Abstract
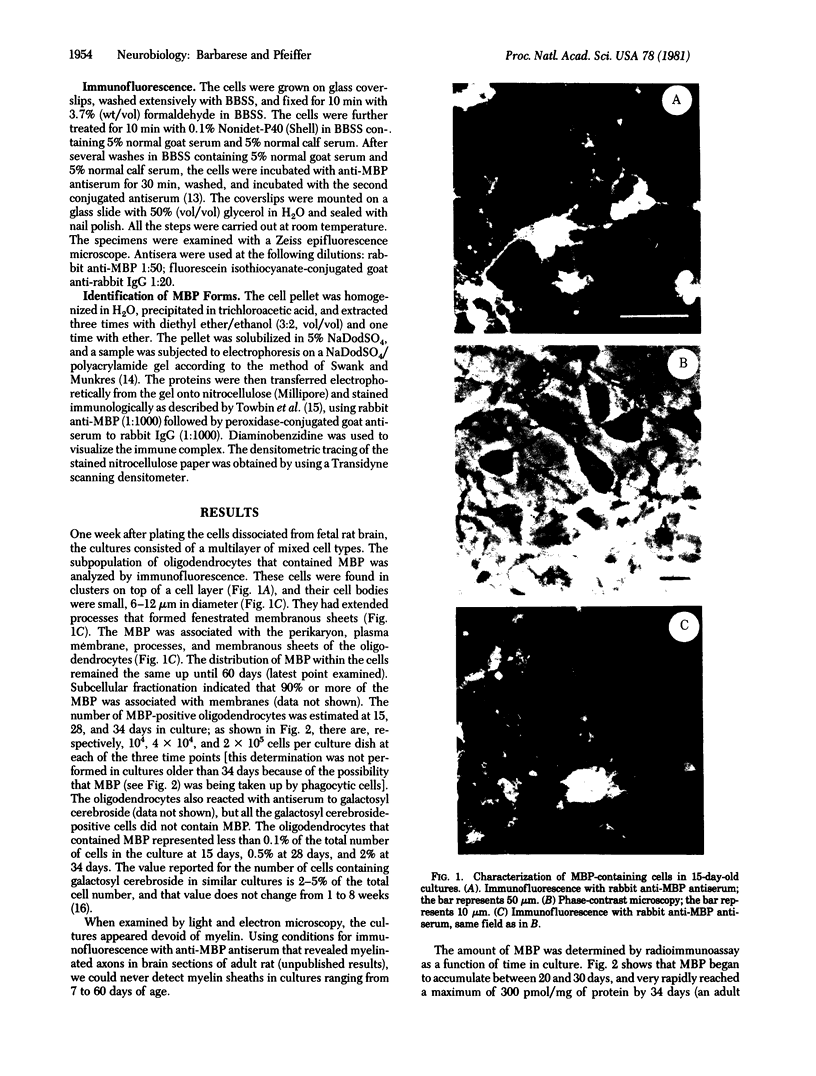
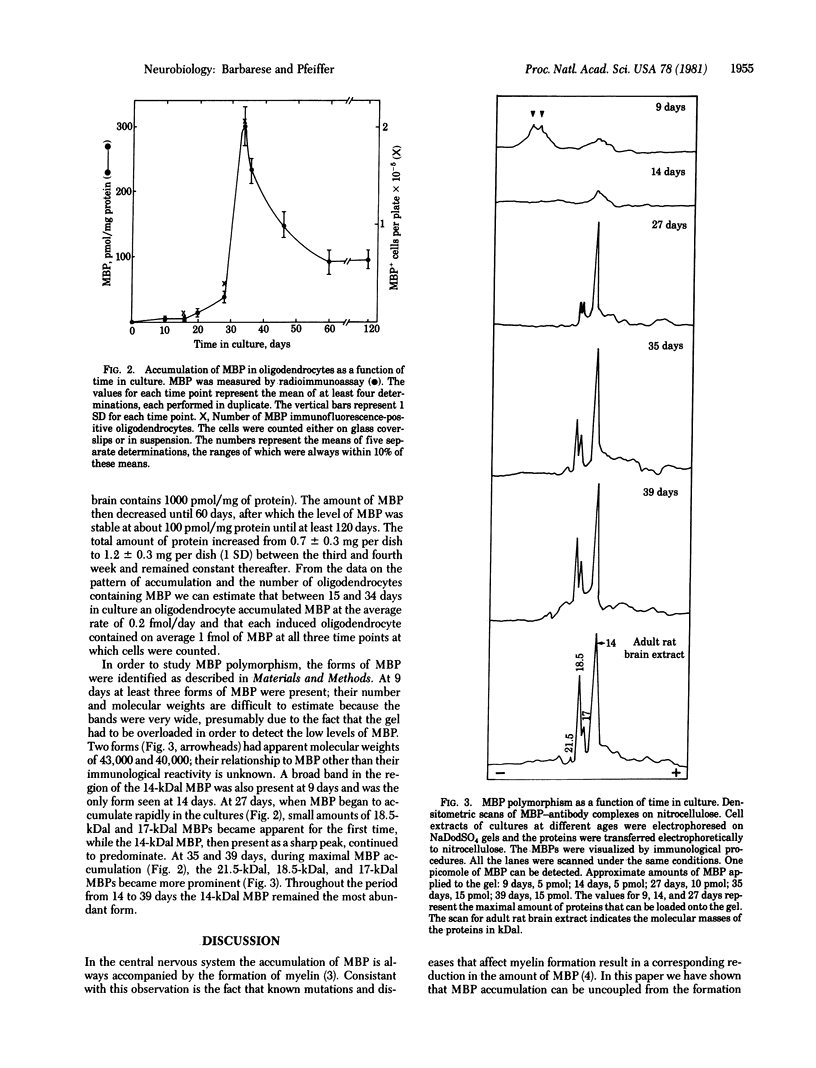
The expression of myelin basic protein, a major component of the myelin membrane, was studied in the absence of myelin formation in a unique situation in which these two processes have been uncoupled. The oligodendrocytes that contained myelin basic protein were identified by immunofluorescence in primary dispersed cultures derived from 20-day-old fetal rat brain. Their number increased 20-fold between 15 and 34 days in culture. Morphologically identifiable myelin was never observed. The oligodendrocytes elaborated a complex network of processes and membranous sheets resembling “unfurled” myelin. Myelin basic protein appeared concomitantly in the perikaryon, processes, and membranous sheets of the oligodendrocytes and remained distributed in these compartments throughout the culture period. The oligodendrocytes synthesized the four forms of myelin basic protein found in rodent brain with molecular weights of 21,500, 18,500, 17,000, and 14,000 and modulated their expression with time in culture. The onset of rapid myelin basic protein accumulation, as measured by radioimmunoassay, took place after 25 days in culture. Myelin basic protein accumulated at the rate of 0.2 fmol per oligodendrocyte per day and reached a level of 300 pmol/mg of protein by 34 days. By 60 days, the amount of myelin basic protein had declined to 100 pmol/mg of protein, a level maintained up until at least 120 days. When the amount of myelin basic protein was correlated with the number of oligodendrocytes, it was estimated that each induced cell contained on average 1 fmol of this protein at the three time points (15, 28, and 34 days in culture) at which cells were counted. Our results indicate that the accumulation, modulation of the molecular forms, and insertion of myelin basic protein into the membrane can occur in the absence of myelin formation, but that continued metabolic stability, subcellular sequestration, and fine control of the relative proportions of the different forms of myelin basic protein may be dependent on myelin morphogenesis.
Keywords: myelination, oligodendrocyte, protein polymorphism
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUNGE M. B., BUNGE R. P., PAPPAS G. D. Electron microscopic demonstration of connections between glia and myelin sheaths in the developing mammalian central nervous system. J Cell Biol. 1962 Feb;12:448–453. doi: 10.1083/jcb.12.2.448. [DOI] [PubMed] [Google Scholar]
- Barbarese E., Braun P. E., Carson J. H. Identification of prelarge and presmall basic proteins in mouse myelin and their structural relationship to large and small basic proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3360–3364. doi: 10.1073/pnas.74.8.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarese E., Carson J. H., Braun P. E. Accumulation of the four myelin basic proteins in mouse brain during development. J Neurochem. 1978 Oct;31(4):779–782. doi: 10.1111/j.1471-4159.1978.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Bourre J. M., Jacque C., Delassalle A., Nguyen-Legros J., Dumont O., Lachapelle F., Raoul M., Alvarez C., Baumann N. Density profile and basic protein measurements in the myelin range of particulate material from normal developing mouse brain and from neurological mutants (Jimpy; quaking; Trembler; shiverer and its mld allele) obtained by zonal centrifugation. J Neurochem. 1980 Aug;35(2):458–464. doi: 10.1111/j.1471-4159.1980.tb06287.x. [DOI] [PubMed] [Google Scholar]
- COONS A. H., LEDUC E. H., CONNOLLY J. M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med. 1955 Jul 1;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. R., Guarnieri M. Immunochemical measurement of myelin basic protein in developing rat brain: an index of myelin synthesis. Dev Biol. 1976 Mar;49(1):294–299. doi: 10.1016/0012-1606(76)90276-1. [DOI] [PubMed] [Google Scholar]
- Fischer C. A., Morell P. Turnover of proteins in myelin and myelin-like material of mouse brain. Brain Res. 1974 Jul 5;74(1):51–65. doi: 10.1016/0006-8993(74)90111-5. [DOI] [PubMed] [Google Scholar]
- Hartman B. K., Agrawal H. C., Kalmbach S., Shearer W. T. A comparative study of the immunohistochemical localization of basic protein to myelin and oligodendrocytes in rat and chicken brain. J Comp Neurol. 1979 Nov 15;188(2):273–290. doi: 10.1002/cne.901880206. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Messer A. The maintenance and identification of mouse cerebellar granule cells in monolayer culture. Brain Res. 1977 Jul 8;130(1):1–12. doi: 10.1016/0006-8993(77)90838-1. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Winter J., Abney E. R., Pruss R. M., Gavrilovic J., Raff M. C. Myelin-specific proteins and glycolipids in rat Schwann cells and oligodendrocytes in culture. J Cell Biol. 1980 Mar;84(3):483–494. doi: 10.1083/jcb.84.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Myelination in rat brain: changes in myelin composition during brain maturation. J Neurochem. 1973 Oct;21(4):759–773. doi: 10.1111/j.1471-4159.1973.tb07520.x. [DOI] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973 Oct;21(4):749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Shortman K., Williams N., Adams P. The separation of different cell classes from lymphoid organs. V. Simple procedures for the removal of cell debris. Damaged cells and erythroid cells from lymphoid cell suspensions. J Immunol Methods. 1972 May;1(3):273–287. doi: 10.1016/0022-1759(72)90005-1. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H., Itoyama Y., Kies M. W., Webster H. D. Myelin basic protein demonstrated immunocytochemically in oligodendroglia prior to myelin sheath formation. Proc Natl Acad Sci U S A. 1978 May;75(5):2521–2524. doi: 10.1073/pnas.75.5.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg H. J., Spencer P. S. Studies on the control of myelinogenesis. II. Evidence for neuronal regulation of myelin production. Brain Res. 1976 Aug 27;113(2):363–378. doi: 10.1016/0006-8993(76)90947-1. [DOI] [PubMed] [Google Scholar]