Abstract
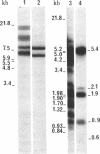
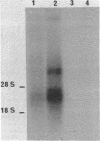
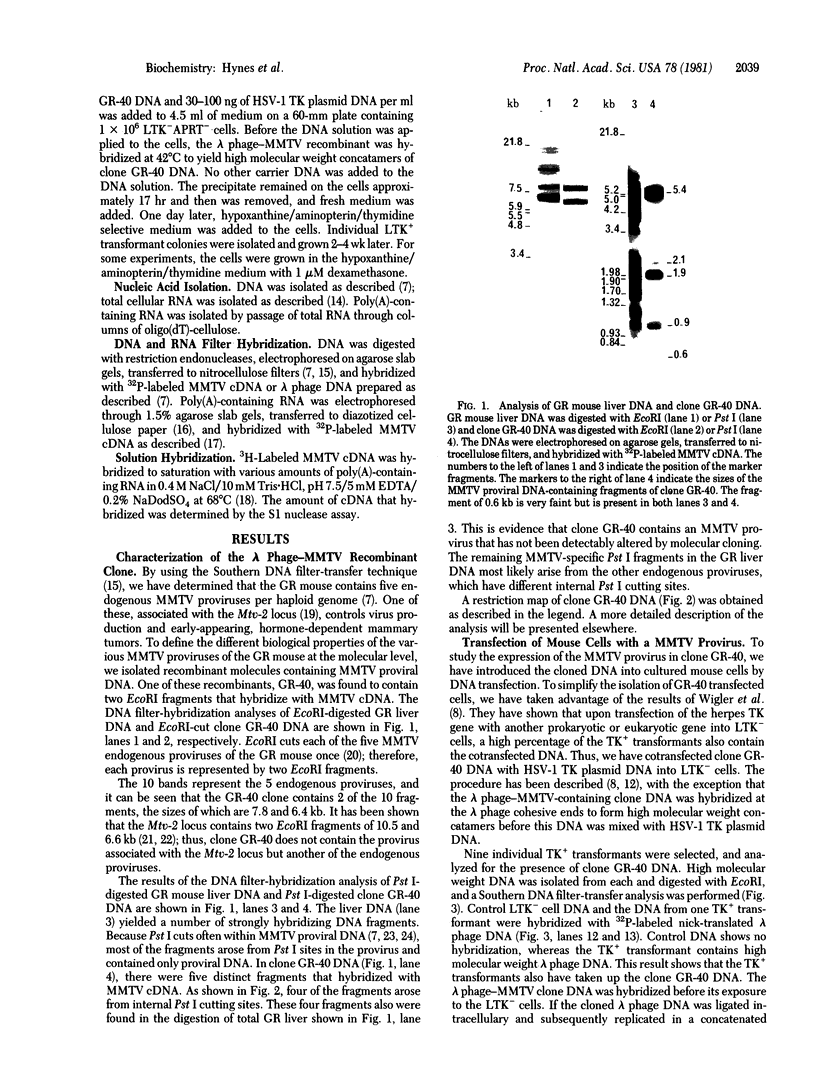
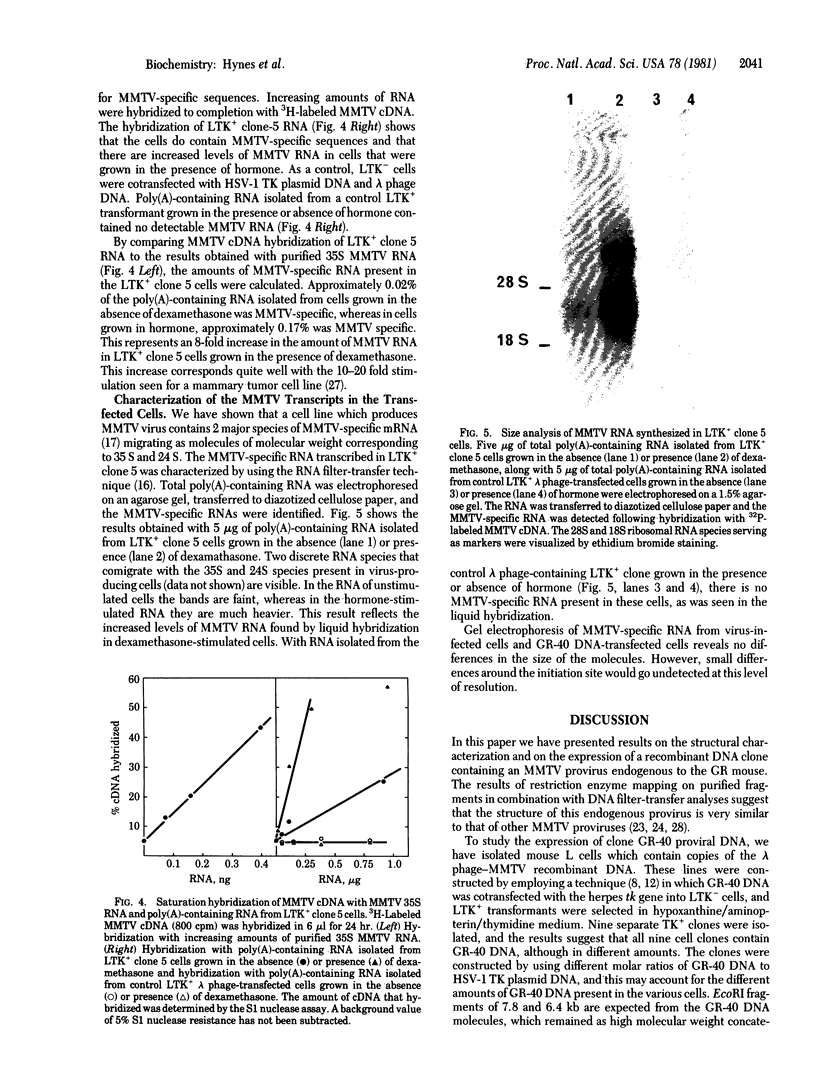
A recombinant lambda phage containing mouse mammary tumor virus (MMTV) proviral DNA was isolated from a gene library constructed from GR mouse liver DNA. Restriction enzyme analyses reveal that the cloned molecule contains a copy of one of the GR endogenous MMTV proviruses flanked on both sides by 2--3 kb of mouse genomic DNA. In this report we have examined the expression of the cloned MMTV provirus after cotransfection with the herpes thymidine kinase (TK; ATP:thymidine 5'-phosphotransferase,, EC 2.7.1.21) gene and integration into mouse LTK- cells. Nine individual TK+ transformants were selected, and all were found to contain MMTV-transfected DNA. One of the TK+ transformants was chosen for further study. Total poly(A)-containing RNA was isolated from the cells, and liquid hybridization analyses with MMTV cDNA showed that it contained 0.02% MMTV-specific RNA. The sizes of the MMTV-specific species were determined and found to correspond to the 35S and 24S mRNAs synthesized in MMTV-infected cells. Glucocorticoid hormones have been shown to increase the concentration of MMTV RNA in virus-infected cultured cells. Therefore, we tested the effect of dexamethasone on the concentration of MMTV-specific RNA in cells transfected with the MMTV proviral DNA. The amount of MMTV-specific poly(A)-containing RNA found in the cells grown in the presence of hormone was 0.17%. Therefore, dexamethasone causes an 8-fold increase in the amount of MMTV-specific RNA in mouse cells containing several copies of a cloned and transfected MMTV proviral gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Dahl H. H., Dickson C. Cell-free synthesis of mouse mammary tumor virus Pr77 from virion and intracellular mRNA. J Virol. 1979 Mar;29(3):1131–1141. doi: 10.1128/jvi.29.3.1131-1141.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Groner B., Buetti E., Diggelmann H., Hynes N. E. Characterization of endogenous and exogenous mouse mammary tumor virus proviral DNA with site-specific molecular clones. J Virol. 1980 Dec;36(3):734–745. doi: 10.1128/jvi.36.3.734-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E., Diggelmann H. Identification of mouse mammary tumor virus-specific mRNA. J Virol. 1979 Apr;30(1):417–420. doi: 10.1128/jvi.30.1.417-420.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Diggelmann H., Van Nie R., Michalides R. Genomic location of mouse mammary tumor virus proviral DNA in normal mouse tissue and in mammary tumors. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1161–1168. doi: 10.1101/sqb.1980.044.01.125. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Nguyen-Huu M. C., Schütz G. mRNA complexity and egg white protein mRNA content in mature and hormone-withdrawn oviduct. Cell. 1977 Aug;11(4):923–932. doi: 10.1016/0092-8674(77)90303-8. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nuss R. R., van Nie R. Identification of the Mtv-2 gene responsible for the early appearance of mammary tumors in the GR mouse by nucleic acid hybridization. Proc Natl Acad Sci U S A. 1978 May;75(5):2368–2372. doi: 10.1073/pnas.75.5.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., van Nie R., Nusse R., Hynes N. E., Groner B. Mammary tumor induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell. 1981 Jan;23(1):165–173. doi: 10.1016/0092-8674(81)90281-6. [DOI] [PubMed] [Google Scholar]
- Nusse R., Asselbergs F. A., Salden M. H., Michalides R. J., Bloemendal H. Translation of mouse mammary tumor virus RNA: precursor polypeptides are phosphorylated during processing. Virology. 1978 Nov;91(1):106–115. doi: 10.1016/0042-6822(78)90359-8. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Cardiff R. D., Varmus H. E., Yamamoto K. R. Infection of cultured rat hepatoma cells by mouse mammary tumor virus. Cell. 1977 Jan;10(1):11–18. doi: 10.1016/0092-8674(77)90134-9. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Varmus H. E. Structural analysis of the intracellular RNAs of murine mammary tumor virus. J Virol. 1979 May;30(2):576–589. doi: 10.1128/jvi.30.2.576-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. A precursor of globin messenger RNA. J Mol Biol. 1976 Sep 15;106(2):403–420. doi: 10.1016/0022-2836(76)90093-0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Young H. A., Parks W. P. Biochemical and physiological mechanisms in glucocorticoid hormone induction of mouse mammary tumor virus. Virology. 1976 Jan;69(1):148–156. doi: 10.1016/0042-6822(76)90202-6. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Smith S. W., Marcus S. L., Sarkar N. H. Identification of the messenger RNAs coding for the gag and env gene products of the murine mammary tumor virus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1736–1740. doi: 10.1073/pnas.76.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Lasfargues E. Y., Heubel G., Lasfargues J. C., Moore D. H. Murine mammary tumor virus: characterization of infection of nonmurine cells. J Virol. 1976 Jun;18(3):911–917. doi: 10.1128/jvi.18.3.911-917.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Young H. A., Scolnick E. M., Parks W. P. Glucocorticoid-receptor interaction and induction of murine mammary tumor virus. J Biol Chem. 1975 May 10;250(9):3337–3343. [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Parks W. P. Steroid induction of mouse mammary tumor virus: effect upon synthesis and degradation of viral RNA. J Virol. 1977 Jan;21(1):139–146. doi: 10.1128/jvi.21.1.139-146.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]