Abstract
The claim that the 6 kDa viral protein (VP) of Tobacco Etch Virus is a marker for ER exit sites (ERES) has been investigated. When transiently expressed as a CFP tagged fusion construct in tobacco mesophyll protoplasts, this integral membrane protein co-localizes with both the COPII coat protein YFP-SEC24 and the Golgi marker Man1-RFP. However, when over-expressed the VP locates to larger spherical structures which co-localize with neither ER nor Golgi markers. Nevertheless, deletion of the COPII interactive N-terminal D(X)E motif causes it to be broadly distributed throughout the ER, supporting the notion that this protein could be an ERES marker. Curiously, whereas brefeldin A (BFA) caused a typical Golgi-stack response (redistribution into the ER) of the VP in leaf epidermal cells, in protoplasts it resulted in the formation of structures identical to those formed by over-expression. However, anomalous results were obtained with protoplasts: when co-expressed with the non-cycling cis-Golgi marker Man1-RFP, a BFA-induced redistribution of the VP-CFP signal into the ER was observed, but, in the presence of the cycling Golgi marker ERD2-YFP, this did not occur. High resolution images of side-on views of Golgi stacks in epidermal cells showed that the 6 kDa VP-CFP signal overlapped considerably more with YFP-SEC24 than with Man1-RFP, indicating that the VP is proportionately more associated with ERES. However, based on a consideration of the structure of its cytoplasmic tail, the scenario that the VP collects at ERES and is transported to the cis-Golgi before being recycled back to the ER, is supported.
Keywords: 6 kDa tobacco etch viral protein, COPII proteins, ERES (ER export sites) markers, Golgi apparatus
Introduction
It is generally accepted that the endoplasmic reticulum (ER) is the gateway to the secretory pathway in all eukaryotic cells (Vitale et al., 1999; Osborne et al., 2005). After undergoing a quality control to assess their export competence (Anelli et al., 2008), secretory proteins and membrane proteins, destined for organelles downstream of the ER, leave the ER through discrete domains known as ER export (or exit) sites (ERES) (Budnik et al., 2009; Hanton et al., 2009). In mammalian cells, ERES are characterized by the presence of the recruiting GTPase Sar1, the dimeric COPII proteins Sec23/24 and Sec13/31, as well as Sec16 (Watson et al., 2006; Hughes et al., 2009). This is also true for yeast (Connerly et al., 2005; Fromme et al., 2008) and higher plants (Hanton et al., 2005; Marti et al., 2010). With the exception of Sec16, the COPII proteins are capable of forming a coat around 60 nm diameter ER-derived transport vesicles (Barlowe et al., 1994; Miller et al., 2010). COPII-coated vesicles have been demonstrated in vivo in both yeast (Fromme et al., 2008) and mammalian cells (Zeuschner et al., 2006). In addition, tubular, partially COPII-coated carriers have also been reported to be released from ERES in mammalian cells (Zeuschner et al., 2006; Saito et al., 2009).
COPII-coated vesicles have not yet been isolated from plants, nor have they successfully been induced in vitro from ER-enriched membrane fractions. The depiction in the electron microscope of vesicle budding events at the ER of higher plant cells has also proved to be very difficult, even when rapid freeze-fixation techniques are employed (Robinson et al., 2007; Kang et al., 2008; Staehelin et al., 2008). On the other hand, an indication of ERES has been possible through the localization of transiently expressed fluorescently tagged COPII homologues (daSilva et al., 2004; Yang et al., 2005; Hanton et al., 2007, 2009), and through immunofluorescence with COPII antibodies (Yang et al., 2005; Zhang et al., 2010). There is an extremely high degree of co-localization between fluorescently tagged COPII proteins and Golgi membrane proteins (e.g. Man1, ERD2) when they are coexpressed in tobacco epidermal cells (daSilva et al., 2004; Hanton et al., 2007, 2008). These observations have led to the concept, that ERES and Golgi stacks move together in a tightly coupled fashion, the so-called ‘secretory unit’ (daSilva et al., 2004; Hawes et al., 2008).
It is well known that infection with plant viruses leads to significant cellular reorganization (Laliberte et al., 2010). Depending on the virus, different organelles may be affected but with one purpose: the production of viral replication complexes (VRCs), which serve to protect the replication machinery from host cell nucleases (Ding et al., 2004; Schwartz et al., 2004). As with poliovirus in mammalian cells (Belov et al., 2007), potyviruses appear to high-jack the early secretory pathway in order to form VRCs. One candidate for the modification of the host cell ER in the case of Tobacco Etch Virus (TEV) is a 6 kDa (53 amino acids) protein which has a putative short luminal domain (11 aa), a 19 aa transmembrane domain, and a 23 aa cytoplasmic domain (Fig. 1A). This is one of eight proteins encoded by the TEV genome (Schaad et al., 1997). According to Wei and Wang (2008), expression of the 6 kDa viral protein (VP)-CFP in leaf epidermal cells of Nicotiana benthamiana leads to the production of two types of signal: punctae, which co-localize with the COPII marker YFP-SEC24 and larger ring-like structures which are not labelled with YFP-SEC24, nor with the Golgi marker ERD2-CFP. The latter probably correspond to the large VRCs described earlier by Schaad et al. (1997). By using non-functional Sar1 and Arf1 mutants, Wei and Wang (2008) also showed that the localization of the 6 kDa VP at putative ERES was dependent upon active anterograde COPII and retrograde COPI machineries.
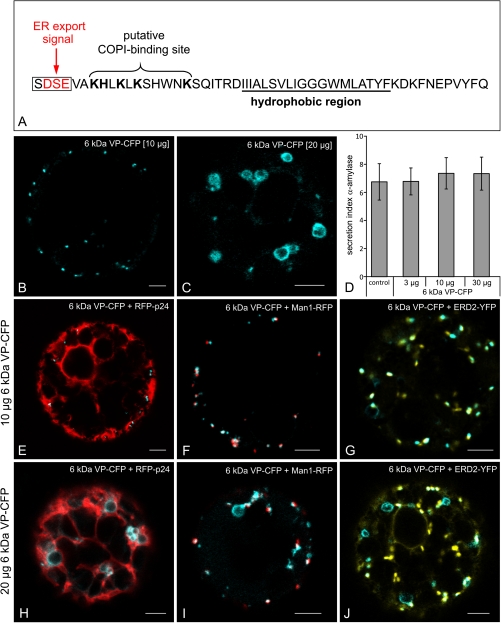
Fig. 1.
(A) Amino acid sequence of the 6 kDa VP-CFP with the ER export signal marked in red and the deleted amino acids (SDSE). (B–J) Effects of 6 kDa overexpression in tobacco mesophyll protoplasts. (B) Punctate expression pattern obtained with 10 μg plasmid DNA after 24 h expression. (C) Large ring-like structures are seen with 20 μg plasmid DNA, in addition to a few punctate signals. (D) Expression of the 6 kDa VP is without effect on the intracellular transport of the secretory reporter α-amylase. Standard deviation is indicated by error bars in each column. (E–J) Comparison of 6 kDA VP-CFP signals at low (10 μg, E–G) and high (20 μg, H–J) plasmid concentrations with ER (RFPp24) and Golgi markers (Man1-RFP; ERD2-YFP). Note the lack of co-localization of the ring-like structures with the fluorescent markers. Bars=5 μm.
It is shown here that, due to the extreme proximity of Golgi stacks to the ER, especially in leaf epidermal cells, the 6 kDa VP-CFP signal overlaps with both YFP-SEC24, and the cis-Golgi marker ManI-RFP. Nevertheless, examination of side-on images of Golgi stacks in the CLSM confirms that the majority of the 6 kDa VP-CFP and YFP-SEC24 label overlap and are separate from the greater portion of the Man1-RFP signal. This confirms that the 6 kDa VP localizes to the interface between ERES and cis-Golgi. Being an integral membrane protein, rather than a COPII coat protein, the 6 kDa VP could therefore have considerable potential as a tool for studies in ER–Golgi transport, but there are drawbacks, especially when using brefeldin A: its redistribution into the ER being dependent upon whether the 6 kDa VP is expressed alone or with a cis-Golgi marker protein.
Materials and methods
Plant material and cultivation
Wild-type Nicotiana benthamiana plants were grown from surface-sterilized seeds on soil in a controlled room at 22 °C with a 16 h day length. Plants of Nicotiana tabacum cv. Petit Havana, were grown from surface-sterilized seeds on Murashige and Skoog medium with 2% (w:w) sucrose in a controlled room at 25 °C with cycles of 16 h light and 8 h darkness.
Transient expression in protoplasts
Preparation of tobacco leaf protoplasts was done exactly as described in Foresti et al. (2006). A total volume of 500 μl of the obtained protoplast suspension was pipetted into a disposable 1 ml plastic cuvette and mixed with an appropriate amount of plasmid DNA: RFP-p24 (Langhans et al., 2008); ERD2-YFP, ST-YFP (Brandizzi et al., 2002a); 6 kDa-CFP (Wei and Wang, 2008); Man1-RFP (Nebenfuehr et al., 1999); and Arf1-Q71L (Pimpl et al., 2003) dissolved in a total of 100 μl of electroporation buffer. The protoplasts were electroporated with stainless steel electrodes at a distance of 3.5 mm, using a complete exponential discharge of a 1000 μF capacitor charged at 160 V. After 30 min of absolute rest, electroporated protoplasts were removed from the cuvettes and transferred to 5 cm Petri dishes with 2 ml of TEX buffer. Protoplasts were then incubated for 20–24 h at 25 °C in a dark chamber. For CLSM analysis, protoplasts were harvested in 15 ml Falcon tubes and allowed to float. For α-amylase assays protoplasts were handled exactly as described in Foresti et al. (2006) and Bubeck et al. (2008). All experiments were performed several times.
Agroinfiltration of markers into tobacco leaves
Four-to-six-week-old tobacco plants (wild type, N. benthamiana) were used for transient expression mediated by Agrobacterium tumefaciens. The relevant binary vectors were transformed into GV3101 (+pSOUP) or ASE and infiltrated into leaf tissues using a 1 ml syringe without a needle by gentle pressure through the stomata on the lower epidermal surface. For leaf infiltration, agrobacteria harbouring the relevant plasmids were grown for 1–2 d in LB plus the appropriate antibiotics, collected by centrifugation, and then resuspended in 1 ml water. The bacterial optical density at 600 nm (OD600) used for plant transformation was 0.1–0.3 for all constructs. Agroinfiltrated plants were incubated under normal growth conditions for 2–3 d at 22–24 °C and small discs of transformed leaf were excised for microscopy.
Treatments with inhibitors
Experiments were performed with 90 μM brefeldin A (BFA). BFA was dissolved in a stock solution in DMSO, leading to a final concentration of DMSO of 0.05%. As demonstrated in Langhans et al (2007), this DMSO concentration is without cytological effect. In our experiments, plant material was pretreated for 30–60 min before observing under the CLSM.
Confocal microscopy
80 μl protoplast solution of N. tabacum var. SR1 was pipetted in an area (10–15 mm) bordered with a frame of 100 μm thick plastic isolating tape on a slide to protect the protoplasts from pressure. The area was covered with a cover slip (24–32 mm). Alternatively, leaves of N. benthamiana were transferred to slides. Cells or plant material were observed under a Zeiss Axiovert LSM510 Meta microscope using a Plan-Neofluar 25×/0.8 Imm corr DIC or a C-Apochromat 63×/1.2 W corr water immersion objective. Special settings were designed for observing single-, double-, and triple expression with different XFP-constructs. Fluorescence was detected by the metadetector using main beam splitter HFTs 488/543 and 458/514. Fluorophores were excited by frame-switching in the multitracking mode of the microscope. Detection of RFP was performed by excitation at 543 nm and an emission at 593-635 nm. CFP and YFP were detected at 464–486 nm and 529–550 nm, respectively, after excitation with a 458 nm or 514 nm LASER beam. Pinholes were adjusted to 1.3 Airy Unit for CFP and to 1 Airy Unit for all other wavelengths. Images were post-processed using the Zeiss LSM Image Browser (Version 4.2.0.121), Corel Draw X4 (Version 14.0.0.567) and ImageJ 1.44i.
α-Amylase assay and secretory index determinations
Preparation of protoplasts and determination of extracellular (secreted) and intracellular α-amylase activities were performed as described earlier (Crofts et al., 1999; Foresti et al., 2006). The secretory index is defined as the ratio of extracellular to intracellular activities (Bubeck et al., 2008).
Plasmid construction
All oligonucleotides were ordered from Sigma-Aldrich (Germany). The expression of the gene of interest is under the control of the 35S promoter and is stopped by the NOS terminator or RBCS terminator. Gateway technology (Invitrogen) was used to generate constructs suitable for expression in tobacco leaves. To generate the 6 kDa VP deletion mutant the 6 kDa VP-CFP (Wei and Wang, 2008) was used as the template. However, primers (forward: GGG GAC AAG TTT GTA CAA AAA AGC AGG CTT CAT GGT GGC TAA GCA TCT G; reverse: GGG GAC CAC TTT GTA CAA GAA AGC TGG GTC TTG GAA ATA GAC TGG) were designed to omit the first 12 nucleotides and cloned into the pJV-113 vector (pGREEN-IIS vector series). Gene sequences were amplified by PCR using Phusion DNA polymerase (Fermentas). The resulting DNA fragments were purified and transferred by recombination into the entry vector pDONR201 (Invitrogen) using BP clonase II (Invitrogen) following the manufacturer's protocol. The insert was then transferred by recombination to the indicated binary destination vector using LR clonase II (Invitrogen) following the standard conditions and procedure recommended by the supplier. RFP-p24 (Langhans et al., 2008) was subcloned into the binary vector pBP33 (Nebenfuehr et al., 1999) using the restriction sites SacI and KpnI. Inserts in the resulting destination clones were verified by sequencing.
Results
6 kDa VP-CFP co-localizes with fluorescently-tagged Golgi markers, but over-expression leads to the production of large spherical membrane structures
In addition to small punctae, Wei and Wang (2008) also observed numerous larger ring-like structures in agro-infiltrated tobacco leaf epidermal cells which were transformed only with the 6 kDa VP-CFP. Since such structures were rarely seen in our agro-infiltrated leaves, it was suspected that their presence in the study of Wei and Wang (2008) might be due to over-expression. To test for this under more controlled conditions, tobacco mesophyll protoplasts were electroporated with increasing amounts of plasmid encoding the 6 kDa VP-CFP construct and then observed in the CLSM after a standard 20–24 h incubation period. Under these conditions it was almost impossible to detect a fluorescent signal with less than 5 μg DNA. A clear punctate signal was first obtained with 10 μg DNA (Fig. 1B); and with 20 μg DNA, in addition to small punctae, there were large ring-like structures present (Fig. 1C). Interestingly, even when higher plasmid concentrations were electroporated, the expression of the 6 kDa VP-CFP had no inhibitory effect on the transport of the secretory reporter α-amylase (Fig. 1D), indicating the non-disruptive nature of short-term expression of the viral protein on the early secretory pathway.
In an attempt to ascertain the nature of the large ring-like structures, the ER marker (RFP-p24) and cis-Golgi markers (Man1-RFP; ERD2-YFP) were co-electroporated with the 6 kDa VP at low (10 μg) and high (20 μg) plasmid concentrations. At the low concentrations, the 6 kDa VP-CFP signal was detected in close proximity to the ER (Fig. 1E) and showed a very high degree of co-localization with both Golgi markers (Fig. 1F, G). Byn contrast, these markers were conspicuously absent from the ring-like structures, which appeared at high plasmid concentrations (Fig. 1H–J).
In response to BFA, the 6 kDa VP behaves like the cis-Golgi marker Man1-RFP in leaves but not in protoplasts
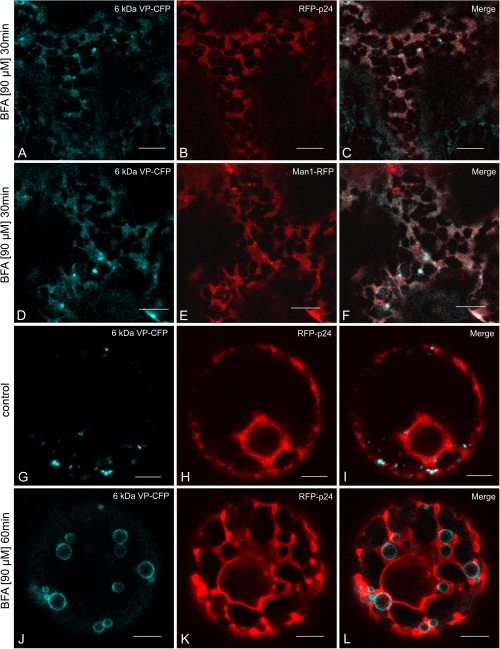
When tobacco leaves were cotransformed with the 6 kDa VP-CFP and either the ER marker RFP-p24 or the cis-Golgi marker Man1-RFP, and then treated with brefeldin A (BFA), the 6 kDa VP-CFP signal was redistributed, like the Man1-RFP signal, throughout the ER network (Fig. 2A–F). Such a re-distribution is typical for Golgi stack markers, as shown on numerous occasions for tobacco leaf epidermal cells (Boevink et al., 1998; Brandizzi et al., 2002b; daSilva et al., 2004; Schoberer et al., 2010). This was not the case with 6 k Da VP-CFP when expressed in protoplasts. In response to BFA, the 6 kDa VP-CFP signal assumed the form of large ring-like structures, very similar to those observed when the 6 kDa VP was over-expressed (see Fig. 1H–J, and Fig. 2J; compare before BFA treatment: Fig. 2G–I). Again, these rings were bordered by, but did not seem to part of the ER (Fig. 2K, L). The formation of these rings was clearly time dependent, being quite small after 20 min BFA treatment (Fig. 3A–C).
Fig. 2.
Effects of BFA on distribution of 6 kDa VP-CFP. (A–F) In leaf epidermal cells after agroinfiltration. (G–L) In mesophyll protoplasts after electroporation. (A–C) Co-expression with the ER marker RFP-p24. (D–F) Co-expression with the Golgi marker Man1-RFP. (G–I) Control protoplasts co-expressing 6 kDa VP-CFP and RFP-p24. (J–L) Protoplasts treated with BFA. Note that the ring-like 6 kDa VP-CFP positive structures do not overlap with the ER marker. Bars=10 μm (A–F), 5 μm (G–L).
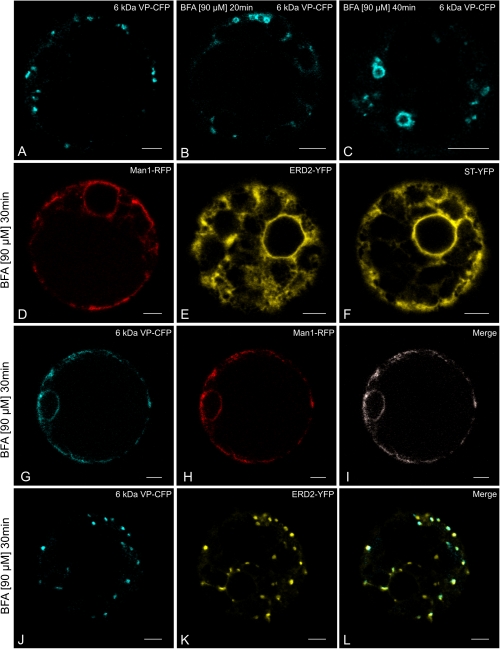
Fig. 3.
Effects of BFA on the distribution of the 6 kDa VP in relation to Golgi marker proteins in tobacco mesophyll protoplasts. (A–C) BFA causes the 6 kDa VP-CFP to form ring-like structures, when the latter is expressed alone. (D–F) The standard Golgi marker proteins (Man1-RFP, ERD2-YFP, ST-YFP) are found in the ER with BFA treatment. (G–I) Both markers are redistributed into the ER after BFA treatment when the 6 kDa VP-CFP is co-expressed with a non-cycling cis-Golgi marker (Man1-RFP). (J–L) BFA fails to relocate the 6 kDa VP-CFP into the ER when co-expressed with the cycling Golgi marker ERD2-YFP. Bars=5 μm.
In contrast to Man1-RFP, coexpressed 6 kDa VP prevents the BFA-induced redistribution of ERD2-YFP into the ER in protoplasts
When expressed singly in tobacco mesophyll protoplasts, both the cis-Golgi markers Man1-RFP and ERD2-YFP and the trans-Golgi marker ST-YFP typically redistribute into the ER after BFA treatment (Fig. 3D–F; Langhans et al., 2011). However, when co-expressed with 6 kDa VP-CFP there is a clear difference between the behaviour of the two cis-Golgi markers towards BFA. Together with Man1-RFP, the 6 kDa VP-CFP signal redistributes into the ER (Fig. 3G–I). By contrast, when coexpressed with ERD2-YFP, both the 6 kDa VP and the Golgi marker remained as punctae (Fig. 3J–L).
ARF1-GTP mutant effects
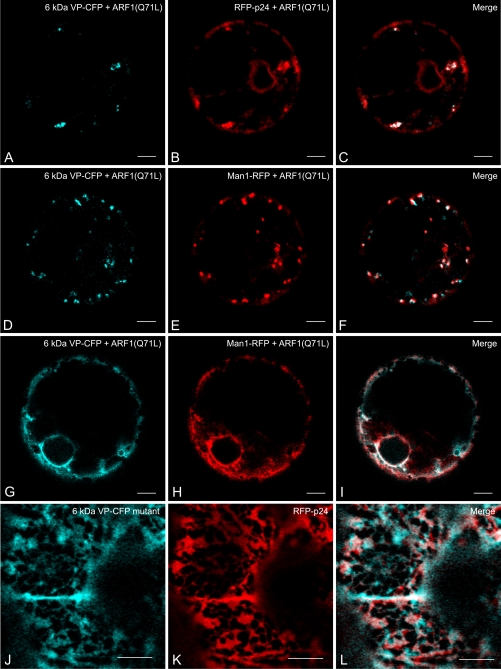
Expression of the GTP-fixed ARF1(Q71L) mutant inhibits COPI vesicle formation (Phillipson et al., 2001). Depending upon the extent of its expression, it can therefore severely reduce retrograde cargo transport from the Golgi, an effect which was previously demonstrated using RFP-p24 which rapidly cycles between the ER and the Golgi apparatus and under steady-state conditions is mainly found in the ER (Langhans et al., 2008). Thus, ARF1(Q71L) expression leads to the retention of RFP-p24 in the Golgi which is reflected by the appearance of fluorescent punctae. When this experiment was performed in the presence of the 6 kDa VP, the VP-CFP signals were seen to co-localize with the RFP-p24 punctae (Fig. 4A–C). Using the same plasmid concentration, expression of the ARF1-GTP mutant did not affect the co-localization of 6 kDa VP-CFP and Man1-RFP punctae (Fig. 4D–F), indicating that the level of ARF1-GTP expression was not sufficient to cause a complete collapse of the Golgi into the ER. This was seen when double the amount of ARF1(Q71L) was electroporated (Fig. 4G–I).
Fig. 4.
(A–I) Effects of low and high expression of the ARF1-GTP mutant ARF1(Q71L) on the 6 kDa VP-CFP and the Golgi marker Man1-RFP, and the ER marker RFP-p24. (A–F) At low levels of expression (0.05 μg), the ARF1-GTP mutant causes a portion of the cycling ER marker RFP-p24 to be retained in the Golgi which is then visible as punctae which co-localize with the 6 kDa VP (A–C). Under these conditions, the 6 kDa VP and Man1-RFP Golgi signals remain punctate (D–F). (G–I) At high levels (0.1 μg) of ARF1-GTP mutant expression both the 6 kDA VP-CFP and Man1-RFP signals redistribute into the ER. (J–L) Deletion of the four N-terminal residues SDSE, causes the 6 kDa VP to be retained in the ER (labelled by RFP-p24). All images are from agro-infiltrated tobacco leaf epidermal cells. Bars=5 μm (A–I), 10 μm (J–L).
An N-terminal deletion mutant of the 6 kDa VP does not exit the ER
The first four amino acids at the N-terminus of the 6 kDa VP contain a classical D(X)E motif (Fig. 1A) for exiting the ER (Hanton et al., 2005; Aniento et al., 2006). When this is deleted, the 6 kDa VP-CFP no longer gives rise to discrete fluorescent punctae, but is distributed throughout the whole of the cortical ER network labelled by RFP-p24 (Langhans et al., 2008) when expressed in tobacco epidermal cells by agroinfiltration (Fig. 4I–L).
6 kDa VP-CFP fluorescence in relation to ERES and Golgi markers at high magnification
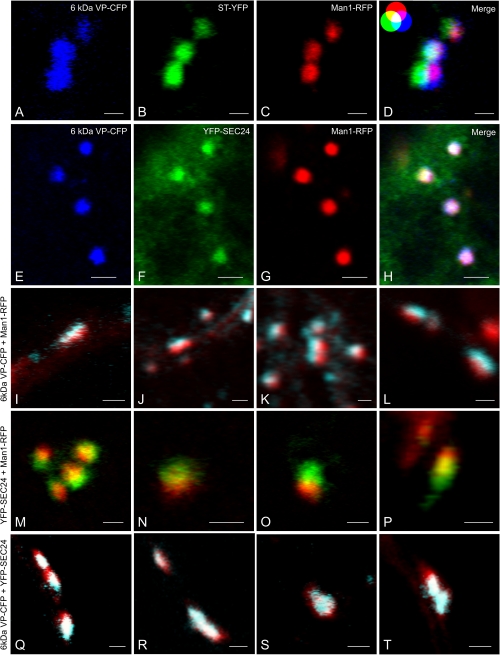
On the basis of the majority of the responses towards BFA and the ARF1(Q71L) mutant expression just described, it would seem that the 6 kDa VP acts like a cis-Golgi located protein. However, since Wei and Wang (2008) came to another conclusion, namely that the 6 kDa VP labelled ERES, it was decided to look more closely at the signals of the 6 kDa VP-CFP versus YFP-SEC24 (COPII), Man1-RFP (cis-Golgi), and ST-YFP (trans-Golgi) in agro-infiltrated tobacco leaf epidermal cells. In doing so, a search was made for both end-on (looking perpendicularly at the cis- or trans-faces), and side-on (looking horizontally at the Golgi stack) views. The former are recognizable by their almost circular profiles, the latter by their more stretched profiles. To eliminate the possibility that the images obtained were a result of optical displacement during the operation of the CLSM, checks were carried out with 0.5 μm and 4 μm TetraSpeck® fluorescent microspheres.
When viewed end-on, the images of the 6 kDa VP-CFP overlapped more or less perfectly with the ERES marker (YFP-SEC24) and the cis-Golgi marker Man1-RFP, but less so with the trans-Golgi marker (ST-YFP) (Fig. 5A–H). The situation was different for side-on images. The side-on images for the 6 kDa VP-CFP and Man1-RFP showed little co-localization: the two lying closely adjacent to one another (Fig. 5I–L). This was also the case with and YFP-SEC24 and Man1-RFP, corresponding to their functions as ERES and cis-Golgi markers, respectively (Fig. 5M–P). Most significantly, the side-one images of the 6 kDa VP-CFP and YFP-SEC24 signals showed a high degree of overlap (Fig. 5Q–T). From the side-on distribution of signals, we can safely say that more 6 kDa VP is present in ERES than in the cis-Golgi.
Fig. 5.
High resolution images of 6 kda VP-CFP labelling in relation to COPII (YFP-SEC24), cis-Golgi (Man1-RFP), and trans-Golgi (ST-YFP) markers. (A–H) End-on views of Golgi stacks and ERES by three-colour imaging. The ‘additive colour model’ is included as an insert in (D) for orientation purposes. Full co-localization between all three colours (blue, green, and red) results in a white colour. (I–P) Side-on views of Golgi and ERES by two-colour imaging. (Q–T) Side-on views of 6 kDa VP and ERES by two-colour imaging. Bars=1 μm (A–D; I–T), 2 μm (E–H).
Discussion
Viral proteins as reporters of membrane trafficking
Viral glycoproteins, such as Vesicular Stomatitis Virus-G protein (VSVG) and Semliki Forest Virus p62 protein, have long been in use as markers to follow membrane trafficking in mammalian cells (Simons et al., 1984; Hirschberg et al., 1998). Although VSVG has been successfully expressed in tobacco protoplasts (Galbraith, 1992) no further work has been performed on plants with this membrane protein. Nor, would it appear, have any attempts been undertaken to exploit the temperature-sensitive mutants of VSVG which have proved to be so useful in visualizing ER–Golgi trafficking in mammals (Presley et al., 1997; Hirschberg et al., 1998). In plants, the use of plant viral proteins as secretory reporters has been minimal. This, in part, reflects a major alternate interest in movement proteins (Benitez-Alfonso et al., 2010; Niehl et al., 2011), but is also due to the fact that plant viral proteins tend to induce the formation of pleiomorphic structures, probably as a consequence of their over-expression. This is certainly the case with the Tomato Spotted Wilt Virus (TSWV) glycoproteins Gn and Gc, which may be regarded as being analagous to VSVG. They appear to use COPII vesicles to exit the ER in tobacco mesophyll protoplasts, but also cause the formation of large ring-like structures, not unlike the ones induced by over-expression of the TEV 6 kDa VP (Ribeiro et al., 2008). However, in contrast to the ring-like structures induced by the TSWV Gn protein which were clearly derived from Golgi membranes (Ribeiro et al., 2008), there was no clear relationship to either the ER or the Golgi apparatus in the case of the TEV 6 kDa VP ring-like structures. Nevertheless, as has been shown, it is possible to avoid the formation of the ring-like structures by expressing the TEV 6 kDa VP at low levels. Since the secretory pathway is otherwise not perturbed, this viral protein could become a useful tool in transient expression studies on membrane traffic in the early secretory pathway of plants.
What is the structure labelled by the 6 kDa VP: ERES, COPII/I vesicles, or the cis-Golgi?
In tobacco leaf cells and protoplasts, fluorescently tagged COPII and cis-Golgi marker proteins co-localize. This has been shown on numerous occasions (see above for literature), and is also the case for the 6 kDa VP. Does the 6 kDa VP therefore reside in ERES or in the cis-Golgi, and if it does enter the cis-Golgi does it remain there or does it recycle via COPI vesicles to the ER?
The distribution of 6 kDa-CFP as against fluorescent COPII and cis-Golgi markers in side-on views of Golgi stacks in the CLSM is strongly in favour of its location at ERES. This is supported by the existence of a di-acidic ER export motif at the N-terminus certainly pointing to the collection of the 6 kDa VP at ERES and to its insertion into a COPII vesicle. However, a closer look at the rest of the cytoplasmic domain of this protein (Fig. 1A) shows the presence of four lysine and two histidine residues. While these positively charged amino acids do not strictly correspond to a canonical dilysine motif for COPI coat binding (Letourneur et al., 1994), they are arranged within a short stretch of 11 amino acids making an interaction with coatomer a likely possibility. If this is indeed so, it would mean that the 6 kDa VP of TEV is a type I membrane protein that constitutively cycles between the ER and the Golgi. This would explain why the 6 kDa VP is never found downstream of the Golgi in the secretory pathway.
The behaviour of the 6 kDa VP towards BFA might be taken as evidence for at least some of it entering the cis-Golgi, although the 6 kDa VP does not behave as a classical Golgi resident protein. Although BFA does give rise to the same phenotype in protoplasts as for Man1-RFP (i.e. redistribution into the ER), it only does so when the 6 kDa VP is expressed together with Man1-RFP. Significantly, it does not when expressed alone. Interestingly, this does not hold for the other Golgi marker ERD2-YFP, which is prevented from entering the ER in the presence of the 6 kDa VP. The reason for this difference may lie in the fact that ERD2, unlike Man1 cycles between the Golgi and the ER (Lee et al., 1993; Townsley et al., 1993), and could point to possible differences in interaction between the 6 kDa VP and the two cis-Golgi membrane proteins. That such interactions must be taking place is, however, an indication that the 6 kDa VP must enter the cis-Golgi, or possibly into a new-subcompartment at the ER/cis-Golgi interface which is created by the expression of the 6 kDa VP.
An unequivocal answer with regard to the location of the 6 kDa VP is probably only possible by immunogold electron microscopy. At present, such data are difficult to deliver. The standard objects previously used for CLSM studies on ERES have been tobacco leaf epidermal cells which, because of their large vacuoles, make them most unsuitable for the preparation of high pressure frozen samples for immunogold electron microscopy. This is not a problem with Arabidopsis roots, and while there should be no great problem in generating a stable transformed myc-tagged 6 kDa VP Arabidopsis line (under the control of an inducible promoter), there is an unexpected caveat: the frequency of visualization of COPII budding events at the ER in Arabidopsis root cells is so low that it makes statistically reliable immunogold labelling virtually impossible to perform.
Acknowledgments
The 6 kDa VP-CFP plasmid was kindly provided by Dr Aiming Wang (Southern Crop Protection and Food Research Centre, AAFC, Canada). We thank Dr Peter Pimpl (Center for Plant Molecular Biology, University of Tübingen, Germany) for cloning RFP-p24 into a binary vector. We also thank Dr Christophe Ritzenthaler (Institut de Biologie Moléculaire des Plantes du CNRS, Université de Strasbourg, France) for providing the binary Man1-RFP construct, and Dr Federica Brandizzi (MSU/DOE Plant Research Laboratory, East Lansing, Michigan, USA) for supplying us with the YFP-SEC24A and ERD2-YFP constructs. Barbara Jesenofsky is thanked for technical assistance. The financial support of the Deutsche Forschungsgemeinschaft (RO 440/14-1) is gratefully acknowledged.
References
- Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO Journal. 2008;27:315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Matsuoka K, Robinson DG. ER-to-Golgi transport: the COPII pathway. In: Robinson DG, editor. The plant endoplasmic reticulum. Plant Cell Monographs. 2006. Vol. 4. 99–124. [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Belov GA, Altan-Bonnet N, Kovtunovych G, Jackson CL, Lippincott-Schwartz J, Ehrenfeld E. Hijacking components of the cellular secretory pathway for replication of poliovirus RNA. Journal of Virology. 2007;81:558–567. doi: 10.1128/JVI.01820-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Alfonso Y, Faulkner C, Ritzenthaler C, Maule AJ. Plasmodesmata: gateways to local and systemic virus infection. Molecular Plant–Microbe Interactions. 2010;23:1403–1412. doi: 10.1094/MPMI-05-10-0116. [DOI] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. The Plant Journal. 1998;15:441–447. doi: 10.1046/j.1365-313x.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Frangne N, Marc-Martin S, Hawes C, Neuhaus JM, Paris N. The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. The Plant Cell. 2002a;14:1077–1092. doi: 10.1105/tpc.000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F, Snapp EL, Roberts AG, Lippincott-Schwartz J, Hawes C. Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: evidence from selective photobleaching. The Plant Cell. 2002b;14:1293–1309. doi: 10.1105/tpc.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck J, Scheuring D, Hummel E, Langhans M, Viotti C, Foresti O, Denecke J, Banfield DK, Robinson DG. The syntaxins SYP31 and SYP81 control ER–Golgi trafficking in the plant secretory pathway. Traffic. 2008;9:1629–1652. doi: 10.1111/j.1600-0854.2008.00803.x. [DOI] [PubMed] [Google Scholar]
- Budnik A, Stephens DJ. ER exit sites: localization and control of COPII vesicle formation. FEBS Letters. 2009;583:3796–3803. doi: 10.1016/j.febslet.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, Glick BS. Sec16 is a determinant of transitional ER organization. Current Biology. 2005;15:1439–1447. doi: 10.1016/j.cub.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Crofts AJ, Leborgne-Castel N, Hillmer S, Robinson DG, Phillipson B, Carlsson LE, Ashford DA, Denecke J. Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. The Plant Cell. 1999;11:2233–2248. doi: 10.1105/tpc.11.11.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva LL, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C, Brandizzi F. Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. The Plant Cell. 2004;16:1753–1771. doi: 10.1105/tpc.022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XS, Liu J, Cheng NH, Folimonov A, Hou YM, Bao Y, Katagi C, Carter SA, Nelson RS. The Tobacco mosaic virus 126-kDa protein associated with virus replication and movement suppresses RNA silencing. Molecular Plant–Microbe Interactions. 2004;17:583–592. doi: 10.1094/MPMI.2004.17.6.583. [DOI] [PubMed] [Google Scholar]
- Foresti O, daSilva LL, Denecke J. Overexpression of the Arabidopsis syntaxin PEP12/SYP21 inhibits transport from the prevacuolar compartment to the lytic vacuole in vivo. The Plant Cell. 2006;18:2275–2293. doi: 10.1105/tpc.105.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme JC, Orci L, Schekman R. Coordination of COPII vesicle trafficking by Sec23. Trends in Cell Biology. 2008;18:330–336. doi: 10.1016/j.tcb.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Galbraith DW, Zeiher CA, Harkins KR, Alfonso CL. Biosynthesis, processing and targeting of the G-protein of vesicular stomatitis virus in tobacco protoplasts. Planta. 1992;186:324–336. doi: 10.1007/BF00195312. [DOI] [PubMed] [Google Scholar]
- Hanton SL, Chatre L, Matheson LA, Rossi M, Held MA, Brandizzi F. Plant Sar1 isoforms with near-identical protein sequences exhibit different localisations and effects on secretion. Plant Molecular Biology. 2008;67:283–294. doi: 10.1007/s11103-008-9317-5. [DOI] [PubMed] [Google Scholar]
- Hanton SL, Chatre L, Renna L, Matheson LA, Brandizzi F. De novo formation of plant endoplasmic reticulum export sites is membrane cargo induced and signal mediated. Plant Physiology. 2007;143:1640–1650. doi: 10.1104/pp.106.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton SL, Matheson LA, Chatre L, Brandizzi F. Dynamic organization of COPII coat proteins at endoplasmic reticulum export sites in plant cells. The Plant Journal. 2009;57:963–974. doi: 10.1111/j.1365-313X.2008.03740.x. [DOI] [PubMed] [Google Scholar]
- Hanton SL, Renna L, Bortolotti LE, Chatre L, Stefano G, Brandizzi F. Diacidic motifs influence the export of transmembrane proteins from the endoplasmic reticulum in plant cells. The Plant Cell. 2005;17:3081–3093. doi: 10.1105/tpc.105.034900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes C, Osterrieder A, Hummel E, Sparkes I. The plant ER–Golgi interface. Traffic. 2008;9:1571–1580. doi: 10.1111/j.1600-0854.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, Phair RD, Lippincott-Schwartz J. Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells. Journal of Cell Biology. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H, Budnik A, Schmidt K, et al. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. Journal of Cell Science. 2009;122:2924–2934. doi: 10.1242/jcs.044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BH, Staehelin LA. ER-to-Golgi transport by COPII vesicles in Arabidopsis involves a ribosome-excluding scaffold that is transferred with the vesicles to the Golgi matrix. Protoplasma. 2008;234:51–64. doi: 10.1007/s00709-008-0015-6. [DOI] [PubMed] [Google Scholar]
- Laliberte JF, Sanfacon H. Cellular remodeling during plant virus infection. Annual Review of Phytopathology. 2010;48:69–91. doi: 10.1146/annurev-phyto-073009-114239. [DOI] [PubMed] [Google Scholar]
- Langhans M, Förster S, Helmchen G, Robinson DG. Journal of Experimental Botany. 2011. Differential effects of the brefeldin A analogue (6R)-hydroxy-BFA in tobacco and Arabidopsis. 10.1093/jxb/err007. [DOI] [PubMed] [Google Scholar]
- Langhans M, Hawes C, Hillmer S, Hummel E, Robinson DG. Golgi regeneration after brefeldin A treatment in BY-2 cells entails stack enlargement and cisternal growth followed by division. Plant Physiology. 2007;145:527–538. doi: 10.1104/pp.107.104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans M, Marcote MJ, Pimpl P, Virgili-Lopez G, Robinson DG, Aniento F. In vivo trafficking and localization of p24 proteins in plant cells. Traffic. 2008;9:770–785. doi: 10.1111/j.1600-0854.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- Lee HI, Gal S, Newman TC, Raikhel NV. The Arabidopsis endoplasmic reticulum retention receptor functions in yeast. Proceedings of the National Academy of Sciences, USA. 1993;90:11433–11437. doi: 10.1073/pnas.90.23.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Marti L, Fornaciari S, Renna L, Stefano G, Brandizzi F. COPII-mediated traffic in plants. Trends in Plant Science. 2010;15:522–528. doi: 10.1016/j.tplants.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Miller EA, Barlowe C. Regulation of coat assembly: sorting things out at the ER. Current Opinion in Cell Biology. 2010;22:447–453. doi: 10.1016/j.ceb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenfuehr A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA. Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiology. 1999;121:1127–1142. doi: 10.1104/pp.121.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehl A, Heinlein M. Cellular pathways for viral transport through plasmodesmata. Protoplasma. 2011;248:75–99. doi: 10.1007/s00709-010-0246-1. [DOI] [PubMed] [Google Scholar]
- Osborne AR, Rapoport TA, van den Berg B. Protein translocation by the Sec61/SecY channel. Annual Review of Cell and Developmental Biology. 2005;21:529–550. doi: 10.1146/annurev.cellbio.21.012704.133214. [DOI] [PubMed] [Google Scholar]
- Phillipson BA, Pimpl P, daSilva LL, Crofts AJ, Taylor JP, Movafeghi A, Robinson DG, Denecke J. Secretory bulk flow of soluble proteins is efficient and COPII dependent. The Plant Cell. 2001;13:2005–2020. doi: 10.1105/TPC.010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpl P, Hanton SL, Taylor JP, Pinto-DaSilva LL, Denecke J. The GTPase ARF1p controls the sequence-specific vacuolar sorting route to the lytic vacuole. The Plant Cell. 2003;15:1242–1256. doi: 10.1105/tpc.010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Ribeiro D, Foresti O, Denecke J, Wellink J, Goldbach R, Kormelink RJ. Tomato spotted wilt virus glycoproteins induce the formation of endoplasmic reticulum- and Golgi-derived pleomorphic membrane structures in plant cells. Journal of General Virology. 2008;89:1811–1818. doi: 10.1099/vir.0.2008/001164-0. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Herranz MC, Bubeck J, Pepperkok R, Ritzenthaler C. Membrane dynamics in the early secretory pathway. Critical Reviews in Plant Sciences. 2007;26:199–225. [Google Scholar]
- Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Schaad MC, Jensen PE, Carrington JC. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO Journal. 1997;16:4049–4059. doi: 10.1093/emboj/16.13.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoberer J, Runions J, Steinkellner H, Strasser R, Hawes C, Osterrieder A. Sequential depletion and acquisition of proteins during Golgi stack disassembly and reformation. Traffic. 2010;11:1429–1444. doi: 10.1111/j.1600-0854.2010.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Chen J, Lee WM, Janda M, Ahlquist P. Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication. Proceedings of the National Academy of Sciences, USA. 2004;101:11263–11268. doi: 10.1073/pnas.0404157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Warren G. Semliki Forest virus: a probe for membrane traffic in the animal cell. Advances in Protein Chemistry. 1984;36:79–132. doi: 10.1016/S0065-3233(08)60296-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA, Kang BH. Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiology. 2008;147:1454–1468. doi: 10.1104/pp.108.120618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley FM, Wilson DW, Pelham HR. Mutational analysis of the human KDEL receptor: distinct structural requirements for Golgi retention, ligand binding and retrograde transport. EMBO Journal. 1993;12:2821–2829. doi: 10.1002/j.1460-2075.1993.tb05943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Denecke J. The endoplasmic reticulum: gateway of the secretory pathway. The Plant Cell. 1999;11:615–628. doi: 10.1105/tpc.11.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ. Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic. 2006;7:1678–1687. doi: 10.1111/j.1600-0854.2006.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Wang A. Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. Journal of Virology. 2008;82:12252–12264. doi: 10.1128/JVI.01329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Elamawi R, Bubeck J, Pepperkok R, Ritzenthaler C, Robinson DG. Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites. The Plant Cell. 2005;17:1513–1531. doi: 10.1105/tpc.104.026757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuschner D, Geerts WJ, van Donselaar E, Humbel BM, Slot JW, Koster AJ, Klumperman J. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nature Cell Biology. 2006;8:377–383. doi: 10.1038/ncb1371. [DOI] [PubMed] [Google Scholar]
- Zhang C, Kotchoni SO, Samuels AL, Szymanski DB. SPIKE1 signals originate from and assemble specialized domains of the endoplasmic reticulum. Current Biology. 2010;20:2144–2149. doi: 10.1016/j.cub.2010.11.016. [DOI] [PubMed] [Google Scholar]