Abstract
A common factor contributing to organ damage in type 2 diabetes mellitus (T2DM) is impaired tissue blood flow caused by damage to vascular endothelial cells (VECs). Damage can occur even before the clinical diagnosis of diabetes. It can be caused by both a high average blood glucose concentration and/or large daily spikes in blood glucose. While much of the present literature focuses on the damage to VECs and organs from these large glucose excursions, this review will focus on the consequence of this damage, that is, how endothelial cell damage in diabetes affects normal daily activities (e.g., exercise, reaction to typical stimuli) and various treatment modalities (e.g.. contrast baths and electrical stimulation therapy). It is important to understand the effects of VEC damage such as poor skin blood flow, compromised thermoregulation, and altered response to skin pressure in designing diabetes technologies as simple as heating pads and as complex as continuous glucose monitors. At the simplest level, people with diabetes have poor circulation to the skin and other organs. In the skin, even the blood flow response to locally applied pressure, such as during standing, is different than for people who do not have T2DM. Simple weight bearing on the foot can occlude the skin circulation. This makes the skin more susceptible to damage. In addition, endothelial damage has far-reaching effects on the whole body during normal activities of daily living, including an impaired response to local heat, such as hot packs and contrast baths, and higher body temperatures during whole body heating due to impaired blood flow and a reduced ability to sweat. Finally, because of multiple organ damage, people with T2DM have poor balance and gait and impaired exercise performance.
Keywords: circulation, diabetes, review, thermoregulation, wounds
Overview
Type 2 diabetes mellitus (T2DM) is associated with serious consequences for health and mortality such as cardiovascular disease, renal disease, and stroke.1,2 While different organ systems are impaired in people with diabetes,3,4 a common factor in many of the complications of diabetes and the natural senescence of the organs due to aging is impaired vascular endothelial function.5 Diabetes is characterized by abnormally high vascular tone6 and impaired vasorelaxation of blood vessels.7 Lack of a typical circulatory response to stress not only damages organs, but affects whole body functions such as balance, gait, and the ability to respond to heat stress.8–11 Many articles have focused on continuous glucose monitoring and its importance in preventing cellular damage in people with diabetes.12–14 This review will concentrate on the implications of poor glucose control on blood flow and on how damage to vascular endothelial cells (VECs) impacts whole body function such as the ability of the skin to handle stressors during daily activities and more gross whole body functions such as thermoregulation, balance, orthostatic tolerance, and gait.
Impact of Diabetes on Vascular Endothelial Cells
In T2DM, the insulin receptor does not appear to be damaged in VECs. The defect is believed to be in the transduction after the binding of insulin on the insulin receptor to activating the phosphoinositide 3-kinase (PI3K) signaling pathway. Phosphoinositide 3-kinase has a central role in mediating the effects of insulin on cellular metabolism,15 and PI3K, in VECs, activates the enzyme endothelial nitric oxide synthetase (ENOS).16 Endothelial nitric oxide synthetase catalyzes the amino acid, L-arginine, to another amino acid, L-citrulline, producing nitric oxide, a free radical that causes relaxation in vascular smooth muscle.8 The net effect of relaxation in vascular smooth muscle cells is to increase blood flow in the skin and other organs as needed by the organs for metabolism or, in the case of the skin, to aid in heat loss in the body.
The diminished effect of insulin on the cells is compensated for by the pancreas increasing the concentration of blood insulin in response to glucose (hyperinsulemia).17 Over time, overproduction of insulin causes pancreatic beta cells to fail and insulin release from the pancreas to decline.8,18 Increased beta-cell apoptosis makes this deficit not reversible.19,20
The damage to the PI3K pathway is believed to be due to elevated glucose concentrations in the blood above 120 mg/dl.21,22 Evidence shows that damage can also be due to spikes in blood glucose during a 24 h period.23,24 The exact relationship between the extent of the variations in blood glucose and endothelial dysfunction has not been clearly shown.12–14,21,22 Tissue culture studies with isolated VECs have shed some interesting light on this question. If two populations of cells are subjected to the same average glucose concentrations, but one population is subjected to spikes while the other is exposed to a constant glucose concentration, it has been shown that the oxidative stress is much more severe in the population subjected to spikes. Further, this same population of cells, subjected to spikes, sustains damage to some cells and apoptosis in many cells.24–27 This then provides evidence that spikes cause more damage to VECs than does simply high average glucose exposure.
With dysfunction in the operation of VECs, there is either reduced production or reduced bioavailability of nitric oxide, the net effect of which is to impair relaxation in vascular smooth muscle. Some studies suggest ENOS28 or transient receptor voltage vanilloid (TRPV)-4 calcium channels are damaged by elevated blood glucose above normal in T2DM.8,29,30 While there is no direct evidence of damage to the TRPV-4 channels, TRPV-1 has been shown in studies in rats to be damaged in T2DM.31 The TRPV-4 calcium channels are a major source of Ca++ ions to activate ENOS. Damaged TRPV-4 channels would reduce the nitric oxide production to a given vasodilator stimulus in VECs. Studies also suggest that, in diabetes, there is a reduced bioavailability of arginine, altering the ability of the endothelial cells to produce nitric oxide.32 Other studies suggest that, once nitric oxide is produced, the high free-radical concentration in the body associated with both obesity and T2DM causes nitric oxide to be immediately converted into peroxynitrite and thus reduce nitric oxide's bioavailability as a vasodilator.23,33,34 Probably all of these mechanisms are present in diabetes. The overall effect is less nitric oxide bioavailability with less production of cyclic guanosine monophosphate in vascular smooth muscle, and thus vasodilatation is reduced.8
To some extent, hyperinsulemia should compensate for impaired nitric oxide bioavailability. In people who do not have T2DM, insulin is a known vasodilator substance.35 Insulin has two effects on the VEC. Its predominant effect is on the nitric-oxide-mediated vasodilator pathway.35 A minor pathway is the mitogen-activated protein kinase signaling pathway. The effect of insulin receptor binding on this later pathway is vasoconstriction.36 This vaso-constrictor pathway normally has little impact on circulation, as the vasodilator pathway predominates, but with dysfunction in the vasodilator pathway in people with diabetes, vasoconstriction predominates. Thus insulin resistance not only reduces vasodilatation, but enhances vasoconstriction of vascular smooth muscle.36
In addition to the altered balance of the vasodilation and vasoconstriction pathways, there are also morphological changes in the endothelial cell–smooth muscle interface that also contribute to reduced vasodilatation in people with T2DM.33,34 Normally, small cellular attachments (electrotonic connections) occur between VECs and vascular smooth muscle.34 When endothelial cells depolarize or hyperpolarize, the electrotonic connection (through gap junctions) depolarize or hyperpolarize smooth muscle cells respectively.37,38 For example, when vasodilator effectors bind to the endothelial membrane, the endothelial cell hyperpolarizes through an increase in potassium permeability. This increase in potassium permeability then causes hyperpolarization in vascular smooth muscle, making it harder to develop action potentials and thus aiding in the process of smooth muscle relaxation.34,37,38 In people with diabetes, these electrotonic connections are impaired or damaged. Therefore, part of the ability of the endothelial cell to relax vascular smooth muscle through these electrical connections is impaired.37–39 Studies on rat retinal preparations of these gap junctions show that the principal gap junction protein, connexin 43, but not connexin 37 and 40, was downregulated by incubation of these cells in high glucose media.40 This may or may not pertain to VECs in the skin.
Above the level of the VEC, there is central damage in the sympathetic nervous system that also leads to a reduced blood flow response to stressors in organs such as the skin. Autonomic damage occurs to the sympathetic ganglia even at the time of the clinical diagnosis of diabetes.5,22,41–46 This has its greatest effect on sympatheti-cally mediated vasodilatation.5,8,22,29,47 A common clinical measure of autonomic nervous system impairment is heart rate variability with the subject at rest.22,48,49 Normally, vasomotor rhythm in the sympathetic and parasympathetic systems causes the heart rate to vary continuously, breath by breath, during respiration.22 These variations in heart rate can be seen by a frequency analysis of the electrocardiogram. As diabetes progresses, heart rate variability is reduced such that, finally, sympathetic damage and parasympathetic damage have occurred to the extent that there is very little variation in heart rate with normal respiration or even a change in body position.22,43,49
Type 2 Diabetes and the Skin Circulatory Response to Pressure and Occlusion
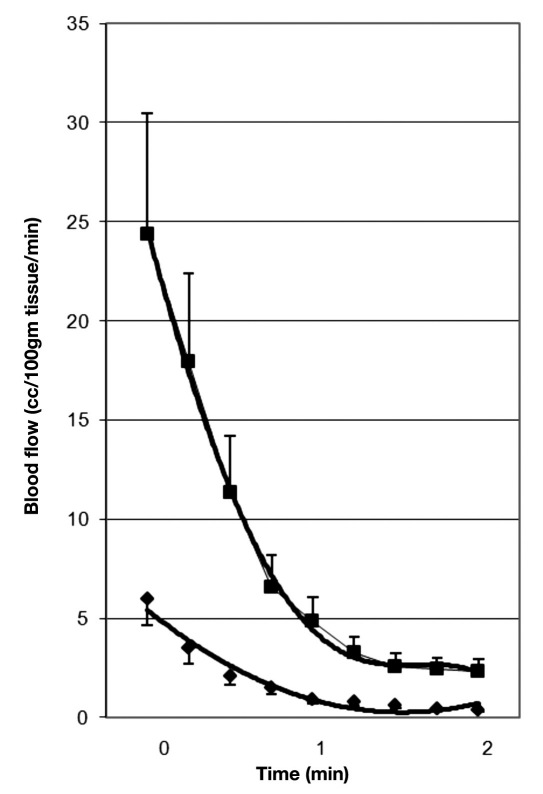
Due to endothelial dysfunction, skin blood flow, at rest, is less in people with diabetes (by almost a 66%) than in age-matched controls.5,29,48,50–53 The skin blood flow response to stressors is also greatly diminished in people with diabetes.5,29,48,50–53 For example, as shown in Figure 1, an occlusion cuff was placed on the upper arm of the subjects and inflated to 250 mm Hg for 4 min. After 4 min of vascular occlusion, there is normally a reactive hyperemia (upper curve, control subjects) measured by venous occlusion plethysmography in the lower arm. This response results from metabolites increasing in and around the cells due to the occlusion. But in people with diabetes, post-occlusion hyperemia is reduced (lower curve).5 Also, the duration of the reactive hyperemia is shorter in people with diabetes compared with age-matched controls. This figure shows whole arm blood flows measured for 2 min after the occlusion to the upper arm was removed. As shown in this figure, the hyperemia lasted for approximately 1 min only in the people with T2DM but for 2 min in the control group.54 Evidence shows that, in the skin, the response is not mediated by nitric oxide.55
Figure 1.
This figure shows the blood flow recorded for 2 min following the release of an arterial occlusion cuff on the brachial artery in nondiabetic control subjects (squares) and subjects with type 2 diabetes (diamonds). Illustrated here are the average results for 15 subjects in each group ± standard deviation. Blood flows are expressed in cc/100 ml muscle per minute, and the time scale on the bottom is in minutes. Blood flows are recorded every 12 s starting at 3 s post-occlusion.
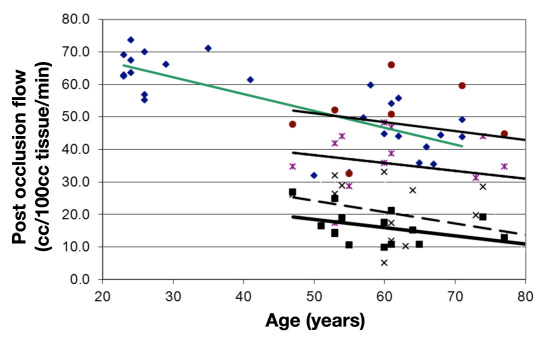
When the area under the post-occlusion blood flow curve (Figure 1) was calculated, the total blood flow increase above the resting blood flow in the arm could be calculated due to the occlusion. This measurement is called the “excess blood flow.” This is the blood flow response caused by the stressor. Figure 2 represents the average excess blood flow for the arm after 4 min of vascular occlusion in subjects of various ages with and without diabetes. As shown in Figure 2, associated with age (upper curve), there is an approximately linear reduction in skin excess blood flow to upper arm occlusion (4 min occlusion). In people with diabetes, excess blood flow after occlusion also decreases with age but at a significantly lower level than was seen for the controls.42 In these studies, one group of subjects had rosiglitazone administered for 3 months and then, at intervals, the effects of 4 min of vascular occlusion was assessed as shown in this figure.42 After administration of rosiglitazone, a drug shown to increase endothelial function in diabetes,5,44,56,57 for three months, the curve moved upward but kept the same age-related slope at each measurement period
Figure 2.
This figure illustrates the excess blood flow above rest during a 2 min period after the release of an occlusion cuff on the brachial artery of control subjects and subjects with diabetes. Individual data points are shown for control subjects (diamonds) and subjects with diabetes prior to initiation of rosiglitazone (squares) and after two weeks (×), four weeks (*), and three months (circles) administration of rosiglitazone. Data are plotted in relationship to the age of the subject. The regression lines are calculated by the method of least squares. For the control subjects, the regression line was blood flow = -0.518 age + 77.78. Prior to administration of rosiglitazone of the subjects with diabetes, the regression equation was blood flow = -0.253 age + 31.01. After two weeks of administration of rosiglitazone, the regression equation was blood flow = -0.348 age + 41.6. After four weeks of rosiglitazone, the regression equation was blood flow = -0.243 age + 50.27. Finally, after three months of rosiglitazone, the regression equation was blood flow = -0.274 age + 64.94.
Effects of Local Pressure
As well as a diminished response to upper arm occlusion, studies have shown that the response of the skin circulation to local pressure in T2DM is also reduced. Normally, when light pressure (up to 4 kPa) is applied to the skin, there is an increase in skin blood flow in people who do not have diabetes.47,58–61 This is a protective mechanism by which, especially during quiet standing, the pressure of the body on the feet does not reduce skin circulation and cause skin damage. When greater pressures are applied to the skin and released, skin circulation is diminished in proportion to the pressure followed by a reactive hyperemia in skin blood flow after the pressure is released.59,61 In people with diabetes, if pressures of 4 kPa are applied to the skin, blood flow was diminished. Even a pressure of 2 kPa reduced skin blood flow. If pressures above 7 kPa were applied to the skin in people with T2DM, circulation was totally occluded compared with total occlusion at vertical pressures of 22 kPa in age-matched controls. When a normal-weight person is standing quietly on both feet, the skin pressure is approximately 15 kPa. For the average person, there is still circulation in the skin during quiet standing. People with diabetes, however, even if they were normal body weight, would display an occlusion to the skin circulation. Because they have higher than normal body weight due to increased body fat, pressures should exceed 20 kPa during quiet standing. This occlusion of skin circulation adds to the potential for skin damage to the foot even during quiet standing. In addition, whereas partial or full circulatory occlusion is followed by a reactive hyperemia in age-matched control subjects, there was no post-occlusive hyperemia from skin pressure in people with T2DM. This may be why the feet are so susceptible to wounds and lack of repair after injury in people with T2DM.47,58
Thermoregulation and Skin Thickness
Whole body effects of diabetes-related endothelial dysfunction are even more pronounced than the effects on single organs such as the skin. One area of concern is thermoregulation in people with T2DM. Endothelial dysfunction coupled with autonomic dysfunction elicits a reduced ability of the skin to vasodilate in response to a global or local thermal stress. There is also a thinning in the dermal layer of the skin in older people and people with diabetes that also hinders heat dissipation.8,41,51,53,62 The thinner skin in older people is reported to be characterized by a thicker stratus corneum and a thinner dermal layer.51,62–65 A thinning in the dermal layer is observed in both older people and people with T2DM and implies less vasculature in the skin compared with younger and nondiabetic people. In people with hypertension or diabetes and hypertension, reduction of capillary density (rarefaction) has been shown in people of many different races.66–68 Rarefaction is a reduction in the capillary density of tissue. Thinner skin and less capillary density makes the skin more susceptible to damage such as burns and injuries and, as is the case for people with diabetes, makes the skin heal more slowly after an injury.
Effect of Diabetes on Global and Local Heat Regulation
The impaired circulation in the skin in people with diabetes makes this population less able to dissipate heat by radiation, convection, and conduction, making skin more prone to heating and reducing the body's ability to dissipate heat. Sweat production, a nitric-oxide-mediated pathway, is also reduced, and therefore, the skin in people with diabetes has less ability to be cooled through evaporative heat loss.44,45 Sweat is not continuously produced by the sweat glands. Due to vasomotor rhythm in the glands, it is produced in pulsatile bursts. When exposed to a 32 °C environmental temperature for 30 min, pulsatile and average sweat rate was lower by approximately half and skin blood flow by a third in subjects with T2DM compared with age-matched control subjects.44 Body temperature was almost 1 °C higher after heat exposure due to impaired thermoregulation. When subjects without diabetes were exposed to even warmer room temperatures (38, 40, and 44 °C), the skin blood flow increased further and sweat rates increased to cope with the higher heat load applied to the body. Body temperature in controls increased in proportion to the room temperature. However, when people with T2DM were exposed for 30 min to these same environmental temperatures, the sweat rate and skin blood flow increased after exposure to the 38 and 40 °C environmental temperatures. Logically, the sweat rate and skin blood flow should even increase more after the subjects were exposed to a 44 °C environmental temperature. For the subjects with diabetes, this did not happen. The sweat rate and skin blood flow seemed to reach its maximum, and core temperature and skin temperature increased, as they failed to thermoregulate in a 44 °C environment.44,48
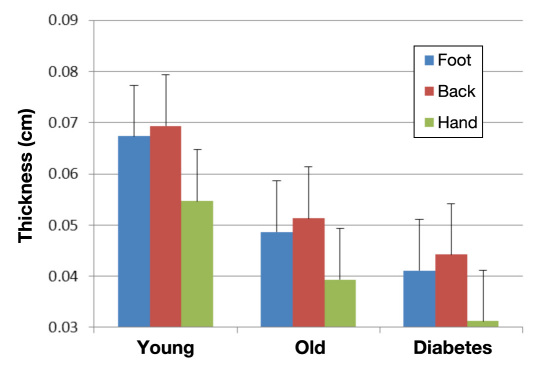
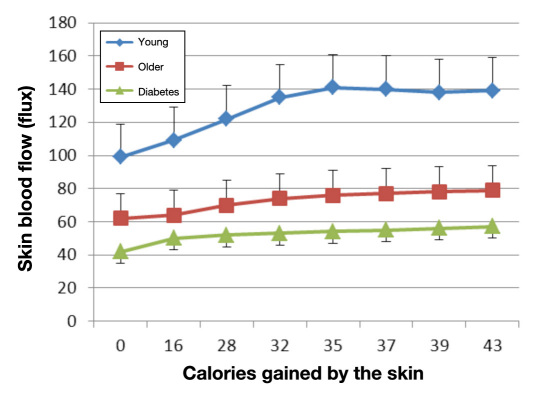
People with diabetes have poor tolerance to locally applied heat as well. Due to a reduction in skin blood flow at rest, a rapid application of heat to the skin results in a fast rise in skin temperature.62 One contributor to this thermal intolerance is the thinner dermal layer and increased subcutaneous fat layer in people with diabetes compared with age-matched controls.62 Thinner skin and more subcutaneous insulation impairs the conductive heat loss through the skin to an applied thermal load. Figure 3 shows the thickness of the skin in three groups of subjects (young, old, and people with diabetes). Skin thickness varies in different parts of the body. The thermal coefficient of the skin is much lower than that seen in age-matched controls without diabetes.51,62 Skin temperature rises faster in people with diabetes when a constant heat source is applied because of circulatory impairment. This, in turn, allows the skin to overheat. An interesting way to display this is to graph the skin blood flow on the y axis and the calories added to the skin on the x axis. As shown in Figure 4, there is disparity in the thermal response of the skin in younger, older, and diabetes subjects.62 Thus, for each calorie added to the skin, there is a reduced response of the skin in people with diabetes compared with the other groups.
Figure 3.
Illustrated here is the thickness of the skin in 3 areas of the body on 3 groups of 15 subjects measured by ultrasound at 10 MHz with a 1 cm standoff. The three groups are young subjects, older subjects, and young and old subjects with type 2 diabetes.
Figure 4.
Illustrating the relationship between skin blood flow and heat gained by the skin in young subjects, older subjects, and subjects with type 2 diabetes. Average ± the standard deviation for each group is shown (n = 15). Experiments involved adding calories of heat to the skin by applying a brass 50 g heated block at different temperatures and measuring the change in skin blood flow for a given caloric load.
The Mechanism of Thermoregulation
The mechanism of the blood flow increase in response to local heat involves two different pathways. When heat above 42 °C is applied to the skin, the skin immediately (first phase) responds with a rapid increase in circulation mediated by sensory nerves in the skin.69,70 The initial vasodilation is believed to be mediated by substance P and/or calcitonin gene-related peptide.71–73 This protects the skin from rapid changes in temperature that might cause damage.74–76 These neuropeptides are released when TRPV-1 voltage-gated calcium channels in skin tactile sensory receptors become active.77,78 The release of neuro-peptides then causes relaxation of vascular smooth muscle. The sustained skin vasodilation that occurs after the first few minutes of heat exposure is mediated by nitric oxide released by activation of the enzyme ENOS, which is activated by calcium released by temperature-sensitive TRPV-4 channels in VECs.69,70,79 The same channels are also sensitive to shear stress in arteries.80 In younger individuals, a second relaxing agent, prostacyclin, is also released, but this is not the case in older people and people with diabetes.69,70,79
In younger individuals, if a local heat source of 44 °C is applied to the skin for a duration of 20 min and repeated each day for 3 days, the blood flow response of the skin in the first day is much greater than that seen in the second and third day for the same increase in skin temperature in the first 2 min after the heat is applied to the skin. The subsequent blood flow response in the last 18 min is the same each day. The mechanism of the increase in blood flow in the first 2 min is through the release of neuropeptides mediated by TRPV-1 thermal receptors on sensory nerve endings.81,82 An enhanced response in these TRPV-1 channels would account for the greater blood flow in the first day in the first minutes after heat was applied, whereas the fact that blood flows were similar in days 1, 2, and 3 in the last 18 min of heat exposure would seem to indicate that the other vaso-dilator pathway mediated by TRPV-4 channels (nitric oxide pathway) remains unaffected by daily heat exposure. Thus some type of adaptive mechanism is probably found in the TRPV-1 channels that alters the response after the first day of heat exposure. A study has provided evidence that oxidized linoleic acid metabolites increase the temperature sensitivity of TRPV-1 channels.83 Perhaps this pathway adapts to repeated exposure with impaired linoleic acid production due to free-radical oxidation and reduces thermal sensitivity.81,84 In contrast, in older individuals and people with diabetes, the initial and sustained blood flow response of the skin was slower and of less amplitude than that seen in the younger individuals for all 3 days. This would seem to indicate that part of the aging and diabetes impairment in increasing skin circulation to heat would be a defect in the TRPV-1 channels since the initial response to heat was slower in older versus younger people and unaltered by repeated daily heat exposure. There is evidence that sensory neurons loose sensitivity with the aging process. The TRPV-1 mediated loss of sensory function to thermal stimuli with age may be related to age-related loss in the ability to oxidize linoleic acid in other parts of the nervous system.85–87 Because thermosensitive properties of TRPV-1 channels have been linked to oxidation of linoleic acid, a loss of this function with age and diabetes would disrupt thermal sensitivity.83
For the sustained heat response of the skin circulation, there has been ample documentation that the TRPV-4 temperature channels on VECs and the nitric oxide pathway in particular show senescence with aging and is impaired in diabetes. Prostacyclin, a major dilator in younger individuals, is largely replaced by nitric oxide for dilation to local heat in older individuals.69,70 This may explain the lower absolute amplitude in circulation in response to heat after a few minutes as seen in the older compared with the younger individuals. While nitric oxide bioavailability also decreases with age,88 the reduction in the prostacyclin pathways decreases more relatively speaking than the nitric oxide pathway.89
Susceptibility to Burns
Because the principal means of removing heat from the skin is the circulation when a warm heat source is applied,62 it is no surprise that people with diabetes are more susceptible to burns than are age-matched control subjects.90 Another contributor to the poor thermal response of the skin in people with diabetes is drier skin. Skin moisture content is about half that of age-matched controls in people with diabetes.51 When the skin is dry, the blood flow response to heat is less, presumably due to TRPV-4 osmotic receptor interaction with normal ENOS activation pathways.51,62 However, even when a moist heat source is used on people with diabetes, the heat tolerance is still less for hot packs compared with age-matched controls.51,62
Effectiveness of Contrast Baths
Contrast baths have been used for this purpose for thousands of years. Because the skin has a diminished response to heat in people with T2DM, modalities such as contrast baths (treatment based on the principle that, by alternate contraction and dilation of the blood vessels through alternating application of heat and cold, the circulation is improved and the removal of waste products is hastened) are ineffective in people with diabetes. Normally, if a limb or even the whole body is immersed in alternating hot and cold water baths, there is an enhanced blood flow response in the skin versus immersion in a continuous warm water bath. But for people with diabetes, the blood flow response is diminished with contrast baths rather than enhanced, making contrast baths ineffective in therapy.48
Response to Electrical Stimulation
In therapy, in addition to contrast baths, modalities such as the application of small electric currents to the skin (electrical stimulation) have been used to increase skin blood flow as an aid in wound healing.41,50,53 Like the response to heat, skin blood flow increases very little, if at all, in response to electrical stimulation in people with diabetes compared with age-matched controls.41,50,53 The mechanism for the increase in skin circulation during electrical stimulation in healthy controls has been linked to the production of nitric oxide in VECs in response to electrical stimulation. It has been suggested that the voltage-gated calcium TRPV-4 channels open in response to electrical stimulation of tissue and thereby increase blood flow to tissue through calcium-activated ENOS.91–93 In people with diabetes and older people, where nitric oxide bioavailability has been shown to be reduced, it is not surprising that the blood flow response to electrical stimulation is diminished.91,92
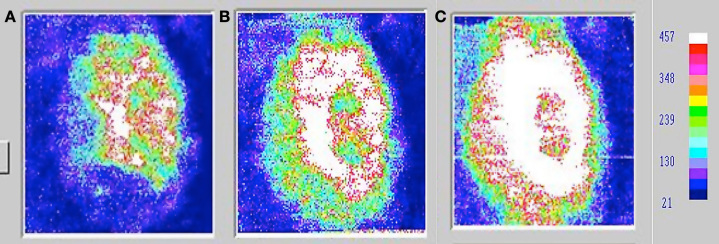
Therefore, while there is a body of literature implicating the use of electrical stimulation as an aid to increasing blood flow for enhanced wound healing, it has been largely ineffective on diabetic ulcers.41,50,53 However, studies may show an interesting effect of combined modalities. The use of local heat and electrical stimulation together has been shown to enhance wound healing. Wounds, which showed no healing for months to years in people with diabetes, have been shown to heal by using local or global heat with electrical stimulation. The mechanism is unknown but may be related to increased nitric oxide production.41,50,53 Both heat and electrical stimulation increase nitric oxide production in the skin VECs. For example, in Figure 5, the three panels show the blood flow in and around a wound that would not heal for 2 years. In previous studies, it was shown that electrical stimulation alone has only a small effect on the blood flow in and around wounds in people with T2DM, increasing blood flow less than 10%.94 However, if the tissue was heated to 37 °C for 20 min prior to the application of electrical stimulation and then, while heat was still applied, electrical stimulation was simultaneously used, blood flow increased greatly by combining the two modalities. Electrical stimulation was applied at a frequency of 30 Hz for 20 min at an amplitude of 20 ma. Nonhealing wounds healed completely in less than 2 months with three treatments per week with the two combined modalities. Interestingly, even 24 h after treatment, wound blood flow was significantly elevated.
Figure 5.
Skin blood flows on a typical subject measured by laser Doppler flow imager (A) before treatment, (B) after 20 min of wound heating to 37 °C, and (C) after 20 min of electrical stimulation at a frequency of 30 Hz and an intensity of 20 ma and heating. The whiter the color, the greater the blood flow. Blue represents the lowest blood flow, as shown on the scale on the right. Heat was applied during the period that electrical stimulation was applied, making the entire heating period 40 min.
Isometric Exercise
Since diabetes impairs the microcirculation, it is reasonable to assume that exercise performance would be compromised in people with diabetes. When isometric strength and endurance of the handgrip muscles was measured in a series of two fatiguing isometric contractions at a tension of 40% of the maximum strength in 10 subjects with T2DM, compared with 10 control subjects who were age matched, strength and endurance for the first contraction was the same in control subjects compared with subjects with T2DM (p > .05).30 However, endurance of the second contraction (accomplished 3 min after the first contraction) was significantly less in subjects with diabetes than age-matched controls (p < .01), showing slower exercise recovery than in the age-matched controls. Heart rate during exercise increased significantly (p < .01) to over 120 beats per minute by the end of the fatiguing isometric exercise in the control subjects, an increase of approximately 50 beats per minute above the resting heart rate. In contrast, for the subjects with diabetes, the heart rate increase was only approximately 15 beats per minute above the resting heart rate. Resting and peak blood pressures were greater in subjects with T2DM compared with control subjects (p < .01). Forearm blood flow was significantly lower at rest, during exercise, and post-exercise in subjects with diabetes compared with control subjects (p < .01).30,45
Orthostatic Tolerance
With an impaired autonomic nervous system and peripheral circulation, orthostatic tolerance is also diminished in people with diabetes. Orthostatic intolerance is an abnormal blood pressure response to standing upright that results from decreased blood pressure compensation to a change in body position. This results in inadequate blood flow to the brain and often dizziness. When tilted from horizontal to the vertical body position or going from sitting to standing, it has been reported that almost 25% of people with diabetes have orthostatic intolerance as judged by a drop of 20 mm Hg in blood pressure 2 min after tilt up or standing from the sitting position. If people stand up or are tilted up in a warm room (39 °C), the combined stress of heat and tilt cause nearly 100% of people with diabetes to have orthostatic intolerance.57 Thus it would appear that, even with an impaired autonomic nervous system and damage to blood vessels, the body can compensate for a single autonomic stressor in most people with diabetes, but the addition of two stressors (heat and tilt) is more than the body can handle in people with diabetes.
Gait and Balance
Balance integrates vision, the vestibular, and the somato-sensory systems of the body. Since all three are impaired in diabetes, it is of no surprise that balance impairments are common in people with diabetes.9–11,43 During quiet standing or during movement, balance is impaired. Incorrect visual cues make matters worse. Thus, in dim light, poor visual cues make balance even worse.9,11,43 People with diabetes have better balance in totally dark rooms than in rooms lit by very dim light. Further, the color of light seen by the eye that shows the greatest impairment with diabetes and age is blue, the very color of most of the newer night lights, making matters worse.9,11 But it is not just visual cues that impair balance. In a study, it was found that the impaired autonomic nervous system in people with diabetes diminishes the normal blood pressure and heart rate response during attempted balance.43 This probably alters the normal maintenance of cerebral blood flow and adds to balance impairments.
It is of no surprise then that gait is also impaired. In diabetes, gait is slower, the steps are wider to increase the base of support, and circumduction of the legs is common to compensate for poor balance and lack of good sensation from the foot.9
Race
There may be racial difference in the complications of diabetes that have not as yet been examined. Studies have shown that even a single high-fat meal in Asians can impair endothelial function within 2 hours.95 This has been attributed to the presence of the “thrifty” gene in Asians, which, while used to protect this population from starvation historically, may be responsible for increased diabetes incidence in the Asian population as well as the Pacific Rim. Further studies should examine balance, gait, and other autonomic impairments in people with diabetes as a function of race to see if these same thrifty genes will worsen the damage from diabetes in other races.
Summary
In summary, when developing new technologies where the skin and skin circulation are important for the proper function of new glucose monitoring and injection systems, the effects of impaired VEC function on poor skin blood flow, altered healing, and response to pressure must be taken into consideration. Poor blood flow may impair the accurate measurement of blood glucose as well as reduce absorption of infused insulin. Impaired healing at the injection or monitoring site can reduce the time an indwelling injection port can be left in place even if the newer technologies allow for better technical stability of the sensors.
Abbreviations
- (ENOS)
endothelial nitric oxide synthetase
- (HbA1c)
hemoglobin A1c
- (PI3K)
phosphoinositide 3-kinase
- (T2DM)
type 2 diabetes mellitus
- (TRPV)
transient receptor voltage vanilloid
- (VEC)
vascular endothelial cells
Funding:
This work was supported by GlaxoSmithKline, Procter & Gamble, and Wyeth Pharmaceuticals.
References:
- 1.Siscovick DS, Sotoodehnia N, Rea TD, Raghunathan TE, Jouven X, Lemaitre RN. Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev Endocr Metab Disord. 2010;11(1):53–59. doi: 10.1007/s11154-010-9133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spooner PM. Sudden cardiac death: influence of diabetes. Diabetes Obes Metab. 2008;10(7):523–532. doi: 10.1111/j.1463-1326.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 3.Grauslund J, Green A, Kawasaki R, Hodgson L, Sjølie AK, Wong TY. Retinal vascular fractals and microvascular and macrovascular complications in type 1 diabetes. Ophthalmology. 2010;117(7):1400–1405. doi: 10.1016/j.ophtha.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 4.Petrofsky J, Lee S, Macnider M, Navarro E. Autonomic, endothelial function and the analysis of gait in patients with type 1 and type 2 diabetes. Acta Diabetol. 2005;42(1):7–15. doi: 10.1007/s00592-005-0168-0. [DOI] [PubMed] [Google Scholar]
- 5.Petrofsky J, Lee S. The effects of type 2 diabetes and aging on vascular endothelial and autonomic function. Med Sci Monit. 2005;11(6):CR247–CR254. [PubMed] [Google Scholar]
- 6.Okon EB, Szado T, Laher I, McManus B, van Breemen C. Augmented contractile response of vascular smooth muscle in a diabetic mouse model. J Vasc Res. 2003;40(6):520–530. doi: 10.1159/000075238. [DOI] [PubMed] [Google Scholar]
- 7.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 8.Farage M, Miller K, Maibach H, editors. Berlin: Springer; 2010. Text book of ageing skin. [Google Scholar]
- 9.Petrofsky J, Lee S, Bweir S. Gait characteristics in people with type 2 diabetes mellitus. Eur J Appl Physiol. 2005;93(5-6):640–647. doi: 10.1007/s00421-004-1246-7. [DOI] [PubMed] [Google Scholar]
- 10.Petrofsky J, Lee S, Cuneo ML. Gait characteristics in patients with type 2 diabetes; improvement after administration of rosiglitazone. Med Sci Monit. 2005;11(6):PI43–PI51. [PubMed] [Google Scholar]
- 11.Petrofsky JS, Cuneo M, Lee S, Johnson E, Lohman E. Correlation between gait and balance in people with and without type 2 diabetes in normal and subdued light. Med Sci Monit. 2006;12(7):CR273–CR281. [PubMed] [Google Scholar]
- 12.Azad N, Emanuele NV, Abraira C, Henderson WG, Colwell J, Levin SR, Nuttall FQ, Comstock JP, Sawin CT, Silbert C, Rubino FA. The effects of intensive glycemic control on neuropathy in the VA cooperative study on type II diabetes mellitus (VA CSDM) J Diabetes Complications. 1999;13(5-6):307–313. doi: 10.1016/s1056-8727(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 13.Cameron FJ, Donath SM, Baghurst PA. Measuring glycaemic variation. Curr Diabetes Rev. 2010;6(1):17–26. doi: 10.2174/157339910790442592. [DOI] [PubMed] [Google Scholar]
- 14.Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, Sommer C, Cleary PA, Lachin JM, Herman WH DCCT/EDIC Research Group. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC) Circulation. 2009;119(22):2886–2893. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulhak AA, Jung C, Ostenson CG, Lundberg JO, Sjöquist PO, Pernow J. PPAR-alpha activation protects the type 2 diabetic myocardium against ischemia-reperfusion injury: involvement of the PI3-Kinase/Akt and NO pathway. Am J Physiol Heart Circ Physiol. 2009;296(3):H719–H727. doi: 10.1152/ajpheart.00394.2008. [DOI] [PubMed] [Google Scholar]
- 16.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37(2):449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- 18.Gepts W, Lecompte PM. The pancreatic islets in diabetes. Am J Med. 1981;70(1):105–115. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- 19.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9(4):151–159. [PubMed] [Google Scholar]
- 20.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 21.Alemzadeh R, Palma-Sisto P, Parton EA, Holzum MK. Continuous subcutaneous insulin infusion and multiple dose of insulin regimen display similar patterns of blood glucose excursions in pediatric type 1 diabetes. Diabetes Technol Ther. 2005;7(4):587–596. doi: 10.1089/dia.2005.7.587. [DOI] [PubMed] [Google Scholar]
- 22.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 23.Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347(8999):444–445. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 24.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 25.Infusino F, Pitocco D, Zaccardi F, Scalone G, Coviello I, Nerla R, Mollo R, Sestito A, Di Monaco A, Barone L, Pisanello C, Ghirlanda G, Lanza GA, Crea F. Low glucose blood levels are associated with abnormal cardiac sympatho-vagal balance in type 2 diabetic patients with coronary artery disease. Eur Rev Med Pharmacol Sci. 2010;14(3):203–207. [PubMed] [Google Scholar]
- 26.Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Inter-mittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281(5):E924–E930. doi: 10.1152/ajpendo.2001.281.5.E924. [DOI] [PubMed] [Google Scholar]
- 27.Ye XY, Tu Q, Tong Z, Weng YJ, Wang YF. [Effects of glucose concentration fluctuation on function of cultured bovine arterial endothelial cells] Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38(3):264–267. [PubMed] [Google Scholar]
- 28.Li H, Wallerath T, Münzel T, Förstermann U. Regulation of endothelial-type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide. 2002;7(3):149–164. doi: 10.1016/s1089-8603(02)00111-8. [DOI] [PubMed] [Google Scholar]
- 29.Maloney-Hinds C, Petrofsky JS, Zimmerman G, Hessinger DA. The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol Ther. 2009;11(1):39–43. doi: 10.1089/dia.2008.0011. [DOI] [PubMed] [Google Scholar]
- 30.Petrofsky JS, Stewart B, Patterson C, Cole M, Al Malty A, Lee S. Cardiovascular responses and endurance during isometric exercise in patients with Type 2 diabetes compared to control subjects. Med Sci Monit. 2005;11(10):CR470–CR477. [PubMed] [Google Scholar]
- 31.Mohammadi-Farani A, Sahebgharani M, Sepehrizadeh Z, Jaberi E, Ghazi-Khansari M. Diabetic thermal hyperalgesia: role of TRPV1 and CB1 receptors of periaqueductal gray. Brain Res. 2010;1328:49–56. doi: 10.1016/j.brainres.2010.02.077. [DOI] [PubMed] [Google Scholar]
- 32.Johnson RA, Durante W, Craig T, Peyton KJ, Myers JG, Stewart RM, Johnson FK. Vascular arginase contributes to arteriolar endothelial dysfunction in a rat model of hemorrhagic shock. J Trauma. 2010;69(2):384–391. doi: 10.1097/TA.0b013e3181e771a3. [DOI] [PubMed] [Google Scholar]
- 33.Potenza MA, Gagliardi S, Nacci C, Carratu' MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16(1):94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 34.Woodman RJ, Playford DA, Watts GF. Basal production of nitric oxide (NO) and non-NO vasodilators in the forearm microcirculation in type 2 diabetes: associations with blood pressure and HDL cholesterol. Diabetes Res Clin Pract. 2006;71(1):59–67. doi: 10.1016/j.diabres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Palaia T, Ragolia L. Impaired insulin-mediated vaso-relaxation in diabetic Goto-Kakizaki rats is caused by impaired Akt phosphorylation. Am J Physiol Cell Physiol. 2009;296(2):C327–C338. doi: 10.1152/ajpcell.00254.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes. 2009;58(10):2238–2245. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Wit C, Boettcher M, Schmidt VJ. Signaling across myoendothelial gap junctions–fact or fiction? Cell Commun Adhes. 2008;15(3):231–245. doi: 10.1080/15419060802440260. [DOI] [PubMed] [Google Scholar]
- 38.Gupta PK, Subramani J, Leo MD, Sikarwar AS, Parida S, Prakash VR, Mishra SK. Role of voltage-dependent potassium channels and myoendothelial gap junctions in 4-aminopyridine-induced inhibition of acetylcholine relaxation in rat carotid artery. Eur J Pharmacol. 2008;591(1-3):171–176. doi: 10.1016/j.ejphar.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 39.Triggle CR, Hollenberg M, Anderson TJ, Ding H, Jiang Y, Ceroni L, Wiehler WB, Ng ES, Ellis A, Andrews K, McGuire JJ, Pannirselvam M. The endothelium in health and disease–a target for therapeutic intervention. J Smooth Muscle Res. 2003;39(6):249–267. doi: 10.1540/jsmr.39.249. [DOI] [PubMed] [Google Scholar]
- 40.Sato T, Haimovici R, Kao R, Li AF, Roy S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes. 2002;51(5):1565–1571. doi: 10.2337/diabetes.51.5.1565. [DOI] [PubMed] [Google Scholar]
- 41.Lawson D, Petrofsky JS. A randomized control study on the effect of biphasic electrical stimulation in a warm room on skin blood flow and healing rates in chronic wounds of patients with and without diabetes. Med Sci Monit. 2007;13(6):CR258–CR263. [PubMed] [Google Scholar]
- 42.Petrofsky J, Lee S, Cuneo M. Effects of aging and type 2 diabetes on resting and post occlusive hyperemia of the forearm; the impact of rosiglitazone. BMC Endocr Disord. 2005;5(1):4. doi: 10.1186/1472-6823-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrofsky JS, Focil N, Prowse M, Kim Y, Berk L, Bains G, Lee S. Autonomic stress and balance–the impact of age and diabetes. Diabetes Technol Ther. 2010;12(6):475–481. doi: 10.1089/dia.2009.0125. [DOI] [PubMed] [Google Scholar]
- 44.Petrofsky JS, Lee S, Cuneo-Libarona M. The impact of rosiglitazone on heat tolerance in patients with type 2 diabetes. Med Sci Monit. 2005;11(12):CR562–CR569. [PubMed] [Google Scholar]
- 45.Petrofsky JS, Lee S, Patterson C, Cole M, Stewart B. Sweat production during global heating and during isometric exercise in people with diabetes. Med Sci Monit. 2005;11(11):CR515–CR521. [PubMed] [Google Scholar]
- 46.Horowitz SH. Recent clinical advances in diabetic polyneuropathy. Curr Opin Anaesthesiol. 2006;19(5):573–578. doi: 10.1097/01.aco.0000245287.37905.c5. [DOI] [PubMed] [Google Scholar]
- 47.McLellan K, Petrofsky JS, Zimmerman G, Lohman E, Prowse M, Schwab E, Lee S. The influence of environmental temperature on the response of the skin to local pressure: the impact of aging and diabetes. Diabetes Technol Ther. 2009;11(12):791–798. doi: 10.1089/dia.2009.0097. [DOI] [PubMed] [Google Scholar]
- 48.Petrofsky J, Lohman E 3rd, Lee S, de la Cuesta Z, Labial L, Iouciulescu R, Moseley B, Korson R, Al Malty A. Effects of contrast baths on skin blood flow on the dorsal and plantar foot in people with type 2 diabetes and age-matched controls. Physiother Theory Pract. 2007;23(4):189–197. doi: 10.1080/09593980701209295. [DOI] [PubMed] [Google Scholar]
- 49.Sridhar B, Haleagrahara N, Bhat R, Kulur AB, Avabratha S, Adhikary P. Increase in the heart rate variability with deep breathing in diabetic patients after 12-month exercise training. Tohoku J Exp Med. 2010;220(2):107–113. doi: 10.1620/tjem.220.107. [DOI] [PubMed] [Google Scholar]
- 50.Malty AM, Petrofsky J. The effect of electrical stimulation on a normal skin blood flow in active young and older adults. Med Sci Monit. 2007;13(4):CR147–CR155. [PubMed] [Google Scholar]
- 51.McLellan K, Petrofsky JS, Bains G, Zimmerman G, Prowse M, Lee S. The effects of skin moisture and subcutaneous fat thickness on the ability of the skin to dissipate heat in young and old subjects, with and without diabetes, at three environmental room temperatures. Med Eng Phys. 2009;31(2):165–172. doi: 10.1016/j.medengphy.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol. 2009;106(2):566–570. doi: 10.1152/japplphysiol.91289.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suh H, Petrofsky J, Fish A, Hernandez V, Mendoza E, Collins K, Yang T, Abdul A, Batt J, Lawson D. A new electrode design to improve outcomes in the treatment of chronic non-healing wounds in diabetes. Diabetes Technol Ther. 2009;11(5):315–322. doi: 10.1089/dia.2008.0092. [DOI] [PubMed] [Google Scholar]
- 54.Maglinger PE, Sessler DI, Lenhardt R. Cutaneous heat loss with three surgical drapes, one impervious to moisture. Anesth Analg. 2005;100(3):738–742. doi: 10.1213/01.ANE.0000143954.98285.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol. 2003;95(2):504–510. doi: 10.1152/japplphysiol.00254.2003. [DOI] [PubMed] [Google Scholar]
- 56.Petrofsky J, Lee S, Cuneo ML. Gait characteristics in patients with type 2 diabetes; improvement after administration of rosiglitazone. Med Sci Monit. 2005;11(6):PI43–PI51. [PubMed] [Google Scholar]
- 57.Petrofsky JS, Besonis C, Rivera D, Schwab E, Lee S. Impairment in orthostatic tolerance during heat exposure in individuals with type I and type II diabetes. Med Sci Monit. 2005;11(4):CR153–CR159. [PubMed] [Google Scholar]
- 58.Petrofsky JS, Bains GS, Prowse M, Mc Lellan K, Ethiraju G, Lee S, Gunda S, Lohman E, Schwab E. The influence of age and diabetes on the skin blood flow response to local pressure. Med Sci Monit. 2009;15(7):CR325–CR331. [PubMed] [Google Scholar]
- 59.Jan YK, Brienza DM, Geyer MJ, Karg P. Wavelet-based spectrum analysis of sacral skin blood flow response to alternating pressure. Arch Phys Med Rehabil. 2008;89(1):137–145. doi: 10.1016/j.apmr.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 60.Nakagami G, Sari Y, Nagase T, Iizaka S, Ohta Y, Sanada H. Evaluation of the usefulness of skin blood flow measurements by laser speckle flowgraphy in pressure-induced ischemic wounds in rats. Ann Plast Surg. 2010;64(3):351–354. doi: 10.1097/SAP.0b013e3181a73078. [DOI] [PubMed] [Google Scholar]
- 61.Noble M, Voegeli D, Clough GF. A comparison of cutaneous vascular responses to transient pressure loading in smokers and nonsmokers. J Rehabil Res Dev. 2003;40(3):283–288. [PubMed] [Google Scholar]
- 62.Petrofsky JS, McLellan K, Bains GS, Prowse M, Ethiraju G, Lee S, Gunda S, Lohman E, Schwab E. Skin heat dissipation: the influence of diabetes, skin thickness, and subcutaneous fat thickness. Diabetes Technol Ther. 2008;10(6):487–493. doi: 10.1089/dia.2008.0009. [DOI] [PubMed] [Google Scholar]
- 63.Fu FH, Fung L. Distribution of subcutaneous fat and equations for predicting percent body fat from skinfold measurements: a comparison between Chinese females from two age cohorts. J Sports Med Phys Fitness. 1995;35(3):224–227. [PubMed] [Google Scholar]
- 64.Puig T, Marti B, Rickenbach M, Dai SF, Casacuberta C, Wietlisbach V, Gutzwiller F. Some determinants of body weight, subcutaneous fat, and fat distribution in 25–64 year old Swiss urban men and woman. Soz Praventivmed. 1990;35(6):193–200. doi: 10.1007/BF01369085. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz RS, Shuman WP, Bradbury VL, Cain KC, Fellingham GW, Beard JC, Kahn SE, Stratton JR, Cerqueira MD, Abrass IB. Body fat distribution in healthy young and older men. J Gerontol. 1990;45(6):M181–M185. doi: 10.1093/geronj/45.6.m181. [DOI] [PubMed] [Google Scholar]
- 66.He FJ, Marciniak M, Markandu ND, Antonios TF, MacGregor GA. Effect of modest salt reduction on skin capillary rarefaction in white, black, and Asian individuals with mild hypertension. Hypertension. 2010;56(2):253–259. doi: 10.1161/HYPERTENSIONAHA.110.155747. [DOI] [PubMed] [Google Scholar]
- 67.Ko SH, Cao W, Liu Z. Hypertension management and micro-vascular insulin resistance in diabetes. Curr Hypertens Rep. 2010;12(4):243–251. doi: 10.1007/s11906-010-0114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pessina AC. Target organs of individuals with diabetes caught between arterial stiffness and damage to the microcirculation. J Hypertens Suppl. 2007;25(1):S13–S18. doi: 10.1097/01.hjh.0000271504.62325.a4. [DOI] [PubMed] [Google Scholar]
- 69.Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86(4):1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 70.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91(4):1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 71.Namer B, Bickel A, Krämer H, Birklein F, Schmelz M. Chemically and electrically induced sweating and flare reaction. Auton Neurosci. 2004;114(1-2):72–82. doi: 10.1016/j.autneu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Petrofsky JS, Al-Malty AM, Prowse M. Relationship between multiple stimuli and skin blood flow. Med Sci Monit. 2008;14(8):CR399–CR405. [PubMed] [Google Scholar]
- 73.Sauerstein K, Klede M, Hilliges M, Schmelz M. Electrically evoked neuropeptide release and neurogenic inflammation differ between rat and human skin. J Physiol. 2000;529(Pt 3):803–810. doi: 10.1111/j.1469-7793.2000.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almalty AM, Petrofsky JS, Al-Naami B, Al-Nabulsi J. An effective method for skin blood flow measurement using local heat combined with electrical stimulation. J Med Eng Technol. 2009;33(8):663–669. doi: 10.3109/03091900903271646. [DOI] [PubMed] [Google Scholar]
- 75.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 76.Rowell LB. Reflex control of the cutaneous vasculature. J Invest Dermatol. 1977;69(1):154–166. doi: 10.1111/1523-1747.ep12497938. [DOI] [PubMed] [Google Scholar]
- 77.Petrofsky J, Bains G, Prowse M, Gunda S, Berk L, Raju C, Ethiraju G, Vanarasa D, Madani P. Does skin moisture influence the blood flow response to local heat? A re-evaluation of the Pennes model. J Med Eng Technol. 2009;33(7):532–537. doi: 10.1080/03091900902952683. [DOI] [PubMed] [Google Scholar]
- 78.Petrofsky JS, Bains G, Raju C, Lohman E, Berk L, Prowse M, Gunda S, Madani P, Batt J. The effect of the moisture content of a local heat source on the blood flow response of the skin. Arch Dermatol Res. 2009;301(8):581–585. doi: 10.1007/s00403-009-0957-3. [DOI] [PubMed] [Google Scholar]
- 79.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vaso- dilation with age during local heating. J Appl Physiol. 2002;93(5):1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 80.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298(2):H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petrofsky J, Goraksh N, Alshammari F, Mohanan M, Soni J, Trivedi M, Lee H, Hudlikar AN, Yang CH, Agilan B, Pai N, Chindam T, Murugesan V, Eun Yim J, Katrak V. The ability of the skin to absorb heat; the effect of repeated exposure and age. Med Sci Monit. 2011;17(1):CR1–CR8. doi: 10.12659/MSM.881315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petrofsky J, Lee H, Trivedi M, Hudlikar AN, Yang CH, Goraksh N, Alshammari F, Mohanan M, Soni J, Agilan B, Pai N, Chindam T, Murugesan V, Yim JE, Katrak V. The influence of aging and diabetes on heat transfer characteristics of the skin to a rapidly applied heat source. Diabetes Technol Ther. 2010;12(12):1003–1010. doi: 10.1089/dia.2010.0152. [DOI] [PubMed] [Google Scholar]
- 83.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120(5):1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ciplak M, Pasche A, Heim A, Haeberli C, Waeber B, Liaudet L, Feihl F, Engelberger R. The vasodilatory response of skin microcirculation to local heating is subject to desensitization. Microcirculation. 2009;16(3):265–275. doi: 10.1080/10739680802595880. [DOI] [PubMed] [Google Scholar]
- 85.Bourre JM. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J Nutr Health Aging. 2004;8(3):163–174. [PubMed] [Google Scholar]
- 86.Freemantle E, Vandal M, Tremblay-Mercier J, Tremblay S, Blachère JC, Bégin ME, Brenna JT, Windust A, Cunnane SC. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):213–220. doi: 10.1016/j.plefa.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 87.McKay DL, Chen CY, Yeum KJ, Matthan NR, Lichtenstein AH, Blumberg JB. Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: a randomized, cross-over pilot study. Nutr J. 2010;9:21. doi: 10.1186/1475-2891-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh N, Prasad S, Singer DR, MacAllister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clin Sci (Lond) 2002;102(5):595–600. [PubMed] [Google Scholar]
- 89.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284(5):H1662–H1667. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- 90.Jose RM, Vidyadharan R, Roy DK, Erdmann M. Hot water bottles and diabetic patients–a cautionary tale. Br J Gen Pract. 2005;55(512):222–223. [PMC free article] [PubMed] [Google Scholar]
- 91.Petrofsky J, Prowse M, Bain M, Ebilane E, Suh HJ, Batt J, Lawson D, Hernandez V, Abdo A, Yang TN, Mendoza E, Collins K, Laymon M. Estimation of the distribution of intramuscular current during electrical stimulation of the quadriceps muscle. Eur J Appl Physiol. 2008;103(3):265–273. doi: 10.1007/s00421-008-0700-3. [DOI] [PubMed] [Google Scholar]
- 92.Petrofsky J, Suh HJ, Fish A, Hernandez V, Abdo A, Collins K, Mendoza E, Yang TN. A multi-channel stimulator and electrode array providing a rotating current whirlpool for electrical stimulation of wounds. J Med Eng Technol. 2008;32(5):371–384. doi: 10.1080/03091900601116994. [DOI] [PubMed] [Google Scholar]
- 93.Loukin S, Su Z, Zhou X, Kung C. Forward genetic analysis reveals multiple gating mechanisms of TRPV4. J Biol Chem. 2010;285(26):19884–19890. doi: 10.1074/jbc.M110.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wissler RW, Strong JP. Risk factors and progression of atherosclerosis in youth. PDAY Research Group. Pathological Determinants of Atherosclerosis in Youth. Am J Pathol. 1998;153(4):1023–1033. doi: 10.1016/s0002-9440(10)65647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bui C, Petrofsky J, Berk L, Shavlik D, Remigio W, Montgomery S. Acute effect of a single high-fat meal on forearm blood flow, blood pressure and heart rate in healthy male Asians and Caucasians: a pilot study. Southeast Asian J Trop Med Public Health. 2010;41(2):490–500. [PMC free article] [PubMed] [Google Scholar]