of receptor binding of apolipoprotein E2. Evidence for modulation of the binding site conformation. J Biol Chem. 1984;259:7261–7267. [PubMed] [Google Scholar]
- Inoue E, Deguchi-Tawarada M, Togawa A, Matsui C, Arita K, Katahira-Tayama S, Sato T, Yamauchi E, Oda Y, Takai Y. Synaptic activity prompts [gamma]-secretase mediated cleavage of EphA4 and dendritic spine formation. J Cell Biol. 2009;185:551–564. doi: 10.1083/jcb.200809151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A, Rollenhagen A, Troncoso E, Kiss JZ, Schachner M. Structural and Functional Aberrations in the Cerebral Cortex of Tenascin-C Deficient Mice. Cereb Cortex. 2005;15:950–962. doi: 10.1093/cercor/bhh195. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Joo JY, Lee SJ, Uemura T, Yoshida T, Yasumura M, Watanabe M, Mishina M. Differential interactions of cerebellin precursor protein (Cbln) subtypes and neurexin variants for synapse formation of cortical neurons. Biochem Biophys Res Commun. 2011;406:627–632. doi: 10.1016/j.bbrc.2011.02.108. [DOI] [PubMed] [Google Scholar]
- Jucker M, Tian M, Ingram D. Laminins in the adult and aged brain. Mol Chem Neuropathol. 1996;28:209–218. doi: 10.1007/BF02815224. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Pavlov I, Võikar V, Lauri SE, Hienola A, Riekki R, Lakso M, Taira T, Rauvala H. Syndecan-3-Deficient Mice Exhibit Enhanced LTP and Impaired Hippocampus-Dependent Memory. Mol Cell Neurosci. 2002;21:158–172. doi: 10.1006/mcne.2002.1167. [DOI] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Fillmore HL, Reeves TM, Phillips LL. Elevation of hippocampal MMP-3 expression and activity during trauma-induced synaptogenesis. Exp Neurol. 2005;192:60–72. doi: 10.1016/j.expneurol.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick LL, Matzuk MM, Dodds DNC, Perin MS. Biochemical Interactions of the Neuronal Pentraxins. J Biol Chem. 2000;275:17786–17792. doi: 10.1074/jbc.M002254200. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14:526–532. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Knott G, Holtmaat A. Dendritic spine plasticity--current understanding from in vivo studies. Brain Res Rev. 2008;58:282–289. doi: 10.1016/j.brainresrev.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Knuesel I. Reelin-mediated signaling in neuropsychiatric and neurodegenerative diseases. Prog Neurobiol. 2010;91:257–274. doi: 10.1016/j.pneurobio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Kohno T, Hattori M. Re-evaluation of protease activity of reelin. Biol Pharm Bull. 2010;33:1047–1049. doi: 10.1248/bpb.33.1047. [DOI] [PubMed] [Google Scholar]
- Kosfeld MD, Frazier WA. Identification of active peptide sequences in the carboxyl-terminal cell binding domain of human thrombospondin-1. J Biol Chem. 1992;267:16230–16236. [PubMed] [Google Scholar]
- Kramar EA, Bernard JA, Gall CM, Lynch G. Integrins Modulate Fast Excitatory Transmission at Hippocampal Synapses. J Biol Chem. 2003;278:10722–10730. doi: 10.1074/jbc.M210225200. [DOI] [PubMed] [Google Scholar]
- Kroger S, Pfister H. Agrin in the nervous system: synaptogenesis and beyond. Future Neurol. 2009;4:67–86. [Google Scholar]
- Ksiazek I, Burkhardt C, Lin S, Seddik R, Maj M, Bezakova G, Jucker M, Arber S, Caroni P, Sanes JR, Bettler B, Ruegg MA. Synapse Loss in Cortex of Agrin-Deficient Mice after Genetic Rescue of Perinatal Death. J Neurosci. 2007;27:7183–7195. doi: 10.1523/JNEUROSCI.1609-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok J, Dick G, Wang D, Fawcett J. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011 doi: 10.1002/dneu.20974. in press. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Sabatini BL. Glutamate induces de novo growth of functional spines in developing cortex. Nature. 2011;474:100–104. doi: 10.1038/nature09986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Barco A, Zablow L, Kandel ER, Siegelbaum SA, Zakharenko SS. Transient expansion of synaptically connected dendritic spines upon induction of hippocampal long-term potentiation. PNAS. 2004;101:16665–16670. doi: 10.1073/pnas.0407581101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Ognibene E, Romano E, Adriani W, Keller F. Gene-environment interaction during early development in the heterozygous reeler mouse: clues for modelling of major neurobehavioral syndromes. Neurosci Biobehav Rev. 2009;33:560–572. doi: 10.1016/j.neubiorev.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- Li Z, Hilgenberg LGW, O'Dowd DK, Smith MA. Formation of functional synaptic connections between cultured cortical neurons from agrin-deficient mice. J Neurobiol. 1999;39:547–557. doi: 10.1002/(sici)1097-4695(19990615)39:4<547::aid-neu8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Libby RT, Champliaud M-F, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ. Laminin Expression in Adult and Developing Retinae: Evidence of Two Novel CNS Laminins. J Neurosci. 2000;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Dahl D, Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J Cell Biol. 1983;96:920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Arai AC, Lynch G, Gall CM. Integrins regulate NMDA receptor-mediated synaptic currents. J Neurophysiol. 2003;89:2874–2878. doi: 10.1152/jn.00783.2002. [DOI] [PubMed] [Google Scholar]
- Lin KT, Sloniowski S, Ethell DW, Ethell IM. Ephrin-B2-induced Cleavage of EphB2 Receptor Is Mediated by Matrix Metalloproteinases to Trigger Cell Repulsion. J Biol Chem. 2008;283:28969–28979. doi: 10.1074/jbc.M804401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, McCroskery S, Ross JM, Chak Y, Neuhuber B, Daniels MP. Induction of filopodia-like protrusions by transmembrane agrin: role of agrin glycosaminoglycan chains and Rho-family GTPases. Exp Cell Res. 2010;316:2260–2277. doi: 10.1016/j.yexcr.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-L, Lei Y-T, Hong C-J, Hsueh Y-P. Syndecan-2 induces filopodia and dendritic spine formation via the neurofibromin-PKA-Ena/VASP pathway. J Cell Biol. 2007;177:829–841. doi: 10.1083/jcb.200608121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P, Larson J, Condie BG, Guidotti A, Costa E. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. PNAS. 2001;98:3477–3482. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucic V, Yang T, Schweikert G, Forster F, Baumeister W. Morphological characterization of molecular complexes present in the synaptic cleft. Structure. 2005;13:423–434. doi: 10.1016/j.str.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Marrs GS, Green SH, Dailey ME. Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nat Neurosci. 2001;4:1006–1013. doi: 10.1038/nn717. [DOI] [PubMed] [Google Scholar]
- Mataga N, Mizuguchi Y, Hensch TK. Experience-Dependent Pruning of Dendritic Spines in Visual Cortex by Tissue Plasminogen Activator. Neuron. 2004;44:1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Miyai K, Sokolowska E, Zurlinden A, Gee CE, Lüscher D, Hettwer S, Wölfel J, Ladner AP, Ster J, Gerber U, Rülicke T, Kunz B, Sonderegger P. Coincident Pre- and Postsynaptic Activation Induces Dendritic Filopodia via Neurotrypsin-Dependent Agrin Cleavage. Cell. 2009;136:1161–1171. doi: 10.1016/j.cell.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Matsuno H, Okabe S, Mishina M, Yanagida T, Mori K, Yoshihara Y. Telencephalin slows spine maturation. J Neurosci. 2006;26:1776–1786. doi: 10.1523/JNEUROSCI.2651-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan Glycoforms Contribute to the Molecular Heterogeneity of Perineuronal Nets. J Neurosci. 2002;22:7536–7547. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S, Bailey A, Lin L, Daniels MP. Transmembrane agrin regulates dendritic filopodia and synapse formation in mature hippocampal neuron cultures. Neuroscience. 2009;163:168–179. doi: 10.1016/j.neuroscience.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S, Chaudhry A, Lin L, Daniels MP. Transmembrane agrin regulates filopodia in rat hippocampal neurons in culture. Mol Cell Neurosci. 2006;33:15–28. doi: 10.1016/j.mcn.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Mecham RP. Overview of extracellular matrix. Curr Protoc Cell Biol. 1998 doi: 10.1002/0471143030.cb1001s00. Chapter 10:Unit 10 11. [DOI] [PubMed] [Google Scholar]
- Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J Neurochem. 2007;102:2085–2096. doi: 10.1111/j.1471-4159.2007.04682.x. [DOI] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J, Jaworski J, Gorecki DC, Ottersen OP, Kaczmarek L. beta-Dystroglycan as a Target for MMP-9, in Response to Enhanced Neuronal Activity. J Biol Chem. 2007;282:16036–16041. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. Matrix Metalloproteinase-9 Controls NMDA Receptor Surface Diffusion through Integrin {beta}1 Signaling. J Neurosci. 2009;29:6007–6012. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaluk P, Wawrzyniak M, Alot P, Szczot M, Wyrembek P, Mercik K, Medvedev N, Wilczek E, De Roo M, Zuschratter W, Muller D, Wilczynski GM, Mozrzymas JW, Stewart MG, Kaczmarek L, Wlodarczyk J. Influence of matrix metalloproteinase, MMP-9 on dendritic spine morphology. J Cell Sci. 2011 doi: 10.1242/jcs.090852. (in press) [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. The integrin family of cell adhesion molecules has multiple functions within the CNS. J Neurosci Res. 2002;69:286–291. doi: 10.1002/jnr.10321. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Molinari F, Rio M, Meskenaite V, Encha-Razavi F, Auge J, Bacq D, Briault S, Vekemans M, Munnich A, Attie-Bitach T, Sonderegger P, Colleaux L. Truncating neurotrypsin mutation in autosomal recessive nonsyndromic mental retardation. Science. 2002;298:1779–1781. doi: 10.1126/science.1076521. [DOI] [PubMed] [Google Scholar]
- Monea S, Jordan BA, Srivastava S, DeSouza S, Ziff EB. Membrane Localization of Membrane Type 5 Matrix Metalloproteinase by AMPA Receptor Binding Protein and Cleavage of Cadherins. J Neurosci. 2006;26:2300–2312. doi: 10.1523/JNEUROSCI.3521-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawski M, Bruckner G, Jager C, Seeger G, Arendt T. Neurons associated with aggrecan-based perineuronal nets are protected against tau pathology in subcortical regions in Alzheimer's disease. Neuroscience. 2010;169:1347–1363. doi: 10.1016/j.neuroscience.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Morris CA. Introduction: Williams syndrome. Am J Med Genet C Semin Med Genet. 2010;154C:203–208. doi: 10.1002/ajmg.c.30266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity Produces Increases in Neurotransmitter Release and Synapse Size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami Y, Abe K, Nishiyama N, Matsuki N. Laminin Degradation by Plasmin Regulates Long-Term Potentiation. J Neurosci. 2000;20:2003–2010. doi: 10.1523/JNEUROSCI.20-05-02003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann FR, Bittcher G, Annies M, Schumacher B, Kroger S, Ruegg MA. An alternative amino-terminus expressed in the central nervous system converts agrin to a type II transmembrane protein. Mol Cell Neurosci. 2001;17:208–225. doi: 10.1006/mcne.2000.0932. [DOI] [PubMed] [Google Scholar]
- Ngo ST, Noakes PG, Phillips WD. Neural agrin: a synaptic stabiliser. Int J Biochem Cell Biol. 2007;39:863–867. doi: 10.1016/j.biocel.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Nicholson C, Syková E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Trana R, Katz Y, Kath WL, Spruston N, Geinisman Y. Distance-Dependent Differences in Synapse Number and AMPA Receptor Expression in Hippocampal CA1 Pyramidal Neurons. Neuron. 2006;50:431–442. doi: 10.1016/j.neuron.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Nickerson E, Greenberg F, Keating MT, McCaskill C, Shaffer LG. Deletions of the elastin gene at 7q11.23 occur in approximately 90% of patients with Williams syndrome. Am J Hum Genet. 1995;56:1156–1161. [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Yasuda R, Oertner TG, Svoboda K. The Number of Glutamate Receptors Opened by Synaptic Stimulation in Single Hippocampal Spines. J Neurosci. 2004;24:2054–2064. doi: 10.1523/JNEUROSCI.5066-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P. Synaptically Targeted Narp Plays an Essential Role in the Aggregation of AMPA Receptors at Excitatory Synapses in Cultured Spinal Neurons. J Neurosci. 2002;22:4487–4498. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi S, Takano K. Amyloid fibrils from the viewpoint of protein folding. Cell Mol Life Sci. 2004;61:511–524. doi: 10.1007/s00018-003-3264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Miwa A, Okado H. Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci. 2001;21:6105–6114. doi: 10.1523/JNEUROSCI.21-16-06105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Oray S, Majewska A, Sur M. Dendritic Spine Dynamics Are Regulated by Monocular Deprivation and Extracellular Matrix Degradation. Neuron. 2004;44:1021–1030. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Pappas GD, Kriho V, Pesold C. Reelin in the extracellular matrix and dendritic spines of the cortex and hippocampus: a comparison between wild type and heterozygous <i>reeler</i> mice by immunoelectron microscopy. J Neurocytol. 2002;30:413–425. doi: 10.1023/a:1015017710332. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Rao BSS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. PNAS. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cruz C, Nolte MW, van Gaalen MM, Rustay NR, Termont A, Tanghe A, Kirchhoff F, Ebert U. Reduced spine density in specific regions of CA1 pyramidal neurons in two transgenic mouse models of Alzheimer’s disease. J Neurosci. 2011;31:3926–3934. doi: 10.1523/JNEUROSCI.6142-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362:239–252. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SK, Kleinman HK. Neuronal laminins and their cellular receptors. Int J Biochem Cell Biol. 1997;29:401–414. doi: 10.1016/s1357-2725(96)00110-0. [DOI] [PubMed] [Google Scholar]
- Prater CA, Plotkin J, Jaye D, Frazier WA. The properdin-like type I repeats of human thrombospondin contain a cell attachment site. J Cell Biol. 1991;112:1031–1040. doi: 10.1083/jcb.112.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential Reelin-Induced Enhancement of NMDA and AMPA Receptor Activity in the Adult Hippocampus. J Neurosci. 2006;26:12943–12955. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchi CC, Wannenes F, Persico AM, Ciafre SA, D’Arcangelo G, Farace MG, Keller F. Reelin Is a Serine Protease of the Extracellular Matrix. J Biol Chem. 2002;277:303–309. doi: 10.1074/jbc.M106996200. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Estructura de los centros nerviosos de las aves. Rev Trim Hist Norm Pat. 1888;1:1–10. [Google Scholar]
- Ramon y Cajal S. La textura del sistema nervioso del hombre y de los vertebrados. Moya, Madrid: 1899. [Google Scholar]
- Ramseger R, White R, Kroger S. Transmembrane form agrin-induced process formation requires lipid rafts and the activation of Fyn and MAPK. J Biol Chem. 2009;284:7697–7705. doi: 10.1074/jbc.M806719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranscht B. Cadherins: molecular codes for axon guidance and synapse formation. Int J Dev Neurosci. 2000;18:643–651. doi: 10.1016/s0736-5748(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Raulo E, Chernousov MA, Carey DJ, Nolo R, Rauvala H. Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM). Identification as N-syndecan (syndecan-3) J Biol Chem. 1994;269:12999–13004. [PubMed] [Google Scholar]
- Reif R, Sales S, Hettwer S, Dreier B, Gisler C, Wolfel J, Luscher D, Zurlinden A, Stephan A, Ahmed S, Baici A, Ledermann B, Kunz B, Sonderegger P. Specific cleavage of agrin by neurotrypsin, a synaptic protease linked to mental retardation. FASEB J. 2007;21:3468–3478. doi: 10.1096/fj.07-8800com. [DOI] [PubMed] [Google Scholar]
- Represa A, Niquet J, Pollard H, Ben-Ari Y. Cell death, gliosis, and synaptic remodeling in the hippocampus of epileptic rats. J Neurobiol. 1995;26:413–425. doi: 10.1002/neu.480260313. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T. Role of the Reelin Signaling Pathway in Central Nervous System Development. Ann Rev Neurosci. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell Migration: Integrating Signals from Front to Back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rodriguez MA, Pesold C, Liu WS, Kriho V, Guidotti A, Pappas GD, Costa E. Colocalization of integrin receptors and reelin in dendritic spine postsynaptic densities of adult nonhuman primate cortex. PNAS. 2000;97:3550–3555. doi: 10.1073/pnas.050589797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin alpha v beta 3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. PNAS. 2003;100:9482–9487. doi: 10.1073/pnas.1633689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberger DJ. Fibronectin. Int J Biochem Cell Biol. 1997;29:939–943. doi: 10.1016/s1357-2725(96)00172-0. [DOI] [PubMed] [Google Scholar]
- Rudelli RD, Brown WT, Wisniewski K, Jenkins EC, Laure-Kamionowska M, Connell F, Wisniewski HM. Adult fragile X syndrome. Clinico-neuropathologic findings. Acta Neuropathol. 1985;67:289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Brain extracellular matrix. Glycobiol. 1996;6:489–492. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Saghatelyan AK, Dityatev A, Schmidt S, Schuster T, Bartsch U, Schachner M. Reduced Perisomatic Inhibition, Increased Excitatory Transmission, and Impaired Long-Term Potentiation in Mice Deficient for the Extracellular Matrix Glycoprotein Tenascin-R. Mol Cell Neurosci. 2001;17:226–240. doi: 10.1006/mcne.2000.0922. [DOI] [PubMed] [Google Scholar]
- Saghatelyan AK, Gorissen S, Albert M, Hertlein B, Schachner M, Dityatev A. The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate perisomatic inhibition and long-term potentiation in the CA1 region of the hippocampus. Eur J Neurosci. 2000;12:3331–3342. doi: 10.1046/j.1460-9568.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- Schapitz IU, Behrend B, Pechmann Y, Lappe-Siefke C, Kneussel SJ, Wallace KE, Stempel AV, Buck F, Grant SGN, Schweizer M, Schmitz D, Schwarz JR, Holzbaur ELF, Kneussel M. Neuroligin 1 Is Dynamically Exchanged at Postsynaptic Sites. J Neurosci. 2010;30:12733–12744. doi: 10.1523/JNEUROSCI.0896-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- Seil FJ. The extracellular matrix molecule, laminin, induces Purkinje cell dendritic spine proliferation in granule cell depleted cerebellar cultures. Brain Res. 1998;795:112–120. doi: 10.1016/s0006-8993(98)00265-0. [DOI] [PubMed] [Google Scholar]
- Serpinskaya AS, Feng G, Sanes JR, Craig AM. Synapse Formation by Hippocampal Neurons from Agrin-Deficient Mice. Dev Biol. 1999;205:65–78. doi: 10.1006/dbio.1998.9112. [DOI] [PubMed] [Google Scholar]
- Sharif KA, Baker H, Gudas LJ. Differential regulation of laminin b1 transgene expression in the neonatal and adult mouse brain. Neuroscience. 2004;126:967–978. doi: 10.1016/j.neuroscience.2004.03.064. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 2006;26:1813–1822. doi: 10.1523/JNEUROSCI.4091-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara Y, Hirase H. Size and Receptor Density of Glutamatergic Synapses: A Viewpoint from Left-Right Asymmetry of CA3-CA1 Connections. Front Neuroanat. 2009;3:10. doi: 10.3389/neuro.05.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia G-M, Béïque J-C, Rumbaugh G, Cho R, Worley PF, Huganir RL. Interaction of the N-Terminal Domain of the AMPA Receptor GluR4 Subunit with the Neuronal Pentraxin NP1 Mediates GluR4 Synaptic Recruitment. Neuron. 2007;55:87–102. doi: 10.1016/j.neuron.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Singhal N, Martin P. Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction. Dev Neurobiol. 2011 doi: 10.1002/dneu.20953. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar DA, Shao Y, Haines JL, Stenger JE, Jaworski J, Martin ER, DeLong GR, Moore JH, McCauley JL, Sutcliffe JS, Ashley-Koch AE, Cuccaro ML, Folstein SE, Gilbert JR, Pericak-Vance MA. Analysis of the RELN gene as a genetic risk factor for autism. Mol Psychiatry. 2004;10:563–571. doi: 10.1038/sj.mp.4001614. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ellis-Davies GC, Magee JC. Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2003;548:245–258. doi: 10.1113/jphysiol.2002.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Strasser V, Fasching D, Hauser C, Mayer H, Bock HH, Hiesberger T, Herz J, Weeber EJ, Sweatt JD, Pramatarova A, Howell B, Schneider WJ, Nimpf J. Receptor Clustering Is Involved in Reelin Signaling. Mol Cell Biol. 2004;24:1378–1386. doi: 10.1128/MCB.24.3.1378-1386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Sun M, Sibbe M, Evers M, Dityatev A, Gass P, Schachner M. Fibronectin Domains of Extracellular Matrix Molecule Tenascin-C Modulate Hippocampal Learning and Synaptic Plasticity. Mol Cell Neurosci. 2002;21:173–187. doi: 10.1006/mcne.2002.1172. [DOI] [PubMed] [Google Scholar]
- Suetsugu M, Mehraein P. Spine distribution along the apical dendrites of the pyramidal neurons in Down's syndrome. Acta Neuropathol. 1980;50:207–210. doi: 10.1007/BF00688755. [DOI] [PubMed] [Google Scholar]
- Syková E, Vorisek I, Mazel T, Antonova T, Schachner M. Reduced extracellular space in the brain of tenascin-R- and HNK-1-sulphotransferase deficient mice. Eur J Neurosci. 2005;22:1873–1880. doi: 10.1111/j.1460-9568.2005.04375.x. [DOI] [PubMed] [Google Scholar]
- Szklarczyk A, Lapinska J, Rylski M, McKay RDG, Kaczmarek L. Matrix Metalloproteinase-9 Undergoes Expression and Activation during Dendritic Remodeling in Adult Hippocampus. J Neurosci. 2002;22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Suzuki J, Kohno M, Oida K, Tamai T, Miyabo S, Yamamoto T, Nakai T. Enhancement of the Binding of Triglyceride-rich Lipoproteins to the Very Low Density Lipoprotein Receptor by Apolipoprotein E and Lipoprotein Lipase. J Biol Chem. 1995;270:15747–15754. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- Takashima S, Iida K, Mito T, Arima M. Dendritic and histochemical development and ageing in patients with Down's syndrome. J Intellectual Disability Research. 1994;38:265–273. doi: 10.1111/j.1365-2788.1994.tb00394.x. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C, Itohara S, Mishina M, Rauvala H, Gahmberg CG. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Hagg T, Denisova N, Knusel B, Engvall E, Jucker M. Laminin-[alpha]2 chain-like antigens in CNS dendritic spines. Brain Res. 1997;764:28–38. doi: 10.1016/s0006-8993(97)00420-4. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62:1984–1989. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin Regulates Dendritic Spine Morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141:1068–1079. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Gerhauser I, Seeliger F, Baumgartner W, Alldinger S. Matrix metalloproteinases and their inhibitors in the developing mouse brain and spinal cord: a reverse transcription quantitative polymerase chain reaction study. Dev Neurosci. 2005;27:408–418. doi: 10.1159/000088455. [DOI] [PubMed] [Google Scholar]
- Uryu K, Butler AK, Chesselet MF. Synaptogenesis and ultrastructural localization of the polysialylated neural cell adhesion molecule in the developing striatum. Journal of Comparative Neurology. 1999;405:216–232. doi: 10.1002/(sici)1096-9861(19990308)405:2<216::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- Verbeek MM, Otte-Holler I, van den Born J, van den Heuvel LP, David G, Wesseling P, de Waal RM. Agrin is a major heparan sulfate proteoglycan accumulating in Alzheimer's disease brain. Am J Pathol. 1999;155:2115–2125. doi: 10.1016/S0002-9440(10)65529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-b, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. PNAS. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Zhang H, Majumdar D, Horwitz AF. [alpha]-5 Integrin Signaling Regulates the Formation of Spines and Synapses in Hippocampal Neurons. J Biol Chem. 2007;282:6929–6935. doi: 10.1074/jbc.M610981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P, Bartsch U, Rasband MN, Czaniera R, Lang Y, Bluethmann H, Margolis RU, Levinson SR, Shrager P, Montag D, Schachner M. Mice Deficient for Tenascin-R Display Alterations of the Extracellular Matrix and Decreased Axonal Conduction Velocities in the CNS. J Neurosci. 1999;19:4245–4262. doi: 10.1523/JNEUROSCI.19-11-04245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, Christian JM, Förster E, Sweatt JD, Herz J. Reelin and ApoE Receptors Cooperate to Enhance Hippocampal Synaptic Plasticity and Learning. J Biol Chem. 2002;277:39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- Wenk GL. Neuropathologic changes in Alzheimer's disease. J Clin Psychiatry. 2003;(64 Suppl 9):7–10. [PubMed] [Google Scholar]
- Wenk GL. Neuropathologic changes in Alzheimer's disease: potential targets for treatment. J Clin Psychiatry 67 Suppl. 2006;3:3–7. quiz 23. [PubMed] [Google Scholar]
- Wilczynski GM, Kaczmarek L. MMP-9/TIMP-1 Extracellular Proteolytic System as AP-1 Target in Response to Neuronal Activity. In: Dudek SM, editor. Transcriptional Regulation by Neuronal Activity. New York, NY: Springer US; 2008. pp. 277–293. [Google Scholar]
- Wilczynski GM, Konopacki FA, Wilczek E, Lasiecka Z, Gorlewicz A, Michaluk P, Wawrzyniak M, Malinowska M, Okulski P, Kolodziej LR, Konopka W, Duniec K, Mioduszewska B, Nikolaev E, Walczak A, Owczarek D, Gorecki DC, Zuschratter W, Ottersen OP, Kaczmarek L. Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol. 2008;180:1021–1035. doi: 10.1083/jcb.200708213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder SJ. The complexities of dystroglycan. Trends Biochem Sci. 2001;26:118–124. doi: 10.1016/s0968-0004(00)01731-x. [DOI] [PubMed] [Google Scholar]
- Winzen U, Cole GJ, Halfter W. Agrin is a chimeric proteoglycan with the attachment sites for heparan sulfate/chondroitin sulfate located in two multiple serine-glycine clusters. J Biol Chem. 2003;278:30106–30114. doi: 10.1074/jbc.M212676200. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk J, Mukhina I, Kaczmarek L, Dityatev A. Extracellular matrix molecules, their receptors and secreted proteases in synaptic plasticity. Dev Neurobiol. 2011 doi: 10.1002/dneu.20958. in press. [DOI] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Choi S, Kim S, Lee JR, Dunah AW, Sheng M, Kim E. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12:428–437. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]
- Woods A. Syndecans: transmembrane modulators of adhesion and matrix assembly. J Clin Invest. 2001;107:935–941. doi: 10.1172/JCI12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth A, Fiete D, Baenziger JU. Spatial and temporal regulation of tenascin-R glycosylation in the cerebellum. J Biol Chem. 2002;277:50941–50947. doi: 10.1074/jbc.M209876200. [DOI] [PubMed] [Google Scholar]
- Woodworth A, Pesheva P, Fiete D, Baenziger JU. Neuronal-specific synthesis and glycosylation of tenascin-R. J Biol Chem. 2004;279:10413–10421. doi: 10.1074/jbc.M312466200. [DOI] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O'Brien RJ, Worley P. Narp and NP1 Form Heterocomplexes that Function in Developmental and Activity-Dependent Synaptic Plasticity. Neuron. 2003;39:513–528. doi: 10.1016/s0896-6273(03)00463-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci. 2009;13:22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- Yagi T, Terada N, Baba T, Ohno S. Immunolocalization of laminin-α1-like antigens around synapses in mouse cerebellar perineuronal nets. Histochem J. 2003;34:559–565. doi: 10.1023/a:1026044517888. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J, Kasai H. Principles of Long-Term Dynamics of Dendritic Spines. J Neurosci. 2008;28:13592–13608. doi: 10.1523/JNEUROSCI.0603-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Kikkawa Y, Mudd JL, Skarnes WC, Sanes JR, Miner JH. Expression of laminin chains by central neurons: Analysis with gene and protein trapping techniques. Genesis. 2003;36:114–127. doi: 10.1002/gene.10206. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Takeuchi M. Expression of fibronectin and laminin by different types of mouse glial cells cultured in a serum-free medium. Cytotechnol. 1991;7:187–196. doi: 10.1007/BF00365930. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- Zamze S, Harvey DJ, Pesheva P, Mattu TS, Schachner M, Dwek RA, Wing DR. Glycosylation of a CNS-specific extracellular matrix glycoprotein, tenascin-R, is dominated by O-linked sialylated glycans and "brain-type" neutral N-glycans. Glycobiology. 1999;9:823–831. doi: 10.1093/glycob/9.8.823. [DOI] [PubMed] [Google Scholar]
- Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60:285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Verkman AS. Microfiberoptic Measurement of Extracellular Space Volume in Brain and Tumor Slices Based on Fluorescent Dye Partitioning. Biophys J. 2010;99:1284–1291. doi: 10.1016/j.bpj.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang J, Wang L. Structural plasticity of dendritic spines. Front Biol. 2010;5:48–58. [Google Scholar]
- Zhou Q, Homma KJ, Poo M-m. Shrinkage of Dendritic Spines Associated with Long-Term Depression of Hippocampal Synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zhou X-H, Brakebusch C, Matthies H, Oohashi T, Hirsch E, Moser M, Krug M, Seidenbecher CI, Boeckers TM, Rauch U, Buettner R, Gundelfinger ED, Fassler R. Neurocan Is Dispensable for Brain Development. Mol Cell Biol. 2001;21:5970–5978. doi: 10.1128/MCB.21.17.5970-5978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Holtzman DM, Li Y, Osaka H, DeMaro J, Jacquin M, Bu G. Role of Tissue Plasminogen Activator Receptor LRP in Hippocampal Long-Term Potentiation. J Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan W-B. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]