Abstract
Elevated homocysteine levels are a known risk factor for Alzheimer’s disease and vascular disorders. Here we applied tensor-based morphometry to brain magnetic resonance imaging scans of 732 elderly individuals from the Alzheimer’s Disease Neuroimaging Initiative study, to determine associations between homocysteine and brain atrophy. Those with higher homocysteine levels showed greater frontal, parietal, and occipital white matter atrophy in the entire cohort, irrespective of diagnosis, age, or sex. This association was also found when considering mild cognitive impairment individuals separately. Vitamin B supplements, such as folate, may help prevent homocysteine-related atrophy in Alzheimer’s disease by possibly reducing homocysteine levels. These atrophy profiles may, in the future, offer a potential biomarker to gauge the efficacy of interventions using dietary folate supplementation.
Keywords: Alzheimer’s disease, atrophy, brain structure, folate, homocysteine, magnetic resonance imaging, vitamin B
Introduction
People with high levels of the amino acid, homocysteine, in the blood have an increased risk for stroke, Alzheimer’s disease (AD), and age-related memory impairment [1]. Recently, dietary supplements of vitamin B, such as folate, have been reported to lower the risk for cognitive decline and AD, possibly by lowering homocysteine levels [2]. To better understand how homocysteine levels might relate to brain degeneration, we studied anatomical magnetic resonance imaging (MRI) scans from 732 elderly individuals, relating homocysteine levels in the blood to brain atrophy. Our goal was to determine which brain systems showed greater atrophy in those with high homocysteine levels, and whether the relationship holds regardless of diagnosis [normal elderly, mild cognitive impairment (MCI), or AD individuals].
Elevated homocysteine levels are a risk factor for cortical and subcortical atrophy in demented and nondemented elderly individuals [3–5]. High homocysteine levels may facilitate a build-up of toxic β-amyloid and tau in the brain, and may compromise DNA repair and promote degenerative changes due to oxidative stress, which may in turn lead to excitotoxicity and apoptosis [6,7].
Prior studies have found associations between homocysteine and atrophy in specific brain regions, as summarized in recent reviews [8,9]. Elevated homocysteine levels are associated with lower overall brain volumes and atrophy in subcortical structures on MRI, but the detailed anatomical pattern of these effects has not been mapped [4,5]. To do this, we aimed to create a three-dimensional map of the structural brain differences associated with homocysteine levels, in a large cohort of elderly individuals scanned as part of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study. We hypothesized that higher plasma levels of homocysteine would be related to lower regional brain volumes, regardless of diagnosis, and also within groups of individuals with AD and MCI. If this is true, some of the brain atrophy that affects elderly populations may be preventable in principle by homocysteine-lowering interventions such as folate and other vitamin B supplements. Homocysteine levels in blood may serve as a potential biomarker, among other neuroimaging and cerebrospinal fluid measures, for AD.
Materials and methods
Individuals
We studied 732 elderly individuals (203 healthy controls, 356 with MCI, 173 with AD; mean age: 75.5±6.8 years) who received a 1.5T anatomical brain MRI scan as part of the ADNI study. ADNI was launched in 2004 by the National Institute of Health, the Food and Drug Administration, private pharmaceutical companies, and non-profit organizations to identify and evaluate biomarkers of AD for use in multisite studies. All ADNI data are publicly available at www.loni.ucla.edu/ADNI. The study was conducted according to the Good Clinical Practice guidelines, the Declaration of Helsinki, and the US 21 CFR Part 50–Protection of Human Subjects, and Part 56–Institutional Review Boards. Written informed consent was obtained from all participants in advance.
At the time of their baseline MRI scan, all individuals had their blood samples collected in the morning before breakfast, after an overnight fast. Total homocysteine levels were measured from plasma samples using a validated enzyme immunoassay [10]. At the time of their MRI scan, all individuals underwent clinical and cognitive evaluations including the Mini-Mental State Examination (MMSE) [11]. The MMSE, with scores ranging from 0 to 30, is a global measure of mental status based on five cognitive domains; lower scores indicate worse performance and scores below 24 are typically associated with dementia. Inclusion and exclusion criteria are detailed in the ADNI protocol [12].
We analyzed Caucasian ADNI individuals (n=732) who had baseline brain MRI scans available at the time of analysis (January 2011) as well as plasma homocysteine levels assessed at the time of the baseline scan (Table 1).
Table 1.
Selected characteristics of the individuals analyzed in the study
| AD | MCI | Controls | All individuals | |
|---|---|---|---|---|
| Sample size (n) | 173 | 356 | 203 | 732 |
| Age (years) | 75.57 ± 7.62 | 75.15 ± 7.26 | 76.13 ± 4.99 | 75.52 ± 6.81 |
| MMSEa | 23.36 ± 2.00 | 27.09 ± 1.78 | 29.16 ± 0.95 | 26.78 ± 2.67 |
| Plasma homocysteine (μM) | 10.78 ± 3.33 | 10.63 ± 2.82 | 10.02 ± 2.85 | 10.49 ± 2.97 |
AD, Alzheimer’s disease; MCI, mild cognitive impairment.
Mini-Mental State Examination (MMSE); maximum score is 30.
Image acquisition and processing
High-resolution structural MRI scans were acquired on 1.5T scanners from General Electric (Milwaukee, Wisconsin, USA), Siemens (Germany), or Philips (The Netherlands) with a standardized MRI protocol [13]. Each scan involved a three-dimensional sagittal magnetization-prepared rapid gradient-echo sequence with the following parameters: repetition time (2400ms), flip angle (8°), inversion time (1000 ms), 24-cm field of view, a 192×192×166 acquisition matrix, a voxel size of 1.25×1.25×1.2mm3, later reconstructed to 1-mm isotropic voxels. To adjust for global differences in brain positioning and size, all scans were first linearly registered to the International Consortium for Brain Mapping template [14] with a nine-parameter transformation using the Minctracc algorithm [15]. Globally aligned images were resampled in an isotropic space of 220 voxels along each axis (x, y, and z) with a final voxel size of 1mm3.
Tensor-based morphometry analysis and statistics
To assess regional brain atrophy in each individual, a minimal deformation template (MDT) was first created from the MRI scans of 40 cognitively healthy ADNI individuals, to serve as an unbiased group average template [16]. All preprocessed MRI images were nonlinearly aligned to the study-specific template so that they would all share a common coordinate system defined by the MDT. The local expansion or compression factor of the three-dimensional elastic warping transform [17], calculated as the determinant of the Jacobian matrix of the deformation, was plotted for each individual. These three-dimensional maps reveal areas of structural volume excess or deficits, relative to the MDT. At each image voxel within the brain, a multiple regression analysis was then carried out to analyze statistical associations between regional brain volumes and plasma homocysteine levels across all individuals, after adjusting for age, sex, and diagnosis. We also analyzed statistical associations within each of the AD, MCI, and control groups, after covarying for age and sex. We then created three-dimensional statistical brain maps to show brain regions where atrophy was significantly associated with homocysteine. To control false-positives, we enforced a standard false discovery rate correction for multiple statistical comparisons across voxels in the brain, using the conventionally accepted false-positive rate of 5% (q=0.05).
Results
Homocysteine and brain volumes
The average morning total plasma homocysteine levels in the ADNI cohort were 10.5±3.0 μM, with mean levels of 10.8±3.3, 10.6±2.8, and 10.0±2.9 μM for AD, MCI, and control groups, respectively (Table 1). Compared with healthy controls, homocysteine levels were higher in AD (two-tailed t-test, P=0.018) and MCI (two-tailed t-test, P=0.015). Agreeing with a previous study [4], homocysteine levels in men (n=434) were higher (two-tailed t-test, P<0.0001) by 1.16 μM on an average, than in women (n=298) in the cohort.
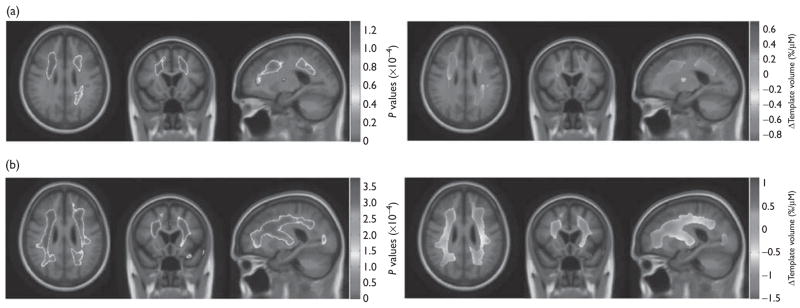
A linear regression analysis across all the individuals covarying for age, sex, and diagnostic group, showed that the regional brain volume association with homocysteine levels was significant (Table 2). Associations were detected in the frontal, parietal, and occipital white matter. After correcting for multiple statistical comparisons, every 5-μM increase in homocysteine level was associated with a relative brain volume deficit of 4.4% (0.88%/μM times 5) and an excess of 3.6% in the cerebrospinal fluid filled areas, relative to the MDT (Fig. 1a).
Table 2.
Critical P values for association between homocysteine and regional brain volumes
| Critical P value | Standard FDR (q =0.05) |
|---|---|
| AD | Did not pass |
| MCI | 0.0038 |
| Controls | Did not pass |
| All individuals | 0.0013 |
| Hyperhomocysteinemia | 0.0002 |
AD, Alzheimer’s disease; FDR, false discovery rate; MCI, mild cognitive impairment.
Fig. 1.
(a and b) Left=three-dimensional P value maps displayed over the mean template (minimal deformation template) show areas where regional brain volumes were significantly associated with homocysteine levels in (a) the entire Alzheimer’s Disease Neuroimaging Initiative cohort (n=732) covaried for age, sex, and diagnosis and (b) in mild cognitive impairment individuals only (n =356) covaried for age and sex. Right=in the significant areas, three-dimensional β value maps show the estimated regional brain volume excess or deficit (percentage relative to minimal deformation template) at each voxel for every 1-μM increase in the homocysteine levels in (a) the entire Alzheimer’s Disease Neuroimaging Initiative cohort (standard false discovery rate, q =0.05, critical P=0.0013); and (b) in mild cognitive impairment individuals only (standard false discovery rate, q=0.05, critical P=0.0038).
A subgroup analysis revealed significant homocysteine associated regional brain volume differences in the MCI group but not in the AD or control groups (Table 2). The MCI group showed similar regions seen in the whole group (frontal, parietal, and occipital white matter), although to a greater extent, with a maximum brain volume deficit of 7.5% per 5-μM increase in homocysteine levels, relative to the MDT. Volume excess was noted relative to the MDT in some regions that corresponded to spaces occupied by cerebrospinal fluid. An excess of up to 5.6% was noted with each 5 μM increase in homocysteine levels in plasma (Fig. 1b).
Hyperhomocysteinemia and brain volumes
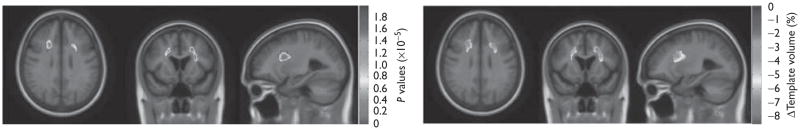
We defined hyperhomocysteinemia as present in those with a plasma homocysteine level greater than or equal to the 95th percentile for controls, which was 14.0 μM. A group difference analysis, between individuals with hyperhomocysteinemia in the cohort (n=70) and individuals without hyperhomocysteinemia in the cohort (n=662) was carried out controlling for age, sex, and diagnosis (Table 2). The three-dimensional beta-Maps (of the correlation coefficients) revealed regional brain volume deficits relative to the MDT (up to 8.6%) in the frontal and parietal white matter associated with the presence of hyperhomocysteinemia (Fig. 2).
Fig. 2.
Left=three-dimensional significance (P value) maps displayed over the mean template (minimal deformation template) show areas where regional brain volumes were lower in those with hyperhomocysteinemia (abnormally high-homocysteine levels) in the Alzheimer’s Disease Neuroimaging Initiative cohort (n=732) after covarying for age, sex, and diagnosis. Right=three-dimensional β value maps show the regression coefficients at each voxel in the significant areas, after correcting for multiple statistical comparisons (standard false discovery rate, q=0.05, critical P=0.0002). Those with hyperhomocysteinemia showed an estimated maximum brain volume reduction of up to 8.6%, relative to the average brain template.
Cognitive performance
The mean baseline MMSE score was 26.78±2.67 points across all the individuals (Table 1). As expected, control group individuals scored more highly than MCI individuals (two-tailed t-test, P<0.0001) and MCI individuals scored higher than AD individuals (two-tailed t-test, P<0.0001). Individuals with hyperhomocysteinemia (n=70) showed lower MMSE scores on average, compared with individuals without hyperhomocysteinemia (n=662) and the difference was marginally significant (two-tailed t-test, P=0.056). In a regression analysis controlling for age and sex, MMSE scores were not significantly associated with homocysteine levels in any of the AD, MCI, control groups, or across the entire cohort.
Discussion
We revealed a three-dimensional profile of strong associations between plasma homocysteine levels and regional brain volumes across all the individuals and in separate analyses of the MCI group specifically, in the white matter regions of the frontal, parietal, and occipital lobes, bilaterally. Those with elevated plasma homocysteine levels (over 14 μM) also showed increased atrophy, irrespective of their disease status. These results may improve understanding of the consequences of having elevated homocysteine levels, and the potential benefits of medications that lower them.
In our study, the group of MCI individuals showed large areas of association between homocysteine levels and brain volume. However, associations were not detected in the AD or the CTL group. This is most likely due to the larger sample size for the MCI group (MCI, n=356 vs. AD, n=173, and controls, n=203), which offered greater power to detect associations.
In a recent randomized control trial, dietary supplementation with vitamin B slowed brain atrophy in individuals with MCI, possibly by lowering homocysteine levels [2]. As those with high homocysteine levels have a profile of greater atrophy, early interventions through homocysteine lowering diets and drugs may be considered as means to resist brain atrophy in the elderly.
Strengths of our study include the large and well-defined cohort (ADNI) with high quality MRI scans of the brain, and well-validated computational methods for volume quantification. As of the ADNI study design, the sample sizes for AD and control groups were not as large as MCI, which could have limited our ability to detect associations in these groups.
Conclusion
Automated whole brain volumetric analysis of brain MRI revealed a three-dimensional pattern of homocysteine-associated brain differences. Hyperhomocysteinemia may promote the magnitude of atrophy in the brain. These statistical associations may help in understanding how neurodegenerative disorders develop. These findings also have implications for drug trials that aim to lower homocysteine levels and thereby slow the onset of dementia or age-related neuro-degeneration.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace Inc., Merck and Co. Inc., Novartis AG, Pfizer Inc., F. Hoffman-La Roche, Schering-Plough, Synarc Inc., as well as nonprofit partners the Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation, with participation from the US Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego, USA. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles, USA. This research was also supported by the NIH Grants P30 AG010129, K01 AG030514, the Dana Foundation, and Grants R01 EB008281 and R01 AG020098 (to P.T.).
Footnotes
Data used in this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative database (www.loni.ucla.edu/ADNI). Investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. For a complete listing of ADNI investigators, see http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Authorship_List.pdf
References
- 1.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 2.Smith AD, Smith SM, De Jager CA, Whitbread P, Johnston C, Agacinski G, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a Randomized Controlled Trial. PLoS ONE. 2010;5:e12244. doi: 10.1371/journal.pone.0012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachdev PS. Homocysteine and brain atrophy. Progr Neuropsychopharmacol Biol Psychiatry. 2005;29:1152–1161. doi: 10.1016/j.pnpbp.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Den Heijer T, Vermeer SE, Clarke R, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MMB. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain. 2002;126:170–175. doi: 10.1093/brain/awg006. [DOI] [PubMed] [Google Scholar]
- 5.Longstroth WT, Katz R, Olson J, Bernick C, Carr JJ, Malinow MR, et al. Plasma total homocysteine levels and cranial magnetic resonance imaging findings in elderly persons. Arch Neurol. 2004;61:67–72. doi: 10.1001/archneur.61.1.67. [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 7.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 8.Morris MS. Homocysteine and Alzheimer’s disease. Lancet Neurol. 2003;2:425–428. doi: 10.1016/s1474-4422(03)00438-1. [DOI] [PubMed] [Google Scholar]
- 9.Van Dam F, Van Gool WA. Hyperhomocysteinemia and Alzheimer’s disease: a systematic review. Arch Gerontol Geriatr. 2009;48:425–430. doi: 10.1016/j.archger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Shaw LM. PENN biomarker core of the Alzheimer’s Disease Neuroimaging Initiative. Neurosignals. 2008;16:19–23. doi: 10.1159/000109755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, MCHugh PR. Mini-Mental State Examination: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 16.Hua X, Leow AD, Parikshak N, Lee S, Chiang MC, Toga AW, et al. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: an MRI study of 676 AD MCI, and normal subjects. Neuroimage. 2008;43:458–469. doi: 10.1016/j.neuroimage.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leow A, Huang S-C, Geng A, Becker J, Davis S, Toga A, Thompson P. Inverse consistent mapping in 3D deformable image registration: its construction and statistical properties. Inf Process Med Imaging. 2005;19:493–503. doi: 10.1007/11505730_41. [DOI] [PubMed] [Google Scholar]