Abstract
Background
We assessed the efficacy, safety, and patient-reported outcomes (PROs) of insulin pump therapy in patients with type 2 diabetes mellitus (T2DM) who were suboptimally controlled with a multiple daily injection (MDI) regimen.
Methods
In this subanalysis of a 16-week multicenter study, 21 insulin-pump-naïve patients [age 57 ± 13 years, hemoglobin A1c (A1C) 8.4 ± 1.0%, body weight 98 ± 20 kg, total daily insulin dose 99 ± 65 U, mean ± standard deviation] treated at baseline with MDI therapy with or without oral antidiabetic agents discontinued all diabetes medications except metformin and initiated insulin pump therapy. Insulin was titrated to achieve the best possible glycemic control with the simplest possible dosing regimen. Outcome measures included A1C, fasting and postprandial glucose, body weight, incidence of hypoglycemia, and PROs.
Results
Glycemic control improved significantly after 16 weeks: A1C 7.3 ± 1.0% (−1.1 ± 1.2%, p < .001), fasting glucose 133 ± 33mg/dl (−32 ± 74 mg/dl, p < .005), and postprandial glucose 153 ± 35 mg/dl (−38 ± 46 mg/dl, p < .001). At week 16, the mean daily basal, bolus, and total insulin doses were 66 ± 36, 56 ± 40, and 122 ± 72 U (1.2 U/kg), respectively, and 90% of patients were treated with two or fewer daily basal rates. Body weight increased by 2.8 ± 2.6 kg (p < .001). Mild hypoglycemia was experienced by 81% of patients at least once during the course of the study with no episodes of severe hypoglycemia. There were significant improvements in PRO measures.
Conclusions
Insulin pump therapy using a relatively simple dosing regimen safely improved glucose control and PROs in patients with T2DM who were unable to achieve glycemic targets with MDI therapy. Controlled trials are needed to further assess the clinical benefits and cost-effectiveness of insulin pumps in this patient population.
Keywords: insulin pump, multiple daily injections, type 2 diabetes
Introduction
The pathophysiology of T2DM is characterized by a variety of defects, including insulin resistance and progressive beta-cell dysfunction.1 Because of this, most patients require insulin therapy at some point during the natural history of their disease in order to achieve and maintain adequate glycemic control. Insulin is generally initiated as a single daily dose of basal insulin targeting fasting and preprandial glucose. Fast-acting mealtime insulin is then added, if required, to address postprandial hyperglycemia.2,3 In T2DM, this “basal–bolus” therapy is almost always administered by multiple daily injections (MDIs) of insulin using a vial and syringe or insulin pen.
Unfortunately, for a variety of reasons, many patients who are treated with MDI therapy are not able to achieve or maintain adequate glycemic control.4 For these patients, an insulin pump may be an important therapeutic option. Few randomized controlled trials have assessed insulin pump therapy in patients with T2DM, and these studies have generally shown similar glycemic control with pumps versus MDIs.5 None of them have specifically assessed insulin pump therapy in patients “failing” treatment with MDIs.
In the present analysis of a published study,6 we assess efficacy, safety, and patient-reported outcomes (PROs) of insulin pump therapy in 21 patients who were suboptimally controlled at baseline with MDIs. This information may help to advance future insulin pump development and inform the design of controlled trials assessing pump therapy in patients with T2DM who are unable to achieve therapeutic targets with MDIs.
Research Design and Methods
This is a subanalysis of a 16-week open-label study conducted at six U.S. study sites between March and December 2008. The study was performed in accordance with the Declaration of Helsinki and was approved by local ethics committees. All patients provided informed consent.
Study Protocol and Treatments
Details of the study design and procedures have been previously reported.6,7 Briefly, the present analysis assessed 21 adults with T2DM who were suboptimally controlled at baseline with MDI therapy [hemoglobin A1c (A1C) 7.0–10.5%]. All patients were insulin pump naïve. Baseline assessments included anti-glutamic-acid decarboxylase (GAD) antibody, A1C, fasting plasma glucose, body weight, and measures of PROs.
Within 5–7 days after the baseline visit, patients discontinued all antidiabetic medications except metformin and began insulin pump therapy (Animas® 2020 insulin pump, Animas Corporation, West Chester, PA) with insulin glulisine (sanofi-aventis, Bridgewater, NJ). Pump therapy was initiated with one daily basal rate and insulin boluses at each major meal. Investigators were instructed to make every effort to safely achieve fasting and preprandial plasma glucose values between 70 and 130 mg/dl and 1.5–2 h postprandial values below 180 mg/dl.
Patients returned to the study site 1, 2, 3, 4, 8, 12, and 16 weeks after pump initiation. During these visits, insulin dose adjustments were made by the investigators based on retrospective glucose readings from both continuous glucose monitoring performed during the first 4 weeks of pump therapy (DexCom™ SEVEN®, DexCom, San Diego, CA) and self-monitored blood glucose readings obtained throughout the 16-week study (OneTouch® Ultra®, LifeScan, Inc., Milpitas, CA) to safely achieve the best possible glycemic control with the simplest possible insulin dosing regimen.
Insulin dose and the number of daily basal rates were assessed at each visit. Two self-monitored seven-point glucose profiles (preprandial, 1.5–2 h postprandial, and bedtime) were performed within 3 days preceding each visit. Hemoglobin A1c was assessed at baseline and at 4, 8, 12, and 16 weeks (Diabetes Control and Complications Trial referenced, normal range 4.0–6.0%, Covance Laboratory, Indianapolis, IN). Fasting plasma glucose was assessed at baseline and week 16 (Covance Laboratory, Indianapolis, IN).
Patient reported outcomes were assessed at baseline and week 16 using two measures of health-related quality of life [HR-QoL; the Diabetes Symptom Checklist-Revised (DSC-R)8 and the EuroQoL-5 Dimensions (EQ-5D)9] and a measure of treatment satisfaction, the Insulin Delivery System Rating Questionnaire (IDSRQ).10
Minor hypoglycemia was defined as symptoms consistent with hypoglycemia that either resolved spontaneously or upon self-treatment with oral carbohydrate. Severe hypoglycemia referred to symptoms consistent with hypoglycemia during which the patient required the assistance of another individual and was associated with a documented glucose concentration less than 56 mg/dl or prompt recovery after oral carbohydrate, intravenous glucose, or glucagon.
Outcome Measures
Outcome measures included change from baseline in A1C, fasting and postprandial glucose, insulin dose, body weight, PROs, and incidence and event rate of hypoglycemia. Insulin dosing patterns, including the number of daily basal rates and the percentage of the total daily insulin dose delivered in a basal and in a bolus fashion, were also assessed at week 16.
Statistical Analysis
In this exploratory study, it was determined that approximately 20 patients would provide sufficient information to assess the insulin doses and dosing patterns. For A1C, a sample size of 20 patients was estimated to produce a 90% confidence interval equal to the sample mean with a precision of 0.44%, with an estimated standard deviation
The data were analyzed using SAS version 8.2 or higher (SAS Institute, Inc., Cary, NC). All statistical tests were two-sided using an alpha of 0.05. The intent-to-treat (ITT) population consisted of all patients who initiated insulin pump therapy. Missing postbaseline values were imputed using the last observation carried forward (LOCF) method.
A comparison to baseline for the change from baseline LOCF at week 16 measurements was performed using a one-sample t-test. Correlations were performed using the Pearson correlation coefficient.
Results
Patient Disposition and Baseline Characteristics
Twenty-two patients receiving MDI therapy at baseline were enrolled, and one withdrew prior to insulin pump initiation, resulting in an ITT population of 21. One patient withdrew from the study due to personal reasons after 4 weeks of pump therapy. One patient who completed the study had a screening A1C of 6.8%, slightly below the lower limit of the A1C inclusion criteria (≥7.0%).
Baseline characteristics are shown in Table 1. All patients were anti-GAD antibody negative. Eight patients were over 65 years old [68 ± 3 years, baseline A1C 8.5 ± 0.9%, mean ± standard deviation (SD), n = 8]. At baseline, 20 patients (95%) were treated with insulin analogs and 1 (5%) with recombinant human insulin. Sixteen patients (76%) were receiving concomitant metformin.
Table 1.
Baseline Demographics and Characteristics of the Intent-To-Treat Populationa
| ITT population (n) | 21 |
| Age (years) | 57 ± 13 |
| Sex (male/female) | 9 (43)/12 (57) |
| Weight (kg) | 98 ± 20 |
| Body mass index (kg/m2) | 34 ± 5 |
| Diabetes duration (years) | 15 ± 6 |
| A1C (%) | 8.4 ± 1.4 |
| Fasting plasma glucose (mg/dl) | 165 ± 58 |
| Fasting C-peptide (ng/dl) | 1.7 ± 1.4 |
| Total daily insulin dose (U) | 99 ± 65 |
Data are means ± SD or n (%).
Glycemic Control
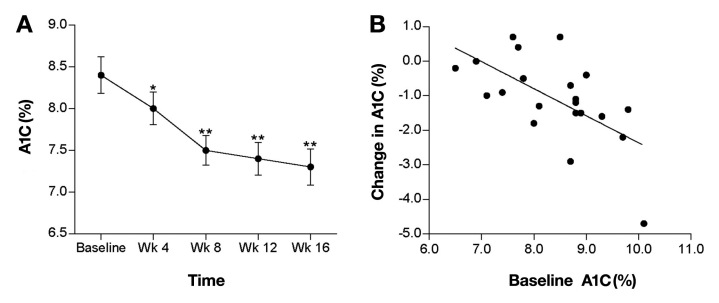
Hemoglobin A1c declined significantly after 4 weeks of pump therapy and continued to decline through week 16. Mean (± SD) A1C reduction at week 16 was 1.1 ± 1.2% (p < .001; Figure 1) and ranged from +0.7 to -4.7%, with a median change of -1.1%. Patients with a baseline A1C greater than 8.5% (n = 11, mean baseline A1C = 9.2 ± 0.5%) experienced a 1.8 ± 1.2% reduction in A1C (p < .001). At week 16, 38% of patients achieved a A1C level of less than 7.0%, and 90% of patients achieved an A1C of less than 7.0% and/or an absolute A1C reduction of greater than 0.5%. A significant correlation was observed between the baseline A1C and the change in A1C from baseline at week 16 (r = 0.62, p < .005; Figure 1).
Figure 1.
(A) Mean (± standard error) change in A1C from baseline to week 16 (*p < .05, **p < .001). (B) Correlation between baseline A1C (on MDI therapy) and change in A1C after 16 weeks of insulin pump therapy (r = 0.62, p < .005) for the ITT population.
Self-monitored seven-point glucose profiles were compared at baseline and week 16. Mean (± SD) fasting (165 ± 43 versus 133 ± 33 mg/dl, p < .005), preprandial (163 ± 43 versus 141 ± 30 mg/dl, p < .05), and postprandial (191 ± 47 versus 153 ± 36 mg/dl, p < .001) glucose values were reduced significantly from baseline at week 16. Pre- to post-meal glucose excursions tended to decline after 16 weeks of pump therapy (28 ± 39 versus 9 ± 50 mg/dl, p = .072). At week 16, fasting glucose levels of less than 130 mg/dl and postprandial levels of less than 180 mg/dl were achieved by 52% and 81% of patients, respectively.
Insulin Dose and Dosing Patterns
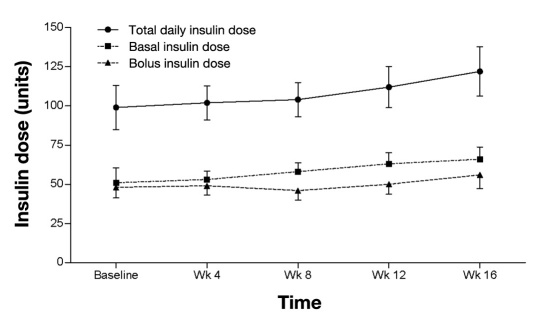
At week 16, the mean daily basal, bolus, and total daily insulin doses were 66 ± 36, 56 ± 40, and 122 ± 72 U (1.2 U/kg), respectively (Figure 2). Approximately 57% of the total daily insulin dose was delivered in a basal fashion and 43% as insulin boluses. At study end, 17 patients (80%) were treated with one daily basal rate, 2 patients (10%) were treated with two daily basal rates, and 2 patients (10%) were treated with greater than two daily basal rates. Individual patient's basal rates at week 16 ranged from 0.9 to 5.5 U/h, with a mean and median of 2.8 ± 1.5 and 2.2 U/h, respectively.
Figure 2.
Mean (± standard error) basal, bolus, and total daily insulin dose from baseline to week 16 for the ITT population.
Eight patients had a reduction in total daily insulin dose during the 16 weeks of pump therapy (mean total daily insulin dose: baseline, 127 ± 84 U; week 16, 102 ± 90 U). Despite the reduction in insulin dose, each of these patients experienced improvement in A1C (mean A1C change = -1.5 ± 1.2%, p = .02).
Hypoglycemia
The incidence (percentage of patients with at least one episode of hypoglycemia) and event rate of minor hypoglycemia was 81% and 21 ± 23 episodes per year, respectively. There was no severe hypoglycemia reported throughout the 16-week study.
Body Weight
Mean (± SD) change in body weight at week 16 was +2.8 ± 2.6 kg (p < .001). On average, patients gained the majority of weight (2.5 kg) during the initial 8 weeks of pump therapy, remaining relatively weight neutral (+0.3 kg) during the remaining 8 weeks of the study.
Patient-Reported Outcomes
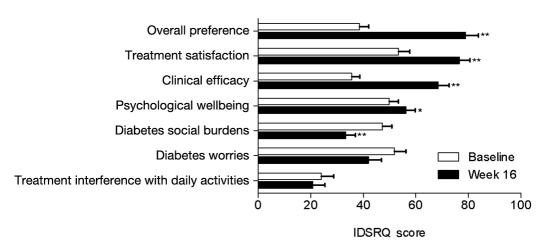
Detailed PRO data from this study were recently published by Rubin and colleagues.7 Improvements from baseline in measures of HR-QoL (DSC-R and EQ-5D) and in perception of treatment (IDSRQ) were observed at week 16. The total DSC-R symptom score improved significantly, as did symptom scores for the psychology/cognitive, hyperglycemia, and hypoglycemia domains. The EQ-5D weighted index improved significantly, and there was no deterioration in the EQ-5D visual analog scale. At week 16, IDSRQ measures of treatment satisfaction, treatment preference, clinical efficacy, diabetes social burden, and psychological wellbeing all demonstrated significant improvement, and there were no significant changes in treatment interference and diabetes worry scores (Figure 3).
Figure 3.
Mean (± standard error) IDSRQ scores at baseline and week 16 (*p < .05, **p < .001) for the ITT population.
Adverse Events
Five “skin reactions” at insulin infusion sites were reported by five separate patients during the course of the study. Two were classified as “mild” and three as “moderate” in intensity. They all resolved without sequela. There were no serious adverse events.
Discussion
In the present subanalysis of a larger study,6,7 we assessed insulin pump therapy in patients who were unable to achieve adequate glycemic control despite basal–bolus therapy with MDIs. Although MDI therapy is widely regarded as the “last step” in insulin intensification for patients with T2DM,2,3 many patients on this regimen are unable to achieve or maintain adequate glycemic control.4 This highlights the importance of assessing alternative therapeutic modalities—including insulin pump therapy—for this patient population.
Although the clinical value of insulin pumps in patients with type 1 diabetes mellitus (T1DM) is well established,11,12 relatively few studies have examined pump therapy in patients with T2DM. Randomized controlled trials of 6–12 months duration comparing pump to MDI therapy have demonstrated similar improvements in A1C from baseline.13,14 In smaller controlled trials of shorter duration, greater improvement in A1C has been shown with insulin pump therapy versus MDI.15,16 Importantly, none of these studies have exclusively assessed patients who at baseline are “failing” intensive insulin therapy with a MDI regimen. Subject inclusion criteria related to baseline insulin regimens have been broad. Raskin et al.13 and Herman et al.14 included patients taking at least one insulin injection per day, and Berthe et al.15 and Wainstein et al.16 studied patients “receiving insulin for more than 6 months” and taking greater than 1 U/kg/day of insulin in two or three divided doses, respectively. To our knowledge, a randomized controlled trial assessing insulin pump therapy in patients suboptimally controlled with MDIs has not been conducted. The results of the present study suggest that use of an insulin pump in this patient population may be an effective therapeutic option.
Patients experienced significant improvements from baseline in various measures of glycemic control. The A1C was reduced by 1.2% and continued to decline at week 16, suggesting that longer treatment may have resulted in a greater clinical benefit. A clinically relevant composite A1C outcome—defined as a reduction of at least 0.5% and/or reaching a A1C less than 7.0%—was achieved by the vast majority of patients (90%). As demonstrated by Pickup and coworkers17 in pump-treated patients with T1DM, our analysis of the correlation between baseline A1C and change in A1C with pump therapy showed that A1C reduction was greatest in patients with the highest baseline A1C levels (Figure 1).
Approximately 40% of the patients (n = 8) were over the age of 65. These patients tolerated pump therapy well and experienced a mean A1C reduction of 0.8% (baseline A1C = 8.5 ± 0.9%, mean ± SD), suggesting that, in otherwise appropriate insulin pump candidates, advancing age should not deter from considering this form of therapy.
The improvement in glycemic control (mean A1C reduction of 1.1%) was achieved with a relatively modest increase from baseline in the average total daily insulin dose (approximately 20%). The finding that, during the course of the study, 40% of patients reduced their total daily insulin dose yet had a concomitant improvement in glycemic control (mean A1C reduction of 1.5%) may indicate improved bioavailability of insulin when administered via continuous subcutaneous infusion. It may also indicate improved dosing compliance when insulin is conveniently available (“attached”) with a pump versus MDIs.
Although we did not formally assess adherence with the prescribed insulin regimen, poor adherence with insulin dosing has been reported to occur in up to 40% of insulin-using patients,18 and skipped insulin injections due to forgotten insulin supplies has been reported to occur frequently.19 Missed injections can have important clinical consequences, as demonstrated by Randlov and Poulsen who estimated that as few as two missed insulin boluses per week can result in an increase in A1C of approximately 0.3–0.4%.20
Past studies have suggested that device convenience may improve adherence with insulin therapy.21 The favorable clinical impact of device ease-of-use and convenience may be especially true for patients with T2DM who often initiate insulin therapy in late adulthood and who may have limited experience with devices and technology. In the present study, the majority of patients were able to be treated with a dosing regimen consisting of one daily basal rate and mealtime insulin boluses. Eleven of the 21 patients were using over 100 U of insulin per day at week 16 (median total daily insulin dose = 112 U/day). In a pump with a 200 or 300 U insulin capacity, this would require replacing the cartridge every 1–2 days—a potentially significant treatment burden. These findings can serve to inform the development of “simple” insulin pumps tailored specifically to patients with T2DM. An effective pump for most patients would likely require the ability to deliver only one to two daily basal rates. Such a pump would ideally have an insulin capacity of at least 300 U and/or the ability to deliver concentrated insulin. Although not currently approved for use in pumps, several retrospective and uncontrolled prospective studies have shown U-500 insulin to be effective in some patients with T2DM.22,23
In the present study, patients did not experience severe hypoglycemia, and the incidence and event rate of nonsevere hypoglycemia was consistent with previous reports.13–16 Despite being significantly less common in T2DM, hypoglycemia can lead to significant morbidity and is an important deterrent to intensification of therapy in this patient population.24,25 This highlights the importance of patient education, including instruction on recognition and treatment of hypoglycemia, especially when there is a change in or intensification of a treatment regimen.
Patients experienced moderate weight gain, which occurred almost exclusively during the initial 8 weeks of pump therapy. Weight gain may have been attenuated by continuation of metformin therapy (used by approximately 75% of patients) and was not associated with any untoward effects on blood pressure or plasma lipids (data not shown). Patients did not receive dietary instruction intended specifically for weight control, although providing this form of education may have helped mitigate weight gain and should be a standard part of clinical practice.
Pump therapy was associated with significant improvements in measures of HR-QoL and treatment preference. This has been a consistent finding in studies comparing pump to MDI therapy in T1DM.26 In patients with T2DM, Raskin and associates13 demonstrated in a randomized controlled trial that use of a pump resulted in significantly greater overall treatment satisfaction and preference compared with MDIs. From a clinical perspective, enhanced patient medication experience may improve outcomes by facilitating improved compliance and persistence.27
The present analysis has limitations that must be taken into consideration. It is a post hoc analysis of a larger pilot study, has no control group, and was of relatively short duration. Given their relatively small sample size and post hoc nature, subset analyses conducted in this study must be interpreted with caution. Even so, the study provides information that may help inform future research and development efforts related to pump therapy in T2DM.
Conclusion
In conclusion, insulin pump therapy using a relatively simple dosing regimen safely improved glucose control and PROs in patients with T2DM unable to achieve glycemic targets with MDI therapy. The development of insulin pumps designed specifically to meet the needs of patients with T2DM is important, and randomized controlled trials will be needed to assess their clinical efficacy and cost-effectiveness in patients who cannot achieve adequate control with MDI therapy.
Glossary
Abbreviations
- (A1C)
hemoglobin A1c
- (DSC-R)
Diabetes Symptom Checklist-Revised
- (EQ-5D)
EuroQoL-5 Dimensions
- (GAD)
glutamic-acid decarboxylase
- (HR-QoL)
health-related quality of life
- (IDSRQ)
Insulin Delivery System Rating Questionnaire
- (ITT)
intent to treat
- (LOCF)
last observation carried forward
- (MDI)
multiple daily injection
- (PRO)
patient-reported outcome
- (SD)
standard deviation
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
Funding
This study was sponsored by Animas Corporation, a Johnson and Johnson company, which was involved in its design and conduct, collection and statistical analysis of data, and provision of study devices and medication.
Disclosures
Juan P. Frias was employed by Johnson and Johnson at the time the study was being conducted. Bruce W. Bode has received consultant fees from Animas Corporation, LifeScan, Inc., DexCom, and sanofi-aventis and research support from Animas Corporation, DexCom, and sanofi-aventis. Timothy S. Bailey has received consultant fees from Animas Corporation, DexCom, and sanofi-aventis and research support from Animas Corporation, LifeScan, Inc., DexCom, and sanofi-aventis. Mark S. Kipnes has received research support from Animas Corporation. Rocco Brunelle has received consultant fees from Animas Corporation. Steven V. Edelman has received consultant fees from Animas Corporation, LifeScan, Inc., DexCom, and sanofi-aventis.
Data from the analysis have been published in abstract form and presented at the 2010 congresses of the American Association of Clinical Endocrinologists (Boston, MA, April 2010) and the European Association for the Study of Diabetes (Stockholm, Sweden, September 2010).
References
- 1.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 4.Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, Paul SK 4-T Study Group. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736–1747. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 5.Bode BW. Insulin pump use in type 2 diabetes. Diabetes Technol Ther. 2010;12(Suppl 1):S17–21. doi: 10.1089/dia.2009.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edelman SV, Bode BW, Bailey TS, Kipnes MS, Brunelle R, Chen X, Frias JP. Insulin pump therapy in patients with type 2 diabetes safely improved glycemic control using a simple insulin dosing regimen. Diabetes Technol Ther. 2010;12(8):627–633. doi: 10.1089/dia.2010.0034. [DOI] [PubMed] [Google Scholar]
- 7.Rubin RR, Peyrot M, Chen X, Frias JP. Patient-reported outcomes from a 16-week open-label, multicenter study of insulin pump therapy in patients with type 2 diabetes. Diabetes Techol Ther. 2010;12(11):901–906. doi: 10.1089/dia.2010.0075. [DOI] [PubMed] [Google Scholar]
- 8.Arbuckle RA, Humphrey L, Vardeva K, Arondekar B, Danten-Viala M, Scott JA, Snoek FJ. Psychometric evaluation of the Diabetes Symptom Checklist-Revised (DSC-R)–a Measure of symptom distress. Value Health. 2009;12(8):1168–1175. doi: 10.1111/j.1524-4733.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- 9.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62) Med Decis Making. 2002;22(4):340–349. doi: 10.1177/0272989X0202200412. [DOI] [PubMed] [Google Scholar]
- 10.Peyrot M, Rubin RR. Validity and reliability of an instrument for assessing health-related quality of life and treatment preferences: the Insulin Delivery System Rating Questionnaire. Diabetes Care. 2005;28(1):53–58. doi: 10.2337/diacare.28.1.53. [DOI] [PubMed] [Google Scholar]
- 11.Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. 2002;324(7339):705. doi: 10.1136/bmj.324.7339.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25(7):765–774. doi: 10.1111/j.1464-5491.2008.02486.x. [DOI] [PubMed] [Google Scholar]
- 13.Raskin P, Bode BW, Marks JB, Hirsch IB, Weinstein RL, McGill JB, Peterson GE, Mudaliar SR, Reinhardt RR. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care. 2003;26(9):2598–2603. doi: 10.2337/diacare.26.9.2598. [DOI] [PubMed] [Google Scholar]
- 14.Herman WH, Ilag LL, Johnson SL, Martin CL, Sinding J, Al Harthi A, Plunkett CD, LaPorte FB, Burke R, Brown MB, Halter JB, Raskin P. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005;28(7):1568–1573. doi: 10.2337/diacare.28.7.1568. [DOI] [PubMed] [Google Scholar]
- 15.Berthe E, Lireux B, Coffin C, Goulet-Salmon B, Houlbert D, Boutreux S, Fradin S, Reznik Y. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res. 2007;39(3):224–229. doi: 10.1055/s-2007-970423. [DOI] [PubMed] [Google Scholar]
- 16.Wainstein J, Metzger M, Boaz M, Minuchin O, Cohen Y, Yaffe A, Yerushalmy Y, Raz I, Harman-Boehm I. Insulin pump therapy vs. multiple daily injections in obese type 2 diabetic patients. Diabet Med. 2005;22(8):1037–1046. doi: 10.1111/j.1464-5491.2005.01597.x. [DOI] [PubMed] [Google Scholar]
- 17.Pickup JC, Kidd J, Burmiston S, Yemane N. Determinants of glycaemic control in type 1 diabetes during intensified therapy with multiple daily insulin injections or continuous subcutaneous insulin infusion: importance of blood glucose variability. Diabetes Metab Res Rev. 2006;22(3):232–237. doi: 10.1002/dmrr.614. [DOI] [PubMed] [Google Scholar]
- 18.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RT, Marrero D, Skovlund SE, Cramer J, Schwartz S. Self-reported compliance with insulin injection therapy in subjects with type 1 and 2 diabetes. Diabetologia. 2003;46(Suppl 2):A275. [Google Scholar]
- 20.Randløv J, Poulsen JU. How much do forgotten insulin injections matter to hemoglobin A1c in people with diabetes? A simulation study. J Diabetes Sci Technol. 2008;2(2):229–235. doi: 10.1177/193229680800200209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28(10):1712–1725. doi: 10.1016/j.clinthera.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Garg R, Johnston V, McNally PG, Davies MJ, Lawrence IG. U-500 insulwhy, when and how to use in clinical practice. Diabetes Metab Res Rev. 2007;23(4):265–268. doi: 10.1002/dmrr.709. [DOI] [PubMed] [Google Scholar]
- 23.Lane WS, Cochran EK, Jackson JA, Scism-Bacon JL, Corey IB, Hirsch IB, Skyler JS. High-dose insulin therapy: is it time for U-500 insulin? Endocr Pract. 2009;15(1):71–79. doi: 10.4158/EP.15.1.71. [DOI] [PubMed] [Google Scholar]
- 24.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28(12):2948–2961. doi: 10.2337/diacare.28.12.2948. [DOI] [PubMed] [Google Scholar]
- 26.Hoogma RP, Hammond PJ, Gomis R, Kerr D, Bruttomesso D, Bouter KP, Wiefels KJ, de la Calle H, Schweitzer DH, Pfohl M, Torlone E, Krinelke LG, Bolli GB 5-Nations Study Group. Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: results of the 5-nations trial. Diabet Med. 2006;23(2):141–147. doi: 10.1111/j.1464-5491.2005.01738.x. [DOI] [PubMed] [Google Scholar]
- 27.Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240–245. doi: 10.2337/dc09-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]