Abstract
After menstruation, the endometrium has a remarkable capacity for repair, but the factors involved remain undefined. We hypothesize adrenomedullin (AM) plays a role in this process. Premenstrually progesterone levels decline, stimulating prostaglandin (PG) synthesis, vasoconstriction, and hypoxia. This study aimed to determine 1) AM expression throughout the menstrual (M) cycle and 2) its regulation by PG and hypoxia. Human endometrial biopsies (n = 51) were collected with ethical approval and consent. AM mRNA expression was examined by quantitative RT-PCR and was found to be selectively elevated in endometrium from the menstrual (M) phase (P < 0.001). AM immunohistochemical staining was maximal in M and proliferative (P) endometrium. Culture of secretory, but not P, explants with 100 nm PGF2α or hypoxia (0.5% O2) increased AM mRNA (P < 0.05). P explants were induced to increase AM expression using in vitro progesterone withdrawal but required the presence of hypoxia (P < 0.05). Short hairpin sequences against hypoxia-inducible factor-1α (HIF-1α) inhibited AM hypoxic up-regulation but did not alter PGF2α-induced expression. The AM receptor was immunolocalized to endothelial cells in both lymphatic and blood vessels. Conditioned medium from PGF2α-treated cells increased endothelial cell proliferation and branching (P < 0.05). This was abolished by AM receptor antagonists. In conclusion, AM is elevated at the time of endometrial repair and induces both angiogenesis and lymphangiogenesis by stimulating endothelial cell proliferation and tube formation. In the human endometrium, AM expression is up-regulated by two mechanisms: a HIF-1α-mediated hypoxic induction and a HIF-1α-independent PGF2α pathway. These physiological mechanisms may provide novel therapeutic targets for disorders such as heavy menstrual bleeding.
The human endometrium is cyclically subjected to inflammation and tissue destruction during menstruation. Its capacity for scar-free repair is remarkable, but the factors involved remain elusive. Delineation of this efficient repair process may reveal novel therapeutic targets for complaints such as heavy menstrual bleeding (HMB). In addition, the physiological mechanisms of endometrial repair may be applicable to tissues elsewhere in the body, where inflammation can lead to pathogenic fibrosis and scarring.
Premenstrually, the endometrium is exposed to high levels of progesterone, secreted from the corpus luteum. In the absence of pregnancy, the corpus luteum regresses and progesterone levels fall dramatically. It is well established that this progesterone withdrawal initiates a cascade of inflammatory mediators that culminates in tissue destruction and menstruation (1). Hormone levels remain low during menstruation, until the proliferative (P) phase commences and estrogen levels rise. Traditionally, endometrial repair was thought to be regulated by estrogen, but recent studies in a mouse model have suggested it is an estrogen-independent event (2). Therefore, we hypothesized that premenstrual progesterone withdrawal simultaneously triggers endometrial breakdown and initiates repair.
Progesterone withdrawal is known to up-regulate cyclo-oxygenase (COX)-2, the enzyme stimulating prostaglandin (PG) synthesis (3, 4). PGF2α is a potent vasoconstrictor and, alongside other vasoactive factors, causes constriction of endometrial spiral arterioles. This leads to an episode of local, transient hypoxia in the uppermost endometrial zones during menstruation. This hypoxic episode was first observed in classic experiments in the rhesus monkey (5) and was recently detected in the mouse model of menstruation (6). Use of pimonidazole, a marker of oxygen levels less than 10 mm Hg, demonstrated intense hypoxia in the luminal portion of the endometrium on d 2 of the cycle, with negligible detection by d 5. In addition, markers of tissue hypoxia [carbonic anhydrase IX and hypoxia-inducible factor (HIF)-1α] have been detected immunohistochemically in the human endometrium during menstruation (7, 8).
Adrenomedullin (AM) is a pluripotent peptide that belongs to the calcitonin gene peptide superfamily. It has a wide range of biological actions, including vasodilation (9–11), induction of angiogenesis (12–14), cell growth (15), and inhibition of apoptosis (16, 17). AM has an attractive but previously undefined role in human endometrial repair. AM acts through a G protein-coupled receptor, the calcitonin receptor-like receptor (CLR). Receptor activity-modifying proteins (RAMP) associate with CLR to determine its ligand binding specificity (18). CLR association with RAMP2 or RAMP3 promotes AM binding, whereas heterodimerization with RAMP1 promotes calcitonin gene-related peptide (CGRP) binding. Hypoxia is known to induce AM gene expression in tumor cells and endothelial cells via the transcription factor, HIF-1 (19, 20). AM is known to be expressed in the human endometrium (12, 21), but its regulation and differential expression across the menstrual cycle remain unknown.
The purpose of this study was to investigate the expression and regulation of AM in human endometrium. We hypothesized that endometrial hypoxia and PG production initiate the up-regulation of factors involved in repair. Here, we have demonstrated that endometrial AM mRNA expression peaked during menstruation, with maximal AM protein observed in M and P phase biopsies. We have identified that progesterone withdrawal, followed by hypoxic conditions and PGF2α synthesis, increased endometrial AM expression. Hypoxia-regulated AM expression was mediated by HIF-1, whereas PGF2α up-regulated AM by a HIF-1-independent pathway. Furthermore, we have demonstrated that endometrial production of AM significantly increased vascular and lymphatic endothelial cell proliferation and branching, suggesting it plays an important role in endometrial repair and regeneration.
Materials and Methods
Tissue collection
Endometrial biopsies were collected with a suction curette (Pipelle; Laboratorie CCD, Paris, France) from women with a subjective complaint of HMB having investigation in the out-patient setting (n = 22) or undergoing hysterectomy (n = 29). At hysterectomy, a full-thickness endometrial biopsy was also collected for immunohistochemical studies. Ethical approval was obtained from the Lothian Research Ethics Committee and written informed consent from all participants. Women were aged 31–52 yr (median, 41; mean, 41) with regular menstrual cycles (21–35 d) and no exogenous hormone or intrauterine device use in the 3 months before biopsy. Women with large (>3 cm) or submucosal fibroids on ultrasound examination were excluded. Women with confirmed endometriosis or with a history of persistent premenstrual dyspareunia or infertility were excluded. Tissue was placed in 1) RNA later, RNA stabilization solution (Ambion Ltd., Warrington, UK); 2) neutral buffered formalin; or 3) PBS for in vitro culture and dated according to the criteria of Noyes et al. (22). The participant's reported last menstrual period was recorded and serum estradiol and progesterone levels measured at the time of biopsy (Table 1). Biopsies were consistent for all three criteria and classified as proliferative (P), early secretory (ES), midsecretory (MS), late secretory (LS), or menstrual (M) for analysis.
Table 1.
Classification of endometrial samples examined in this study
| Histological stage of cycle | Mean age | Day of cycle | Mean E2 pmol/litre (range) | Mean P4 nmol/litre (range) |
|---|---|---|---|---|
| M (n = 8) | 41 | 2–3 | 192.25 (55–514) | 3.71 (1.24–10.59) |
| P (n = 16) | 42 | 6–16 | 441.18 (79–1105) | 2.81 (0.97–7.1) |
| ES (n = 10) | 42 | 17–21 | 497.50 (289–841) | 59.60 (23.2–112.91) |
| MS (n = 11) | 40 | 21–25 | 638.00 (242–1949) | 64.30 (25.47–114.53) |
| LS (n = 6) | 42 | 22–29 | 318.22 (59.09–819) | 8.22 (1.06–16.95) |
Immunohistochemistry
AM and CLR localization was studied by immunohistochemistry, using standard methods. Antigen retrieval was performed by pressure cooking in boiling 0.01 m citrate buffer (pH 6.0), and endogenous peroxidase activity was quenched with 10% (vol/vol) H2O2 in methanol. After blocking for 30 min in nonimmune goat serum, sections were incubated overnight at 4C with 1:1000 rabbit antihuman AM (Phoenix Pharmaceuticals, Karlsruhe, Germany) or rabbit antihuman CLR, previously described in Ref. 15. An avidin-biotin peroxidase detection system was used (Dako Ltd., Cambridge, UK) with 3,3-diaminobenzidine. Controls were incubated with isotype-matched IgG or nonimmune serum instead of primary antibody.
CLR, CD31 (platelet endothelial cell adhesion molecule), and podoplanin expression was studied by triple immunofluorescence immunohistochemistry. Sections were prepared as above, blocked with 20% nonimmune serum, and incubated sequentially with rabbit anti-CLR antibody (1:3000), rabbit anti-CD31 antibody (1:1000) (Abcam, Cambridge, UK), and sheep antihuman podoplanin (1:500) (R&D Systems, Abingdon, UK), for 18 h at 4 C. Control sections were incubated with an equivalent concentration of normal IgG or nonimmune serum. Detection was performed using goat antirabbit or donkey antisheep Fab fragment-peroxidase, as appropriate, at a dilution of 1:500 (Dako Ltd.) followed by incubation with Tyr fluorescence (CLR; green), TyrCy5 (CD31; blue), and TyrCy3 (podoplanin; red) for 10 min at a dilution of 1:50 (PerkinElmer, Wokingham, UK). Between antibodies, sections were microwaved for 2.5 min in 0.01 m citrate buffer (pH 6.0). Sections were counterstained with 4′,6-diamidino-2-phenylindole (Invitrogen, Carlsbad, CA) in PBS and mounted in PermaFluor (Immunotech-Coulter, Brea, CA) and coverslipped. Controls included replacement of the antibody to CLR with an antibody that does not bind to vessels (antihuman CD56 as the first antibody) and preimmune serum from the same rabbit that was used to raise the anti-CLR antibody. Fluorescent images were visualized and photographed using a Carl Zeiss (Jena, Germany) laser scanning microscope Meta LM 510.
Scoring of immunohistochemical staining
Localization and intensity of AM immunostaining was evaluated blindly by two independent observers using a previously validated semiquantitative scoring system (23). Intensity and distribution of staining was rated as described previously (24). Intensity was graded using a three-point scale (0, no staining; 1, mild staining; 2, strong staining). The percentage of cells stained at each of these intensities was assessed in the glandular epithelial cells and stromal cells in both the basal and functional layer of the endometrium. The surface epithelial cells and endothelial cells were also assessed where visible. A value was derived for each of the cellular compartments by using the sum of these percentages after multiplication by the intensity of staining.
Cell culture
Human Ishikawa endometrial adenocarcinoma cells (European Collection of Cell Culture, Centre for Applied Microbiology, Wiltshire, UK) stably expressing the EP2 receptor (EP2S) or the FP receptor (FPS) were maintained as previously described (25). Approximately 4 × 105 EP2S or FPS cells were seeded in six-well plates. The next day, cells were washed in PBS and incubated in serum-free culture medium containing antibiotics and 8.4 μm indomethacin for at least 16 h. Cells were treated with vehicle, 100 nm PGE2 (EP2S cells), or 100 nm PGF2α (FPS cells) and placed in either normoxia (21% O2, 5% CO2, 37 C) or hypoxic conditions (0.5% O2, 5% CO2, 37 C) in a sealed chamber (Coy Laboratory Products, Inc., Grass Lake, MI) for 24 h.
To examine the contribution of HIF-1, cells were pretreated with vehicle or 1, 2, 5, or 10 nm echinomycin (an inhibitor of HIF-1 DNA binding activity) for 1 h. Cells were then stimulated with vehicle, 100 nm PGF2α ± echinomycin, or hypoxia ± echinomycin for 8 h.
Tissue culture
Endometrial biopsies (secretory phase, n = 4; P phase, n = 3) were divided into four equal explants and incubated on raised platforms in 24-well plates, with 1 ml of serum-free RPMI 1640 medium and 8.4 μm indomethacin for 16 h. One explant was then treated with vehicle and one with 100 nm PGF2α in normoxia (21% O2, 5% CO2, 37 C). The remaining two explants were treated in the same way and placed in a sealed hypoxic chamber (Coy Laboratory Products) set at 0.5%O2, 5% CO2, 37 C for 24 h.
Five further P biopsies were divided into eight equal-sized explants and placed on raised platforms. All explants were treated with 1 μm medroxyprogesterone acetate (MPA) for 24 h. Explants were then treated with either 1) 1 μm MPA plus vehicle, 2) 1 μm MPA plus 8.4 μm indomethacin (a COX enzyme inhibitor), 3) 1 μm MPA and 1 μm RU486 (a progesterone receptor antagonist) plus vehicle, or 4) 1 μm MPA and RU486 plus 8.4 μm indomethacin. One plate was placed in normoxic conditions and the other in hypoxic conditions for 48 h.
Quantitative RT-PCR (Q-RT-PCR)
Total RNA from cells and homogenized endometrial tissue was extracted using the RNeasy Mini kit (QIAGEN Ltd., Sussex, UK) according to the manufacturer's instructions. Samples were quantified and assessed for quality as previously described (7, 26). RNA samples were reverse transcribed as described previously (26). A tube with no reverse transcriptase was included to exclude DNA contamination. Real-time quantitative PCR was carried out using an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Warrington, UK) as previously described (26). Primer and probe sequences are listed in Table 2. Data were analyzed and processed using Sequence Detector version 2.3 (PE Biosystems, Foster City, CA). Expression of target mRNA was normalized to RNA loading for each sample using the 18S ribosomal RNA as an internal standard.
Table 2.
Sequences of primers and probes used for TaqMan RT-PCR analysis
| AM | Forward | 5′-CCGACGGCCGCGTTGAC-3′ |
| Reverse | 5′-GACACAACTCCTCTCTCTT-3′ | |
| Probe | 5′-FAM-CAAACTCCCTTGGCTCACC-TAMRA-3′ | |
| CLR | Forward | 5′-GGACTCAATTCAGTTGGGAGTTACTAG-3′ |
| Reverse | 5′-GAGCCATCCATCCCAGGTT-3′ | |
| Probe | 5′-FAM- CCCCATTCAACAAGCAGAAGGCGTTT-3′-TAMRA |
Short hairpin RNA (shRNA) against HIF-1α
Two different 19-nucleotide shRNA sequences derived from human HIF-1α mRNA (U22431; bp 1470–1489 and bp 2192–2211) were used and termed HIF-1α/shRNA1470 and HIF-1α/shRNA2192 (27, 28). In addition, control oligonucleotides (TIB MOLBIOL) were used and termed (ShSCR). FPS cells were transiently transfected with lentivirus at multiplicity of infection 10 for 24 h. Cells were incubated in serum-free medium overnight before treatment with 100 nm PGF2α or placement in the hypoxic chamber for 8 h. Cells were washed with PBS and harvested and RNA or protein extracted for PCR or Western blot analysis.
Adrenomedullin enzyme immunoassay
AM concentration in conditioned media was measured using a human AM (1-52) enzyme immunoassay kit from Phoenix Peptides (Karlsruhe, Germany) according to manufacturer's instructions. All samples were analyzed on the same plate. Minimum detectable concentration, 0.13 ng/ml; interassay error, less than 14%; intraassay error, less than 5%. There was 100% cross-reactivity for rat AM (1-50) and human AM (13-52) and mouse AM (1-50) but no reported cross-reactivity for endothelin-1, amilin, CGRP, CGRP-2, α-atrial natriuretic peptide, or brain natriuretic peptide-32.
Functional assays
Human umbilical vein endothelial cells (HUVEC) (Lonza, Wokingham, UK) and human dermal lymphatic endothelial cells (HDLEC) (TCS CellWorks, Buckingham, UK) were maintained in endothelial cell growth medium, EGM-2 (Lonza) and MV2 (PromoCell, Heidelberg, Germany), respectively, with recommended growth supplements. Flasks for maintenance of HDLEC were precoated with fibronectin (1 mg/ml; Sigma, Poole, UK).
Preparation of conditioned medium
The 1 × 107 FPS cells were seeded in a 162-cm2 flask and treated with vehicle or 100 nm PGF2α in 30 ml of serum-free medium containing indomethacin for 48 h. Vehicle-treated conditioned medium (control conditioned medium, CCM) or PGF2α-treated conditioned medium (PCM) was then centrifuged and the supernatant frozen at −20 C until use.
Proliferation assay
HUVEC and HDLEC cells were seeded in 96-well plates at a concentration of 3 × 103 cells per well and starved overnight in EBM-2 basal medium supplemented with 1% fetal bovine serum and antibiotic. Cells were treated (six replicates per sample) with vehicle, 1 nm AM, CCM, PCM, or the same in the presence of one of two CLR antagonists, CGRP8–37, or AM22–52 (each used at 10 nm) for 96 h, with reagents replenished at 48 and 72 h. Proliferation was then measured with Cell Titer 96 Aqueous One solution reagent (Promega, Southampton, UK) according to the manufacturer's instructions. Results are displayed as PCM-treated cells divided by relative controls, i.e. CCM-treated cells.
Network assay
If stimulated, HUVEC and HDLEC cells branch toward each other to forming capillary tubes or networks in vitro. The degree of network formation can be assessed by measurement of the number of branch points between the tube-like structures formed, providing a quantification of angiogenic potential. Inserts of 12-well 0.4 mm transwell plates (Corning Costar, Cambridge, UK) were coated in 80 μl of growth factor reduced Matrigel (BD Biosciences, Oxford, UK) either alone or in the presence of 10 nm CGRP8–37 or 1 nm AM22–52. Matrigel was incubated at 37 C for 30 min; 2.5 × 104 HUVEC or HDLEC cells in suspension were then added to the inserts in a 500 μl volume of 1% medium; 1 ml of control medium, AM, CCM, or PCM was added to the bottom chamber and incubated for 16 h at 37 C.
Cells were fixed in ice-cold methanol and stained with hematoxylin. Networks were photographed using an Axiovert 200 microscope (Zeiss, Welwyn Garden City, UK), and the number of branch points was counted by an observer blinded to the treatment of the well. As each treatment was carried out in duplicate, the mean branch point count was calculated after unblinding.
Statistics
For mRNA expression in tissue explants, cell culture, or functional assays, results were expressed as fold increase, for which relative expression of mRNA in treated samples was divided by the relative expression in vehicle-treated samples. Data are presented as mean ± sem. Significant difference was determined using one-way ANOVA of delta cycle threshold values, with Tukey's post hoc test analysis. For endometrial biopsies from across the menstrual cycle, results were expressed as quantity relative to a comparator, a sample of RNA from the liver. Significant difference was determined using Kruskal-Wallis nonparametric test with Dunn's multiple comparison post hoc test (Instat, GraphPad Software, Inc., San Diego, CA).
Results
AM expression across the M cycle
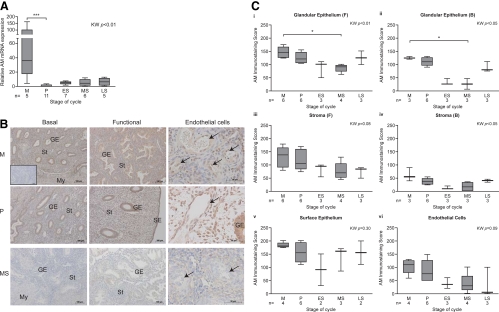
AM mRNA expression in homogenized endometrial biopsies was low in samples from the P and secretory phases of the cycle. Expression peaked at menstruation, when AM mRNA levels were significantly higher than during the P phase (P < 0.001) (Fig. 1A). Immunostaining for AM was detected in a cytoplasmic location in the surface epithelium (SE), glandular epithelium (GE), stromal compartment (St), and endothelial cells (Fig. 1B). More positive staining was observed in the functional endometrial layer than in cells located in the basal portion of the endometrium (Figs. 1, B and C, i–iv). Strongest staining was observed in the SE, and this did not vary significantly throughout the cycle (Fig. 1C, v). In contrast, semiquantitative assessment of staining in the GE revealed significantly greater staining in the M phase, compared with MS endometrium, in both the functional and basal layers (P < 0.05). There was also increased staining of P phase GE cells, but this did not reach statistical significance (Fig. 1C, i and ii). There was no significant difference in staining score of the stromal cell compartment across the cycle (Fig. 1C, iii and iv). Endothelial cells from all phases stained positively for AM (Fig. 1C, vi).
Fig. 1.
AM levels in the human endometrium at different stages of the menstrual cycle. A, AM mRNA expression was determined by Q-RT-PCR, normalized using 18S rRNA as an internal standard. B, AM protein was present in the surface epithelial (SE), glandular epithelial (GE) cells, the stromal compartment (St), and endothelial cells (arrows) during the M and P phase. Staining was markedly decreased during the secretory phase. Inset, Negative control. Scale bar, 100 or 50 μm as marked. C, Scoring of immunohistochemical staining for AM in GE cells of the functional (F) layer (i), GE cells in the basal (B) layer (ii), the St of the functional endometrial layer (iii), the St in the basal layer (iv), SE cells (v), and endothelial cells in both endometrial layers (vi). My, Myometrium; KW, Kruskal Wallis statistical analysis. *, P < 0.05.
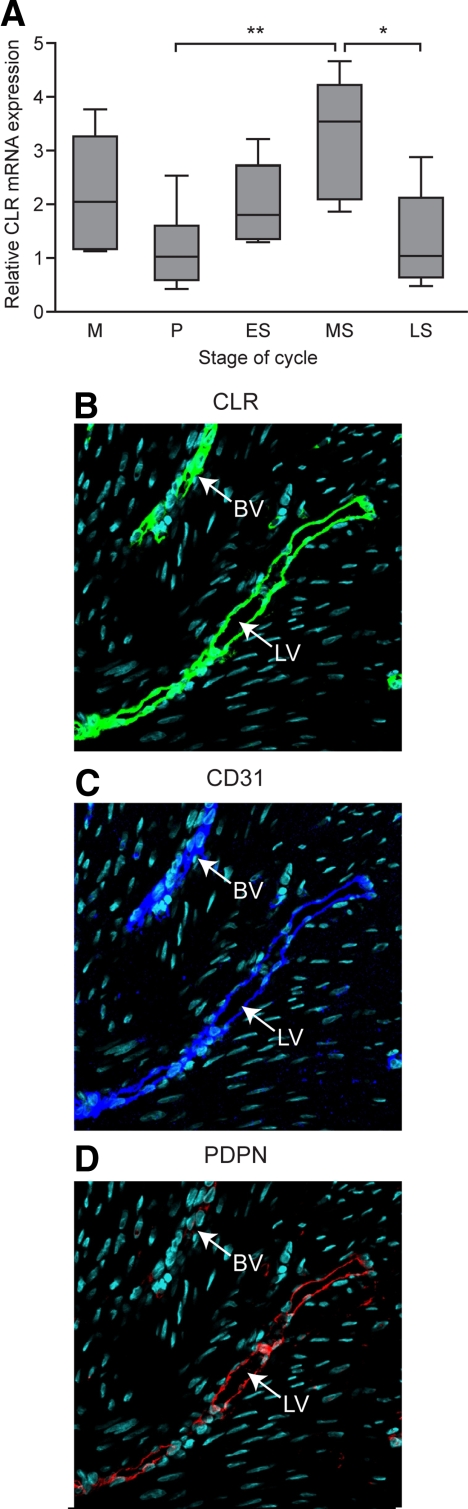
Endometrial CLR expression
CLR mRNA was present throughout the menstrual cycle (Fig. 2A). Highest levels were observed in the MS phase, when CLR expression was significantly higher than in the P (P < 0.01) and LS stage (P < 0.05) (Fig. 2A). To characterize the site of CLR expression, sections were stained sequentially with antibodies to CLR, CD31 (a pan-endothelial marker), and podoplanin (a lymphatic endothelial marker). CLR and CD31 were expressed in all vessels (Fig. 2, B and C). In contrast, podoplanin was expressed in only a proportion of vessels, predominantly in the basal layer of the endometrium (Fig. 2D). This shows CLR was present both in the blood vessels (podoplanin-negative, CD31-, and CLR-positive vessels) and lymphatics (CD31-, CLR-, and podoplanin-positive vessels).
Fig. 2.
CLR in human endometrium from across the menstrual cycle. A, CLR mRNA expression. ns, No significant difference. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Immunolocalization of CLR (B) (green) was present in the vasculature of the endometrium and colocalized both with endothelial cell marker CD31 (C) (blue) and lymphatic vessel marker podoplanin (PDPN) (D) (red). Blood vessel (BV) is negative for podoplanin, but lymphatic vessel (LV) is positive for all three antibodies.
Regulation of endometrial AM expression
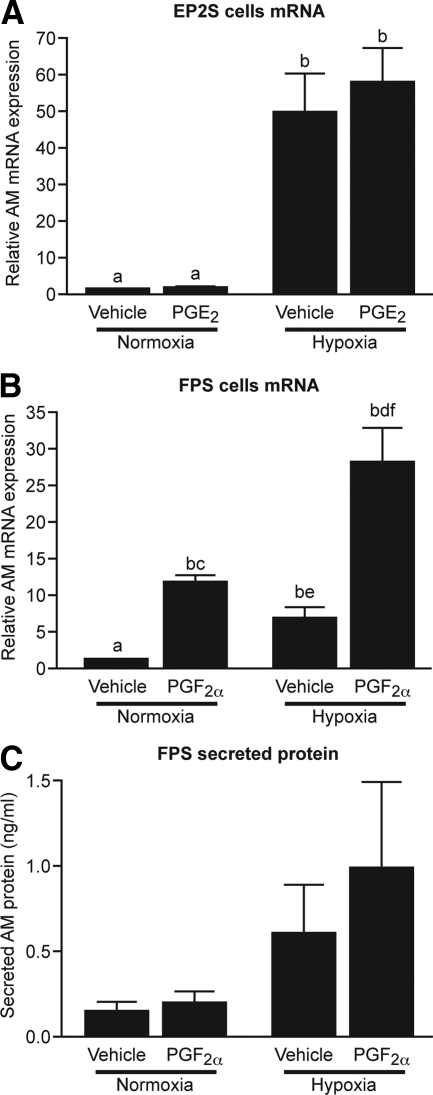
PGF2α and hypoxic conditions up-regulate AM expression in endometrial epithelial cells
To determine the impact of PG and hypoxia on AM expression, we first used an endometrial epithelial cell line stably transfected with either the PGE2 receptor (EP2S) or with the PGF2α receptor (FPS). Treatment of EP2S cells with 100 nm PGE2 had no significant impact on AM expression at 8 h (Fig. 3A) or 2, 4, 24, or 48 h (data not shown). EP2S cells placed in hypoxic conditions (0.5% O2) displayed a significant up-regulation of AM mRNA expression, with a 50-fold increase over cells in normoxic conditions (P < 0.001) (Fig. 3A). There was no further increase when cells were cotreated with hypoxia and 100 nm PGE2. Treatment with 100 nm PGF2α significantly increased AM mRNA expression in FPS cells, resulting in a more than 11-fold increase over vehicle-treated cells after 8 h (P < 0.001) (Fig. 3B). Hypoxic conditions significantly increased AM expression in FPS cells more than 6-fold (P < 0.001). Interestingly, cotreatment of FPS cells with PGF2α and hypoxia resulted in a synergistic increase in AM mRNA expression (>28-fold increase over normoxic vehicle) that was significantly greater than treatment with PGF2α or hypoxia alone (P < 0.05 and P < 0.001, respectively). Analysis of culture supernatants by ELISA revealed no significant differences in AM-secreted protein levels after 24 h, but a similar pattern emerged, with increased secreted AM protein from cells treated with both PGF2α and hypoxia compared with vehicle treatment in normoxia (Fig. 3C).
Fig. 3.
PGF2α and hypoxia up-regulate AM expression in an endometrial epithelial cell line. A, 100 nm PGE2 did not up-regulate AM expression in EP2S cells after 8 h, whereas hypoxic conditions (0.5% O2) significantly increased AM expression. B, 8-h treatment with 100 nm PGF2α or hypoxia significantly increased AM expression in FPS cells. Cotreatment with both factors revealed a synergistic increase in AM expression. C, Secreted AM protein levels in conditioned media from FP cells treated with 100 nm PGF2α and/or hypoxia for 24 h. a and b, P < 0.001; c and d, P < 0.05; e and f, P < 0.01.
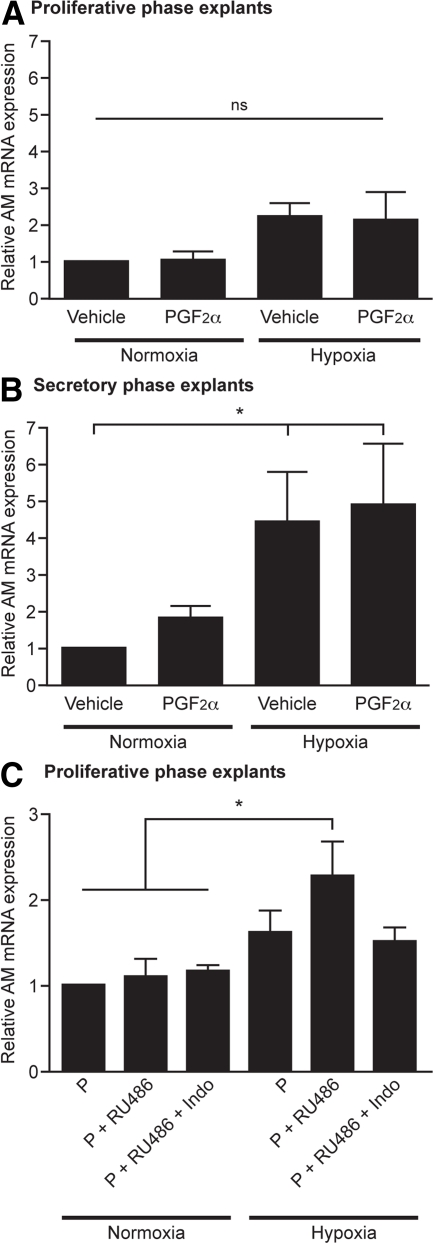
PGF2α and hypoxia up-regulate AM expression in secretory endometrial tissue
To further investigate PGF2α and hypoxia-mediated AM expression, we treated endometrial tissue explants with 1) vehicle, 2) 100 nm PGF2α, 3) hypoxic conditions, or 4) PGF2α and hypoxia. P biopsies revealed no significant differences in AM mRNA expression with these treatments (n = 3) (Fig. 4A). In contrast, endometrial tissue from the secretory phase did show a significant increase in AM expression when placed in hypoxic conditions (n = 4) (>4-fold, P < 0.05) (Fig. 4B). In addition, there was a nonsignificant 2-fold increase in AM expression after treatment with PGF2α in normoxia. This suggests that previous exposure to progesterone is a prerequisite to enable up-regulation of AM by PGF2α and hypoxia.
Fig. 4.
Hypoxia and PGF2α significantly increase AM expression in endometrial tissue previously exposed to progesterone. A, P phase explants of endometrium exposed to vehicle, 100 nm PGF2α, 0.5% O2 or both for 24 h (n = 3). B, Identical treatment of secretory phase biopsies (n = 4). C, P phase explants (n = 5) were pretreated with1 μm MPA and then 1) maintained in 1 μm MPA (P), 2) cotreated with 1 μm MPA and 1 μm RU486, a progesterone receptor antagonist (P + RU486), or 3) cotreated with 1 μm MPA, 1 μm RU486, and 8.4 μm indomethacin, a COX enzyme inhibitor (P + RU486 + Indo). Identical treatments were incubated in either normoxia (21% O2) or hypoxia (0.5% O2). ns, Nonsignificant. *, P < 0.05.
Progesterone withdrawal, in the presence of hypoxia and PG, up-regulates AM expression in P endometrial tissue
To examine the contribution of progesterone exposure and withdrawal, we used an in vitro model of progesterone antagonism. P explants were treated with progesterone followed by progesterone and mifepristone (RU486, a progesterone receptor antagonist), to mimic progesterone withdrawal. There was no significant difference in endometrial AM mRNA expression in explants subjected to progesterone withdrawal when compared with those maintained in progesterone. However, when progesterone withdrawal was carried out in hypoxic conditions, a significant increase in AM mRNA expression was observed (P < 0.05) (Fig. 4C). The contribution of PGs, which are up-regulated downstream of progesterone withdrawal, was assessed by coculture of explants with indomethacin, a COX enzyme inhibitor. Addition of indomethacin resulted in abrogation of the AM increase observed after progesterone withdrawal in hypoxic conditions (Fig. 4C).
AM induction by hypoxia, but not by PGF2α, is dependent on HIF-1α
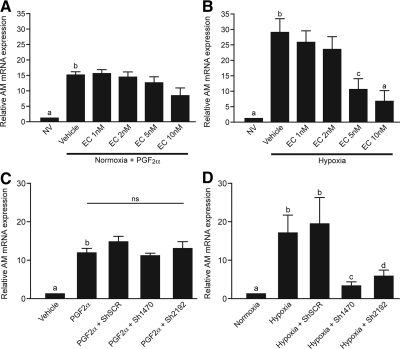
To assess the contribution of the transcription factor HIF-1α, we treated endometrial epithelial cells with echinomycin. This small molecule inhibits HIF-1 binding to hypoxic response elements on target genes, thereby preventing HIF-1-induced transcription (29). Cotreatment of FPS cells with 100 nm PGF2α and 1–10 nm echinomycin had no significant effect on AM mRNA expression (Fig. 5A). In contrast, echinomycin prevented AM up-regulation by hypoxia in a dose-dependent manner (Fig. 5B). AM expression was significantly reduced in cells treated with hypoxia and 5–10 nm echinomycin when compared with hypoxia treatment in the absence of echinomycin.
Fig. 5.
Inhibition of HIF-1 prevents hypoxia-mediated AM expression but not PGF2α-induced expression. A, Endometrial epithelial cells (FPS) treated with 100 nm PGF2α and echinomycin (EC) (a pharmacological inhibitor of HIF-1 binding) showed no significant difference in AM expression (n = 3). B, Cells treated with 5–10 nm echinomycin in hypoxic conditions significantly abrogated the hypoxic induction of AM expression (n = 3). C, Silencing of HIF-1α with two short hairpin sequences against HIF-1α (Sh1470 and Sh2192) had no significant effect on PGF2α-mediated AM expression. D, Cells transfected with shRNA against HIF-1α had a significantly abrogated response to hypoxic stimulation, with low levels of AM expression compared with untransfected cells or those transfected with a scrambled sequence (ShSCR). a and b, P < 0.001; b-c, P < 0.01; b-d, P < 0.05. ns, Nonsignificant; NV, normoxia and vehicle.
To confirm these findings, we used two different shRNA sequences against HIF-1α (HIF-1α/Sh1470 and HIF-1α/Sh2192). HIF-1α silencing was confirmed by Western blot analysis and TaqMan Q-RT-PCR (Supplemental Fig. 1, A and B, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Specificity of the shRNA sequences was confirmed with measurement of Lamin A/C mRNA levels (Supplemental Fig. 1C). HIF-1α silencing had no significant impact on PGF2α-induced AM mRNA expression (Fig. 5C). However, hypoxic induction of AM was significantly lower in those cells in which HIF-1α was silenced, when compared with untransfected cells or cells transfected with HIF-1α/ShSCR (P < 0.05) (Fig. 5D).
Endometrial AM regulates endothelial cell function
To investigate downstream effects of endometrial AM production, we treated endothelial cells (HUVEC/HDLEC) for 96 h with 1 nm AM, PCM, and the same reagents in the presence of two AM receptor antagonists CGRP8–37 (10 nm) and AM22–52 (10 nm). Endothelial cell proliferation and network formation were assessed in both cell types and displayed in Fig. 6 as fold increase over controls (vehicle treatment or cells treated with control conditioned medium).
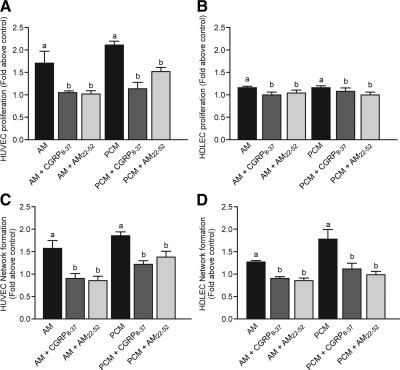
Fig. 6.
PGF2α-induced AM secretion up-regulates proliferation and network formation in human vascular endothelial cells and in HDLEC. Proliferation in HUVEC cells (A) and HDLEC cells (B) in response to AM, AM in the presence of CLR antagonists (CGRP8–37 and AM22–52), PGF2α treated conditioned medium (PCM), or PCM in the presence of CLR antagonists (n = 9–10). Network formation in HUVEC cells (C) and HDLEC cells (D) in response to AM, PCM, and AM or PCM in the presence of CLR antagonists (CGRP8–37 and AM22–52) (n = 4–5). a and b, P < 0.05.
Treatment of HUVEC cells with AM or PCM induced 1.69 (±0.28)- and 2.1 (±0.1)-fold increases in HUVEC proliferation, respectively. These effects were inhibited by the AM antagonists (Fig. 6A). Similarly, treatment of lymphatic endothelial cells (HDLEC) with AM and PCM induced small but significant increases in HDLEC proliferation, which were inhibited by chemical antagonists (Fig. 6B).
To further investigate the effect of PGF2α-mediated endometrial AM expression on endothelial cell function, network formation of endothelial cells was studied in the presence of AM and PCM. AM induced a 1.56 (±0.19)-fold increase in the number of networks formed by HUVEC, whereas PCM caused a 1.84 (±0.05)-fold increase. These effects were significantly inhibited by the AM receptor antagonists (P < 0.05) (Fig. 6C). A similar effect of AM and conditioned media on network formation was observed in HDLEC. In this case AM and PCM induced 1.25 (±0.05)- and 1.75 (±0.23)-fold increases in network formation, respectively, which were again significantly inhibited by the antagonists (P < 0.05) (Fig. 6D).
Discussion
This study demonstrates significant, novel advances in our understanding of human endometrial repair. Firstly, we show significant menstrual up-regulation of AM mRNA expression. Protein levels are also highest during the M and P stages, a temporal pattern consistent with a role in repair and regeneration. Secondly, we demonstrate that endometrial AM is regulated by two distinct mechanisms: a HIF-1-mediated hypoxic induction and a novel, HIF-1 independent, PGF2α pathway. Both these pathways are induced by progesterone withdrawal during the LS phase. Finally, we show that AM produced by endometrial cells has a significant impact on both vascular and lymphatic endothelial cell function in vitro. These data suggest that AM may have an active role in endometrial repair.
The expression of AM in the human endometrium has been studied previously (12, 21, 30, 31), but we present the first detailed analysis of endometrial AM expression at well-defined stages of the menstrual cycle. We show, for the first time, that AM mRNA and protein are high during menstruation, when repair is initiated. Immunolocalization revealed AM in the SE, GE, St, and endothelial cells, in agreement with previous in situ hybridization studies (30). We acknowledge that our study population had a subjective complaint of HMB. Previous studies have shown that 64% of women with subjective heavy loss actually had a normal objective measurement (32). However, further studies are required to determine whether blood loss has an impact on endometrial AM expression. Maximal AM localization was in the functional endometrial layer. A recent study comparing the functional and basal endometrial layers at menstruation found that genes associated with extracellular matrix biosynthesis were greatest in the lysed functional layer (33), suggesting fragments of this layer have an active role in endometrial repair. Our finding of high levels of AM protein in P endometrium is consistent with findings in the mouse and rat uterus (34, 35). However, our study also revealed high levels of AM protein during the M phase, when estradiol levels are low. This suggests an additional estradiol independent mechanism of AM up-regulation. This is consistent with observations in the mouse model of simulated menstruation (2). The absence of estradiol support in ovariectomized mice, with additional removal of extraovarian estrogenic influence using an aromatase inhibitor, had no significant impact on the rate of endometrial repair. Notwithstanding the limitations of this mouse model, these data support our findings in the human endometrium, suggesting factors with a role in initial repair are not reliant upon estrogen for their expression.
Nikitenko et al. (30) previously identified the endometrial expression of CLR by RT-PCR but did not assess its level of expression across the cycle. We observed high CLR expression during the ES phase, with low levels during the M and P phases. This inversely correlates to the pattern of endometrial AM expression. Internalization and desensitization of the endogenous CLR in endothelial cells occurs after exposure to AM (15), suggesting the function of CLR is tightly regulated by its ligand. A similar pattern of reduced CLR expression was seen in the mouse model of sepsis, where AM expression is markedly up-regulated (36). Further studies of this receptor, in particular investigation of the endometrial expression of the receptor activating binding proteins, are required to confirm its functional role and affect on AM responsiveness.
Our finding that CLR protein is localized to endothelial cells of blood vessels in the human endometrium is consistent with previous studies (30, 37, 38). In addition, we have demonstrated CLR localization in the lymphatic vasculature. Nikitenko et al. (15) have previously shown that treatment of primary endometrial endothelial cells with a synthetic AM peptide increased their growth in vitro. Our study supports and extends this work. We have demonstrated significantly increased proliferation and network formation of macrovascular and lymphatic endothelial cells treated with AM produced endogenously by endometrial cells. Confirmation of these findings in microvascular endothelial cells, as found in the human endometrium, is necessary. These processes are essential for the repair of damaged blood vessels at menstruation (39, 40). Endometrial lymphangiogenesis has recently emerged as an important process in the normal menstrual cycle (40). The lymphatics play a vital role in tissue fluid balance and immune surveillance. A study of endometrial lymphatic vessels identified small sparsely distributed vessels in the functional layer, with larger lymphatic vessels in the basal layer, often closely associated with spiral arterioles (41). This association may indicate that the lymphatics have a regulatory role in spiral arteriole function. Importantly, AM has been shown to promote the proliferation, migration, and network formation of cultured lymphatic microvascular endothelial cells and reduces secondary lymphoedema in mice with tail injury (42). Our results suggest that endometrial epithelial cell AM production increases blood and lymphatic endothelial cell proliferation and network formation. An alternative explanation is that PGF2α has an autocrine effect on endothelial cell AM production to increase their proliferation (15). Either way, these data provide further support for a functional role for AM in human endometrial repair.
The regulation of endometrial AM expression has not previously been defined. Here, we have demonstrated that endometrial cell AM expression is induced by hypoxia and by a novel PGF2α-mediated pathway. As our immunohistochemistry data showed strong staining in epithelial cells, we used an endometrial epithelial cell line stably transfected with either the PGE2 receptor (EP2) or the PGF2α receptor (FP). These cell lines were used because primary endometrial epithelial cells have a limited capacity for proliferation in vitro but do express PG receptors in vivo (43, 44). We have shown that PGF2α significantly up-regulated endometrial AM expression, whereas PGE2 had no such effect. Inflammatory cytokines, such as TNF-α and IL-1β, have previously been shown to regulate AM expression (45), but our report is the first on AM regulation by PGF2α. PGF2α is emerging as a potential key player in the initiation of endometrial repair factor expression. We have previously demonstrated PGF2α induction of a host of endometrial angiogenic factors, including vascular endothelial growth factor, fibroblast growth factor-2, and IL-8 (46–48). In addition to this novel pathway of AM regulation, we also confirmed hypoxic induction of endometrial AM expression, an effect previously demonstrated in other cell types (49, 50).
To further delineate the mechanisms controlling endometrial AM expression, we assessed the contribution of HIF-1α. HIF-1 is a transcription factor composed of two subunits: the oxygen-destructible HIF-1α subunit and the constitutively expressed HIF-1β. As a heterodimer, it binds to hypoxic response elements on target genes, which include angiogenic and mitogenic factors, such as AM, vascular endothelial growth factor, connective tissue growth factor, and IL-8 (51–54). In addition to its stabilization in hypoxic conditions, there is evidence that inflammatory mediators, e.g. PG, can induce HIF-1 in normoxia (7, 55). Therefore, we examined the contribution of HIF-1 to both hypoxia and PGF2α-mediated up-regulation of AM in our endometrial cell line. Both pharmacological inhibition of HIF-1 binding and silencing of HIF-1α with shRNA elicited a significant abrogation of hypoxia-induced AM up-regulation. These results concur with findings in cardiomyocytes and human tumor cell lines (53, 56). In contrast, we found that inhibition of HIF-1 had no impact on the ability of PGF2α to increase endometrial cell AM expression. Therefore, we propose that two independent pathways are active in the endometrium to regulate AM expression: a HIF-1-dependent hypoxic pathway and a novel PGF2α pathway that is not reliant on HIF-1 activity.
Interestingly, when examining the effect of PGF2α and hypoxic treatment of primary endometrial explants, increases in AM expression were only observed in explants taken during the secretory phase of the cycle. This suggested that previous progesterone exposure is necessary for endometrial AM up-regulation. To test this hypothesis, we subjected P endometrium to a previously described ex vivo model of progesterone antagonism (57), to mimic progesterone withdrawal in the LS phase. When explants were cotreated with progesterone and a progesterone receptor antagonist, there was no significant increase in AM expression over explants maintained in progesterone alone. However, when progesterone is withdrawn in vivo the spiral arterioles constrict, resulting in a hypoxic episode that was not accounted for in our model. Therefore, we treated explants from the same biopsies in an identical manner but placed them in a hypoxic chamber. Progesterone withdrawal in hypoxic conditions significantly increased endometrial AM expression when compared with explants treated in normoxic conditions. Addition of the COX inhibitor indomethacin abrogated this AM up-regulation. These data suggest that progesterone withdrawal up-regulates endometrial AM expression but only in the presence of hypoxia and PGs. Although the contribution of hypoxia to the initiation of menstruation is currently controversial (58), our results suggest that endometrial hypoxia has an important role in the initiation of repair.
In summary, our work describes the expression of AM and its receptor in human endometrium across the menstrual cycle. Our study identified a peak in AM mRNA and protein expression at menstruation, coinciding with the initiation of endometrial repair. We provide evidence that AM produced endogenously by endometrial cells is functionally active, in that it can stimulate proliferation and capillary branching of vascular and lymphatic endothelial cells. In addition, we demonstrate regulation of endometrial AM expression via two separate pathways: a novel PGF2α pathway and hypoxic induction via HIF-1. We propose that these pathways are activated in the endometrium after progesterone withdrawal in the LS phase, to up-regulate AM for endometrial repair. Aberrant AM expression and regulation in the endometrium may contribute to defective repair and subsequent clinical presentations of prolonged bleeding or HMB. AM expression in women with objective measurement of their menstrual loss remains to be determined. Further work in this area may aid development of effective medical treatments for this common complaint.
Supplementary Material
Acknowledgments
We thank Sharon McPherson and Catherine Murray for their help with recruitment, Sarah McDonald and Omaima Idris for technical assistance, Ronnie Grant for his help with illustrations, and Sheila Milne (all affiliated with The University of Edinburgh, Edinburgh, United Kingdom), for secretarial support. shRNA constructs were a kind donation from Professor T. Cramer (Charité-Universitätsmedizin, Berlin, Germany), and we thank Dr. Pamela Brown for her help using them.
This work was supported by Medical Research Council Grants U.1276.00.004.00002.01 and G0600048 and, in part, by the Wellcome Trust (L.L.N.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AM
- Adrenomedullin
- CCM
- control conditioned medium
- CD31
- platelet endothelial cell adhesion molecule
- CGRP
- calcitonin gene-related peptide
- CLR
- calcitonin receptor-like receptor
- COX
- cyclo-oxygenase
- ES
- early secretory
- GE
- glandular epithelium
- HDLEC
- human dermal lymphatic endothelial cell
- HIF
- hypoxia-inducible factor
- HMB
- heavy menstrual bleeding
- HUVEC
- human umbilical vein endothelial cell
- LS
- late secretory
- M
- menstrual
- MPA
- medroxyprogesterone acetate
- MS
- midsecretory
- P
- proliferative
- PCM
- PGF2α-treated conditioned medium
- PG
- prostaglandin
- Q-RT-PCR
- quantitative RT-PCR
- RAMP
- receptor activity-modifying protein
- SE
- surface epithelium
- shRNA
- short hairpin RNA
- St
- stromal compartment.
References
- 1. Jabbour HN, Kelly RW, Fraser HM, Critchley HO. 2006. Endocrine regulation of menstruation. Endocr Rev 27:17–46 [DOI] [PubMed] [Google Scholar]
- 2. Kaitu'u-Lino TJ, Morison NB, Salamonsen LA. 2007. Estrogen is not essential for full endometrial restoration after breakdown: lessons from a mouse model. Endocrinology 148:5105–5111 [DOI] [PubMed] [Google Scholar]
- 3. Critchley HO, Jones RL, Lea RG, Drudy TA, Kelly RW, Williams AR, Baird DT. 1999. Role of inflammatory mediators in human endometrium during progesterone withdrawal and early pregnancy. J Clin Endocrinol Metab 84:240–248 [DOI] [PubMed] [Google Scholar]
- 4. Hapangama DK, Critchley HO, Henderson TA, Baird DT. 2002. Mifepristone-induced vaginal bleeding is associated with increased immunostaining for cyclooxygenase-2 and decrease in prostaglandin dehydrogenase in luteal phase endometrium. J Clin Endocrinol Metab 87:5229–5234 [DOI] [PubMed] [Google Scholar]
- 5. Markee JE. 1940. Menstruation in intraocular endometrial transplants in the rhesus monkey. Contrib Embryol 177:220–230 [DOI] [PubMed] [Google Scholar]
- 6. Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML, Brenner RM, Giudice LC, Nayak NR. 2008. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J 22:3571–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Critchley HO, Osei J, Henderson TA, Boswell L, Sales KJ, Jabbour HN, Hirani N. 2006. Hypoxia-inducible factor-1α expression in human endometrium and its regulation by prostaglandin E-series prostanoid receptor 2 (EP2). Endocrinology 147:744–753 [DOI] [PubMed] [Google Scholar]
- 8. Punyadeera C, Thijssen VL, Tchaikovski S, Kamps R, Delvoux B, Dunselman GA, de Goeij AF, Griffioen AW, Groothuis PG. 2006. Expression and regulation of vascular endothelial growth factor ligands and receptors during menstruation and post-menstrual repair of human endometrium. Mol Hum Reprod 12:367–375 [DOI] [PubMed] [Google Scholar]
- 9. Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. 1993. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Bioph Res Co 192:553–560 [DOI] [PubMed] [Google Scholar]
- 10. Shindo T, Kurihara Y, Nishimatsu H, Moriyama N, Kakoki M, Wang Y, Imai Y, Ebihara A, Kuwaki T, Ju KH, Minamino N, Kangawa K, Ishikawa T, Fukuda M, Akimoto Y, Kawakami H, Imai T, Morita H, Yazaki Y, Nagai R, Hirata Y, Kurihara H. 2001. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation 104:1964–1971 [DOI] [PubMed] [Google Scholar]
- 11. Wilkinson IB, McEniery CM, Bongaerts KH, MacCallum H, Webb DJ, Cockcroft JR. 2001. Adrenomedullin (ADM) in the human forearm vascular bed: effect of neutral endopeptidase inhibition and comparison with proadrenomedullin NH2-terminal 20 peptide (PAMP). Br J Clin Pharmacol 52:159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao Y, Hague S, Manek S, Zhang L, Bicknell R, Rees MC. 1998. PCR display identifies tamoxifen induction of the novel angiogenic factor adrenomedullin by a non estrogenic mechanism in the human endometrium. Oncogene 16:409–415 [DOI] [PubMed] [Google Scholar]
- 13. Martinez A, Vos M, Guedez L, Kaur G, Chen Z, Garayoa M, Pio R, Moody T, Stetler-Stevenson WG, Kleinman HK, Cuttitta F. 2002. The effects of adrenomedullin overexpression in breast tumor cells. J Natl Cancer I 94:1226–1237 [DOI] [PubMed] [Google Scholar]
- 14. Oehler MK, Hague S, Rees MC, Bicknell R. 2002. Adrenomedullin promotes formation of xenografted endometrial tumors by stimulation of autocrine growth and angiogenesis. Oncogene 21:2815–2821 [DOI] [PubMed] [Google Scholar]
- 15. Nikitenko LL, MacKenzie IZ, Rees MC, Bicknell R. 2000. Adrenomedullin is an autocrine regulator of endothelial growth in human endometrium. Mol Hum Reprod 6:811–819 [DOI] [PubMed] [Google Scholar]
- 16. Shichiri M, Kato H, Doi M, Marumo F, Hirata Y. 1999. Induction of max by adrenomedullin and calcitonin gene-related peptide antagonizes endothelial apoptosis. Mol Endocrinol 13:1353–1363 [DOI] [PubMed] [Google Scholar]
- 17. Oehler MK, Norbury C, Hague S, Rees MC, Bicknell R. 2001. Adrenomedullin inhibits hypoxic cell death by upregulation of Bcl-2 in endometrial cancer cells: a possible promotion mechanism for tumour growth. Oncogene 20:2937–2945 [DOI] [PubMed] [Google Scholar]
- 18. McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. 1998. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333–339 [DOI] [PubMed] [Google Scholar]
- 19. Nakayama M, Takahashi K, Murakami O, Shirato K, Shibahara S. 1998. Induction of adrenomedullin by hypoxia and cobalt chloride in human colorectal carcinoma cells. Biochem Bioph Res Co 243:514–517 [DOI] [PubMed] [Google Scholar]
- 20. Nakayama M, Takahashi K, Murakami O, Shirato K, Shibahara S. 1999. Induction of adrenomedullin by hypoxia in cultured human coronary artery endothelial cells. Peptides 20:769–772 [DOI] [PubMed] [Google Scholar]
- 21. Laoag-Fernandez JB, Otani T, Maruo T. 2000. Adrenomedullin expression in the human endometrium. Endocrine 12:15–19 [DOI] [PubMed] [Google Scholar]
- 22. Noyes RW, Hertig AT, Rock J. 1950. Dating the endometrial biopsy. Fertil Steril 1:3–25 [DOI] [PubMed] [Google Scholar]
- 23. Wang H, Critchley HO, Kelly RW, Shen D, Baird DT. 1998. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol Hum Reprod 4:407–412 [DOI] [PubMed] [Google Scholar]
- 24. McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS., Sr 1985. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109:716–721 [PubMed] [Google Scholar]
- 25. Sales KJ, Maudsley S, Jabbour HN. 2004. Elevated prostaglandin EP2 receptor in endometrial adenocarcinoma cells promotes vascular endothelial growth factor expression via cyclic 3′,5′-adenosine monophosphate-mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase 1/2 signaling pathways. Mol Endocrinol 18:1533–1545 [DOI] [PubMed] [Google Scholar]
- 26. Smith OP, Jabbour HN, Critchley HO. 2007. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum Reprod 22:1450–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizukami Y, Li J, Zhang X, Zimmer MA, Iliopoulos O, Chung DC. 2004. Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res 64:1765–1772 [DOI] [PubMed] [Google Scholar]
- 28. Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL. 2003. Predominant role of hypoxia-inducible transcription factor (Hif)-1α versus Hif-2α in regulation of the transcriptional response to hypoxia. Cancer Res 63:6130–6134 [PubMed] [Google Scholar]
- 29. Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G. 2005. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res 65:9047–9055 [DOI] [PubMed] [Google Scholar]
- 30. Nikitenko LL, Brown NS, Smith DM, MacKenzie IZ, Bicknell R, Rees MC. 2001. Differential and cell-specific expression of calcitonin receptor-like receptor and receptor activity modifying proteins in the human uterus. Mol Hum Reprod 7:655–664 [DOI] [PubMed] [Google Scholar]
- 31. Michishita M, Minegishi T, Abe K, Kangawa K, Kojima M, Ibuki Y. 1999. Expression of adrenomedullin in the endometrium of the human uterus. Obstet Gynecol 93:66–70 [DOI] [PubMed] [Google Scholar]
- 32. Wyatt KM, Dimmock PW, Walker TJ, O'Brien PM. 2001. Determination of total menstrual blood loss. Fertil Steril 76:125–131 [DOI] [PubMed] [Google Scholar]
- 33. Gaide Chevronnay HP, Galant C, Lemoine P, Courtoy PJ, Marbaix E, Henriet P. 2009. Spatiotemporal coupling of focal extracellular matrix degradation and reconstruction in the menstrual human endometrium. Endocrinology 150:5094–5105 [DOI] [PubMed] [Google Scholar]
- 34. Watanabe H, Takahashi E, Kobayashi M, Goto M, Krust A, Chambon P, Iguchi T. 2006. The estrogen-responsive adrenomedullin and receptor-modifying protein 3 gene identified by DNA microarray analysis are directly regulated by estrogen receptor. J Mol Endocrinol 36:81–89 [DOI] [PubMed] [Google Scholar]
- 35. Cameron VA, Autelitano DJ, Evans JJ, Ellmers LJ, Espiner EA, Nicholls MG, Richards AM. 2002. Adrenomedullin expression in rat uterus is correlated with plasma estradiol. Am J Physiol 282:E139–E146 [DOI] [PubMed] [Google Scholar]
- 36. Ono Y, Okano I, Kojima M, Okada K, Kangawa K. 2000. Decreased gene expression of adrenomedullin receptor in mouse lungs during sepsis. Biochem Bioph Res Co 271:197–202 [DOI] [PubMed] [Google Scholar]
- 37. Nikitenko LL, Blucher N, Fox SB, Bicknell R, Smith DM, Rees MC. 2006. Adrenomedullin and CGRP interact with endogenous calcitonin-receptor-like receptor in endothelial cells and induce its desensitisation by different mechanisms. J Cell Sci 119:910–922 [DOI] [PubMed] [Google Scholar]
- 38. Nikitenko LL, Cross T, Campo L, Turley H, Leek R, Manek S, Bicknell R, Rees MC. 2006. Expression of terminally glycosylated calcitonin receptor-like receptor in uterine leiomyoma: endothelial phenotype and association with microvascular density. Clin Cancer Res 12:5648–5658 [DOI] [PubMed] [Google Scholar]
- 39. Girling JE, Rogers PA. 2005. Recent advances in endometrial angiogenesis research. Angiogenesis 8:89–99 [DOI] [PubMed] [Google Scholar]
- 40. Rogers PA, Donoghue JF, Girling JE. 2008. Endometrial lymphangiogensis. Placenta 29(Suppl A):S48–S54 [DOI] [PubMed] [Google Scholar]
- 41. Donoghue JF, Lederman FL, Susil BJ, Rogers PA. 2007. Lymphangiogenesis of normal endometrium and endometrial adenocarcinoma. Hum Reprod 22:1705–1713 [DOI] [PubMed] [Google Scholar]
- 42. Li B, Ogasawara AK, Yang R, Wei W, He GW, Zioncheck TF, Bunting S, de Vos AM, Jin H. 2002. KDR (VEGF receptor 2) is the major mediator for the hypotensive effect of VEGF. Hypertension 39:1095–1100 [DOI] [PubMed] [Google Scholar]
- 43. Milne SA, Jabbour HN. 2003. Prostaglandin (PG) F(2α) receptor expression and signaling in human endometrium: role of PGF(2α) in epithelial cell proliferation. J Clin Endocrinol Metab 88:1825–1832 [DOI] [PubMed] [Google Scholar]
- 44. Milne SA, Perchick GB, Boddy SC, Jabbour HN. 2001. Expression, localization, and signaling of PGE(2) and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J Clin Endocrinol Metab 86:4453–4459 [DOI] [PubMed] [Google Scholar]
- 45. Hofbauer KH, Schoof E, Kurtz A, Sandner P. 2002. Inflammatory cytokines stimulate adrenomedullin expression through nitric oxide-dependent and -independent pathways. Hypertension 39:161–167 [DOI] [PubMed] [Google Scholar]
- 46. Sales KJ, List T, Boddy SC, Williams AR, Anderson RA, Naor Z, Jabbour HN. 2005. A novel angiogenic role for prostaglandin F2α-FP receptor interaction in human endometrial adenocarcinomas. Cancer Res 65:7707–7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sales KJ, Maldonado-Pérez D, Grant V, Catalano RD, Wilson MR, Brown P, Williams AR, Anderson RA, Thompson EA, Jabbour HN. 2009. Prostaglandin F(2α)-F-prostanoid receptor regulates CXCL8 expression in endometrial adenocarcinoma cells via the calcium-calcineurin-NFAT pathway. Biochim Biophys Acta 1793:1917–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Keightley MC, Brown P, Jabbour HN, Sales KJ. 2010. F-Prostaglandin receptor regulates endothelial cell function via fibroblast growth factor-2. BMC Cell Biol 11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nagata D, Hirata Y, Suzuki E, Kakoki M, Hayakawa H, Goto A, Ishimitsu T, Minamino N, Ono Y, Kangawa K, Matsuo H, Omata M. 1999. Hypoxia-induced adrenomedullin production in the kidney. Kidney Int 55:1259–1267 [DOI] [PubMed] [Google Scholar]
- 50. Gratton RJ, Gluszynski M, Mazzuca DM, Nygard K, Han VK. 2003. Adrenomedullin messenger ribonucleic acid expression in the placentae of normal and preeclamptic pregnancies. J Clin Endocrinol Metab 88:6048–6055 [DOI] [PubMed] [Google Scholar]
- 51. Ahn JK, Koh EM, Cha HS, Lee YS, Kim J, Bae EK, Ahn KS. 2008. Role of hypoxia-inducible factor-1α in hypoxia-induced expressions of IL-8, MMP-1 and MMP-3 in rheumatoid fibroblast-like synoviocytes. Rheumatology 47:834–839 [DOI] [PubMed] [Google Scholar]
- 52. Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garayoa M, Martínez A, Lee S, Pío R, An WG, Neckers L, Trepel J, Montuenga LM, Ryan H, Johnson R, Gassmann M, Cuttitta F. 2000. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: a possible promotion mechanism of carcinogenesis. Mol Endocrinol 14:848–862 [DOI] [PubMed] [Google Scholar]
- 54. Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. 2004. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol 287:F1223–F1232 [DOI] [PubMed] [Google Scholar]
- 55. Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF, Levine AC. 2002. Prostaglandin E2 induces hypoxia-inducible factor-1α stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem 277:50081–50086 [DOI] [PubMed] [Google Scholar]
- 56. Cormier-Regard S, Nguyen SV, Claycomb WC. 1998. Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J Biol Chem 273:17787–17792 [DOI] [PubMed] [Google Scholar]
- 57. Critchley HO, Kelly RW, Brenner RM, Baird DT. 2003. Antiprogestins as a model for progesterone withdrawal. Steroids 68:1061–1068 [DOI] [PubMed] [Google Scholar]
- 58. Zhang J, Salamonsen LA. 2002. Expression of hypoxia-inducible factors in human endometrium and suppression of matrix metalloproteinases under hypoxic conditions do not support a major role for hypoxia in regulating tissue breakdown at menstruation. Hum Reprod 17:265–274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.