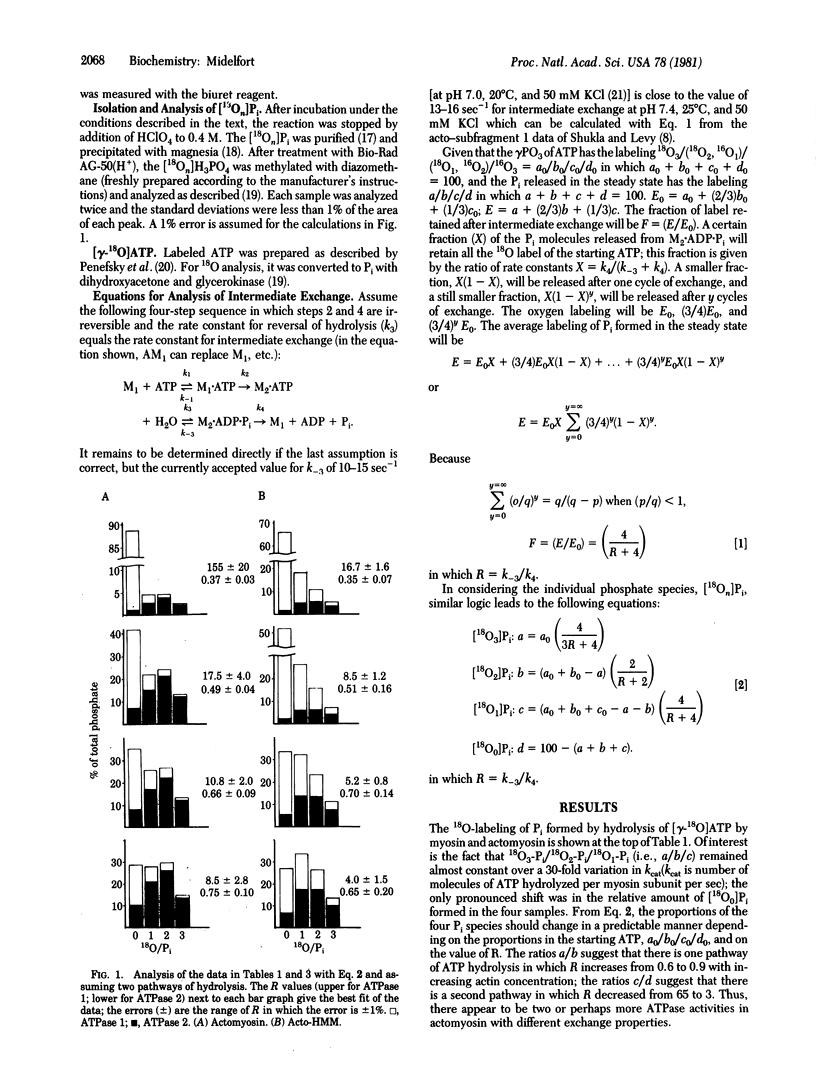
Abstract
The labeled inorganic phosphate formed by enzymatic hydrolysis of [gamma-18O]ATP in normal water was derivatized to trimethyl phosphate and analyzed for the proportions of [18O3]Pi, [18O2]Pi, [18O1]Pi, and [18O0]Pi. The proportions observed were correlated with the kinetics of intermediate exchange by using a kinetic relationship in which it is assumed that binding of ATP and subsequent release of products are irreversible. Actomyosin and acto-heavy meromyosin catalyze intermediate exchange at a mean rate that is more than 1 order of magnitude slower than that predicted by rapid kinetic studies or implied by the essentially complete intermediate exchange observed with myosin alone. The reason for the slow apparent exchange is that there are two ATPase activities with different exchange properties. The effect of varying heavy meromyosin concentrations at a constant actin concentration shows that the two activities are interrelated and suggests further that one is due to ATP hydrolysis by the undissociated actomyosin crossbridge.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw C. R., Trentham D. R., Wolcott R. G., Boyer P. D. Oxygen exchange in the gamma-phosphoryl group of protein-bound ATP during Mg2+-dependent adenosine triphosphatase activity of myosin. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2592–2596. doi: 10.1073/pnas.72.7.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms A. S., Boyer P. D. Occurrence and characteristics of 18 O exchange reactions catalyzed by sodium- and potassium-dependent adenosine triphosphatases. J Biol Chem. 1973 May 10;248(9):3155–3162. doi: 10.2172/4485348. [DOI] [PubMed] [Google Scholar]
- EBASHI S. Calcium binding activity of vesicular relaxing factor. J Chir (Paris) 1961 Sep;82:236–244. doi: 10.1093/oxfordjournals.jbchem.a127439. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Dobkin L., Kielley W. W. Heavy meromyosin: evidence for a refractory state unable to bind to actin in the presence of ATP. Proc Natl Acad Sci U S A. 1972 Mar;69(3):667–671. doi: 10.1073/pnas.69.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Inoue A., Shigekawa M., Tonomura Y. Direct evidence for the two route mechanism of the acto-H-meromyosin-ATPase reaction. J Biochem. 1973 Nov;74(5):923–934. [PubMed] [Google Scholar]
- LEVY H. M., KOSHLAND D. E., Jr Mechanism of hydrolysis of adenosinetriphosphate by muscle proteins and its relation to muscular contraction. J Biol Chem. 1959 May;234(5):1102–1107. [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- MOMMAERTS W. F. H. M., PARRISH R. G. Studies on myosin. I. Preparation and criteria of purity. J Biol Chem. 1951 Feb;188(2):545–552. [PubMed] [Google Scholar]
- Midelfort C. F., Rose I. A. A stereochemical method for detection of ATP terminal phosphate transfer in enzymatic reactions. Glutamine synthetase. J Biol Chem. 1976 Oct 10;251(19):5881–5887. [PubMed] [Google Scholar]
- Moos C., Eisenberg E. Effect of myosin on actin-bound nucleotide exchange in the presence and absence of ATP. Biochim Biophys Acta. 1970 Dec 8;223(2):221–229. doi: 10.1016/0005-2728(70)90179-9. [DOI] [PubMed] [Google Scholar]
- PENEFSKY H. S., PULLMAN M. E., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. II. Participation of a soluble adenosine tolphosphatase in oxidative phosphorylation. J Biol Chem. 1960 Nov;235:3330–3336. [PubMed] [Google Scholar]
- Shukla K. K., Levy H. M. Mechanism of oxygen exchange in actin-activated hydrolysis of adenosine triphosphate by myosin subfragment 1. Biochemistry. 1977 Jan 11;16(1):132–136. doi: 10.1021/bi00620a022. [DOI] [PubMed] [Google Scholar]
- Shukla K. K., Levy H. M. Oxygen exchange by single-headed myosin. Further support for the hypothesis that the active site of myosin is near the (subfragment 1)-(subfragment 2) hinge. J Biol Chem. 1978 Dec 10;253(23):8362–8365. [PubMed] [Google Scholar]
- Sleep J. A., Hackney D. D., Boyer P. D. The equivalence of phosphate oxygens for exchange and the hydrolysis characteristics revealed by the distribution of [18O]Pi species formed by myosin and actomyosin ATPase. J Biol Chem. 1980 May 10;255(9):4094–4099. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]
- YOUNG D. M., HIMMELFARB S., HARRINGTON W. F. ON THE STRUCTURAL ASSEMBLY OF THE POLYPEPTIDE CHAINS OF HEAVY MEROMYOSIN. J Biol Chem. 1965 Jun;240:2428–2436. [PubMed] [Google Scholar]


