Abstract
Single-domain antibodies (sdAbs) found in camelids, lack a light chain and their antigen-binding site sits completely in the heavy-chain variable domain (VHH). Their simplicity, thermostability, and ease in expression have made VHHs highly attractive. While this has been successfully exploited for macromolecular antigens, their application to the detection of small molecules is still limited to a very few reports, mostly describing low affinity VHHs. Using triclocarban (TCC) as a model hapten, we found that conventional antibodies, IgG1 fraction, reacted with free TCC with a higher relative affinity (IC50 51.0 ng/mL) than did the sdAbs (IgG2 and IgG3, 497 and 370 ng/mL, respectively). A VHH library was prepared, and by elution of phage with limiting concentrations of TCC and competitive selection of binders, we were able to isolate high-affinity clones, KD 0.98–1.37 nM (SPR) which allowed development of a competitive assay for TCC with an IC50 = 3.5 ng/mL (11 nM). This represents a 100-fold improvement with regard to the performance of the sdAb serum fraction, and it is 100-fold better than the IC50 attained with other anti-hapten VHHs reported thus far. Despite the modest overall anti-hapten sdAbs response in llamas, a small subpopulation of high affinity VHHs are generated that can be isolated by carefully design of the selection process.
Keywords: sdAbs, triclocarban, phage display, small-molecule, competitive ELISA
INTRODUCTION
Since their discovery 1, single domain antibodies (sdAbs) have emerged as highly attractive novel candidates for therapeutic and biotechnological applications. Indeed, there are several unique features of sdAbs that make them particularly useful for this purpose. The variable domain of sdAbs (VHH) is the smallest antibody-binding fragment (about 16 kDa) that retains antibody specificity, and despite the fact that only three CDRs conform the antigen-binding site, they bind their cognate antigen with similar affinity as conventional antibodies 2. VHHs are readily expressed in soluble form in the E. coli periplasm at much higher expression levels than conventional antibody fragments 3. Their small size and easy production has prompted their use as fusion partners to produce recombinant chimeric constructs 4, or multimeric formats that reinforce the avidity of the interaction 5. These antibody fragments are also unique in that they possess a remarkable thermostability 2. This unusual stability has been interpreted on the basis of the reversibility of their thermal unfolding, which could also occur at a high temperature, through an antigen-induced folding mechanism 6. In addition to their biophysical features, a major advantage of sdAbs is that highly complex libraries of VHH expressed on the tip of the filamentous phage M13 can be easily assembled and selected. Contrary to what happens with conventional antibodies, where the original pairing of the heavy and light chain is shuffled during the amplification and cloning steps of library construction, the single-chain nature of mono-domain antibodies assures that the original specificity of the parent antibody is always retained, and is therefore highly represented in the VHH library.
The advantageous characteristics of VHHs have led to their use in countless applications, where they exceed the performance of conventional antibody fragments 3. In the vast majority of these developments the target antigens are macromolecules and there are only a few reports on the generation of VHHs against small molecules (haptens). Among other reported small molecules, sdAbs have been prepared to the plant hormone auxin 7, the Reactive Red azo-dye 8, caffeine 9, trinitrotoluene 10, the herbicide picloram 11 and the drug methotrexate 12. Despite the fact that a large and important group of compounds of medical, toxicological and environmental analytical interest are small molecules, the practical aspects and usefulness of the generation of anti-hapten sdAbs has not been clearly established and remains controversial. Studies that compared the conventional (IgG1) or mono-domain (IgG2 and IgG3) antibody response to haptens have consistently found that the anti-hapten IgG2 and IgG3 titers are much lower than that of IgG1 10, 13. This comes as no surprise, considering that conventional antibodies can conveniently accommodate their cognate haptens in deep pockets built at the interface of the heavy and light chain variable domains. In this way up to 85% of the hapten can be buried in the binding pocket, providing a large contact area that accounts for the high affinity of the interaction 14.
Apparently to compensate for the lack of light chain, sdAbs use all CDRs (complementarity determining regions) to create hapten binding cavities that also include some framework residues 8. Thus, the especially long CDR3 commonly found in anti-hapten VHHs appears to play a key role in the formation of this pocket, by bending towards the face of the VHH that would pair with the light chain variable domain in conventional antibodies 15. This hapten-binding approach does not appear very efficient, at least based on the modest affinity of anti-hapten VHHs reported in the literature (table 1). With the exception of the anti-Reactive Red azo-dye antibodies, the affinities of all other VHHs are in the micromolar range. It is important to note that in most cases the affinity was measured by surface plasmon resonance (SPR) using immobilized hapten-carrier conjugates on the chip. Therefore, the reported values correspond to the affinity of the VHH antibody for the chemically modified hapten molecule. This is not a minor point because the cross-reactivity between the immobilized hapten and the free compound may differ dramatically, as can be seen in table 1. When the SPR measurement was done using the free compound (values in parentheses) the Kd values were much higher. This was also observed when the VHHs were used in competitive assays (IC 50 column of table 1), high concentrations of the free compound were necessary to inhibit the binding of the VHH to the immobilized hapten.
Table 1.
Main features of anti-hapten VHHs
| Hapten (MW) | Isolation method | Biopanning elution | IC 50 (nM) | KD (KDf) (nM) | CDR3 Length (mer) |
|---|---|---|---|---|---|
| Picloram11 (241 Da) | Ribosome display (naïve llama) | RNA | ND | 3000 (8.0 105) | 5 |
| IAA7 (175 Da) | Phage display (naïve llama) | TEA | ND | 5000 (20000) | 19 |
| DON 16 (296 Da) | Phage display | TEA | 1240 | 5000 | 14 |
| Methotrexate12 (454 Da) | Phage display (lib size 106) | TEA | 10000 | 29 (80) | 17 |
| Caffeine9 (194 Da) | Phage display (lib size 106) | TEA | 67000 | ND | 12 |
| Caffeine17 (194 Da) | Grafting of anti-caffeine CDR in VHH anti- RNase A | TEA | 1200 | ND | 12 |
| Reactive red azo dye18 (728 Da) | Episomal expression in S. cerevisiae | Unselected library | ND | 18 | 14 |
| Reactive red azo dye8 (728 Da) | Episomal expression in S. cerevisiae | Unselected library | ND | 22 | 14 |
ND: Not determined; TEA: 0.1 M Triethylamine pH 11; IC50: Concentration of hapten causing 50% inhibition of the antibody binding in competitive ELISA; KD: Dissociation constant for the immobilized hapten; KDf: KD for the free hapten.
In this work we used triclocarban (TCC) 3,4,4′-trichlorocarbanilide, a broad spectrum bactericide and fungicide widely used in soaps, disinfectants, and other household products, as a model hapten to further explore the practical aspects and usefulness of producing VHH against haptens. We focused our study on the llama antibody response to the free hapten. The anti-hapten responses were dominated both quantitatively and qualitatively by conventional IgG1 antibodies. We demonstrated that high affinity sdAbs developed, but they are a minor fraction of the sdAbs response and require careful design of the panning strategy to allow their isolation from VHH libraries.
MATERIALS & METHODS
Materials
Triclocarban (TCC), TCC analog (Carbanilide), TCC metabolites (2′-OH TCC and Sulfate of 2′-OH TCC), TCC related compounds(3-Trifluoromethyl-4,4′-dichlorocarbanilide, triclosan, dinuron, 4,4′-Dichlorocarbanilide, 3,3′,4,4′-Tetrachloro-carbanilide, soluble epoxyhydrolase inhibitors (sEHi, #1709 and sEHi, #1555), TCC coupled to BSA (TCC-BSA), thyroglobulin (Thy) were kindly provided by Dr. Shirley Gee (Entomology Department, UC Davis, CA, USA). In this conjugate the chlorine atom of TCC at position 4′ is substituted by the sulfur atom of a 3-mercaptopropionic acid moiety that is used for conjugation to the carrier protein. The mouse anti-llama total IgG was prepared by immunization of mice with repeated injections of 40 μg of each IgG subclass. All other reagents were from Sigma-Aldrich (Saint Louis, MO, USA) unless otherwise stated.
Llama immunization
Two adult male llamas (Lama glama) #807 and #856 from the Montevideo municipal zoo were immunized according to animal welfare regulations, intramuscularly with 600 μg of the TCC-Thy conjugate in Freund Incomplete Adjuvant at days 0, followed by 4 booster injections every 3 weeks. One month after the last booster 150 mL of blood was collected in double blood collection bags with anticoagulant. Additionally, 10 mL was collected in plastic tubes to obtain the serum fraction.
Purification of IgG subclasses
Llama IgG isotypes were obtained from the sera using protein A and protein G (GE healthcare) as described1 with minor modifications. After dialysis against PBS, serum fractions were analyzed by SDS-PAGE under reducing conditions. The reactivity of IgG subclasses against TCC and thyroglobulin were evaluated by ELISA assays as described in the ELISA protocol.
Lymphocyte isolation, RNA extraction and library construction
Peripheral blood lymphocytes were isolated from 150 mL of blood by centrifugation on Histopaque 1077 gradients (Sigma) according to the manufacturer’s instructions. Total RNA was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) from about 107 cells. The concentration of RNA was calculated measuring the absorbance at 260 nm in a Nano Drop (Thermo Scientific, Fremont, CA). The superscript III first-strand synthesis system for RT-PCR (Invitrogen) and the primer JH (Supporting information) were used to synthesize cDNA. DNA fragments encoding the VH and VHH IgG variable domains were amplified by PCR using the set of primers forward (Supporting information). The SfiI sites introduced with the primers were used for subsequent cloning of the fragments into the phagemid pComb3X vector, a kind gift from Dr. Barbas (The Scripps Research Institute, La Jolla, USA). This vector encodes an in-frame 6xHis tag downstream of the cloning site, followed by the encoding sequence of the HA (hemagglutining epitope), a stop amber codon, and a truncated version of the pIII coat protein of M13. The expression of the inserted gene is under the control of the lacZ promoter and after induction with isopropyl-β-d-thiogalactopyranoside (IPTG), even in suppressor cells, an important proportion of the recombinant protein is secreted to the media as VHH-6xHis-HA recombinant protein. The ligated vector was electroporated in ER2738 E. coli cells.
Panning and selection of high affinity anti-TCC clones
Microtiter ELISA (Maxisorp, Nunc,) plates were coated with 1 μg/mL of TCC-BSA overnight at 4 °C. After coating, wells were blocked with 5% skimmed milk-PBS (250 μL). The antibody library (1012 transducing units) was mixed with PBS, 0.05% Tween 20 (PBST) containing 1% of BSA, and dispensed into 3 microtiter wells coated with the conjugate. After incubating for 2 h at 4 °C, plates were washed 10 times, incubated half an hour in PBST at 4 °C, and washed again 10 times, after which the bound phages were eluted by incubation with 100 μL per well of 100 mM glycine-HCl, pH 2.2 for 10 min. The eluted phage was then neutralized by addition of 2 M Tris Base. Alternatively, we performed competitive elution using low concentrations of free hapten by adding 100 μL of 100, 20 and 5 ng/mL of TCC in PBST and incubating for 1 hour at room temperature in the first, second and third round of panning, respectively. Individual clones were selected and cultured overnight. The induced supernatants were diluted two-fold in 5% skimmed milk-PBS and added to ELISA microplates wells coated with BSA, or TCC-BSA in the presence of different concentrations of TCC, ranging from 0 to 1 μg/mL. After 1 h of incubation, and washing 5 times with PBST, positive clones were detected using an anti-HA antibody conjugate to peroxidase. A detailed explanation of the selection method is shown in scheme S-1, supporting information.
Expression and purification of VHHs
For expression, the VHH genes were cloned in the pET 28a(+), flanked by the coding sequences of the ompA signal peptide at the 5′ end, and the 6 x His and the HA epitope coding sequences. The vector was transformed into BL21(DE3) E. coli, individual clones were grown in LB-kanamicyn (40 μg/mL) plates and antibody expression was induced with 1mM of IPTG during 4 hours at 37 °C. Cells were pelleted and the periplasmic proteins were extracted by osmotic shock as described previously 19. Antibody purification was performed on Ni-NTA columns in the ÄKTA purification system (GE Healthcare, Uppsala, Sweden) according to the manufacturer’s instructions.
ELISA protocol
ELISA plates (Nunc Maxysorb) were coated with 100 μL/well of 1 μg/mL of TCC-BSA, BSA, or Thy in phosphate-buffered saline (PBS) overnight at 4 °C. After blocking with 5% skimmed milk-PBS, serial dilutions of purified IgG subclasses or serum were added and incubated 1 h at room temperature. After washing, a 1/1000 dilution of the mouse anti-llama IgG serum was added and incubated 1 h, followed by washing and incubation with anti-mouse HRP (Pierce, Rockford, IL, USA) for 1 h. After extensive washing, the peroxidase activity was developed by adding 100 μl of peroxidase substrate (0.4 ml of 6 mg 3,3′,5,5′-tetramethylbenzidine in 1 ml of DMSO-0.1 ml of 1% H2O2 in water in a total of 25 ml of 0.1 M acetate buffer, pH 5.5) and incubated for 20 min at room temperature. The enzyme reaction was stopped by the addition of 50 μl of 2 N H2SO4, and the absorbance was read at 450 nm, For competitive ELISA, checkerboard assays were performed for each clone to determine the optimal amounts of coating antigen and VHH concentration. Anti-HA HRP was used for detection of VHH (Roche, Boulder, Colorado, USA).
RESULTS AND DISCUSSION
Total antibody response to TCC and Thy
Two llamas were immunized with TCC-Thy as described. Thirty days after the fourth booster, the sera were analyzed on ELISA plates coated with TCC-BSA or the carrier protein (figure S-1, supporting information). Both animals raised antibodies to TCC and the carrier, but the antibody titer was moderate, probably due to the fact that for animal care reasons only FIA adjuvant was used throughout the immunizing protocol. In both cases the response to TCC was much stronger than that against the carrier. The anti-TCC antibody titer of the llama 807 serum was about four-fold higher than that of llama 856, and therefore the material obtained from this animal was selected for this work.
Subclass antibody response to TCC
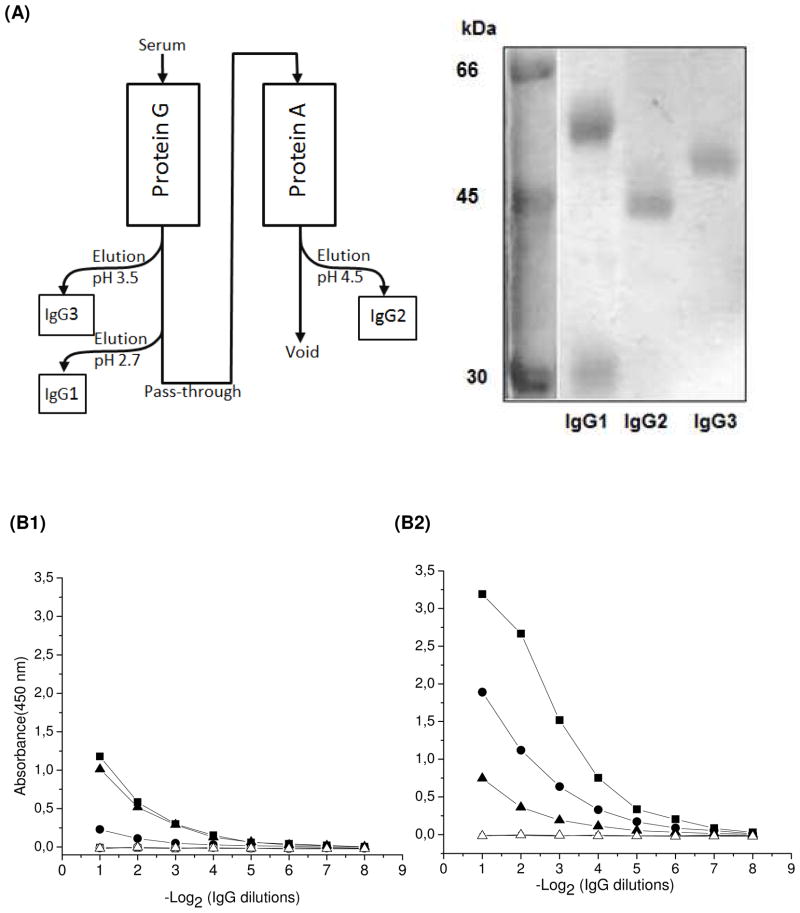
In order to study this response, the IgG1, IgG2 and IgG3 subclasses were purified from the serum collected from the last bleeding of llama 807, using the protein G-protein A protocol as described by Hamer-Casterman et al. (figure 1A). The right panel of the figure displays the SDS-PAGE analysis of the purified antibodies showing that homogeneous preparations were obtained for each subclass, with their characteristic band pattern: IgG1 25 and 50 kDa, IgG2 43 kDa, and IgG3 46 kDa. As shown in figure 1B, all subclasses reacted with the hapten. Among the IgG subclasses, the IgG1 response was markedly stronger, followed by IgG2, and IgG3. A similar response has also been obtained for other haptens 10, 13. Comparatively, the individual reactivity of each IgG fraction with the carrier was weaker except for IgG3, which showed a similar poor response to both antigen moieties (TCC and Thy).
Figure 1. Subclass antibody responses to TCC and Thy.
Top: (A), left panel, scheme of the protocol used for IgG separation by protein G and protein A chromatography 1. Right panel, electrophoretic analysis of the purified IgG fractions on a 10% SDS-PAGE gel run under reducing conditions and stained with coomassie blue; molecular markers are shown on the left lane. Bottom: Reactivity of the three IgG subclasses against Thy (B1) or TCC-BSA (B2), using BSA as control (white symbols). The starting concentration of all antibodies was adjusted to 1 mg/mL. IgG1 (squares), IgG2 (circles) and IgG3 (triangles).
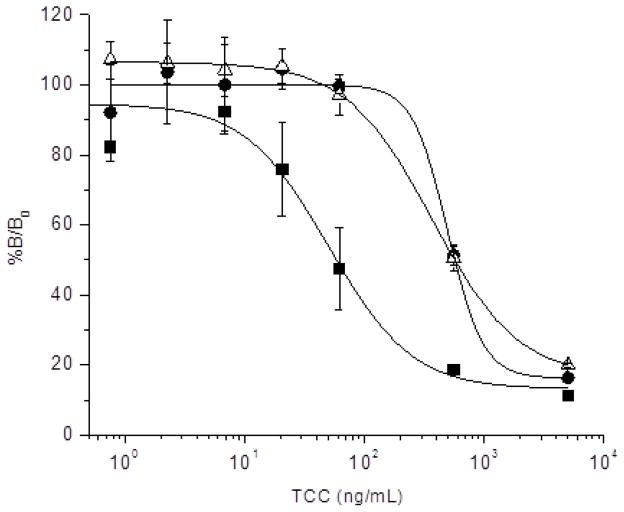
Hapten conjugates are needed in order to raise anti-hapten antibodies. However, in this process the target compound is chemically modified so it can be coupled to the carrier protein, and it is presented, as such, to the immune system of the animal host. Therefore, the anti-hapten antibodies are actually raised against the modified compound. Thus, to what extent these antibodies will cross-react with the free hapten needs to be studied. To do this, in addition to their binding to the conjugated hapten, the individual IgG subclasses were tested for their capacity to bind free TCC in a competitive format. After checkerboard optimization of the TCC-BSA coating amount and antibody concentration, competitive assays were set up with each of the three IgG fractions (figure 2). The concentration of TCC causing 50% inhibition (IC50) was 51.0 for IgG1, 497 for IgG2, and 370 ng/mL for IgG3. These values are rather high, particularly with regard to the potential use of these antibodies for analytical purposes, where IC50 values in the low ng/mL range would be needed. The conventional antibody response provided the best recognition of TCC, with an overall binding strength several-fold higher than IgG3 and IgG2. To conclude whether this is case-specific or a generic result requires further analysis, but a priori, it is not surprising that the pocket formed between the heavy- and light-chain variable domains of conventional antibodies provides a larger surface that contributes to the energetics of the binding. In spite of the unpromising overall affinity of the IgG2 and IgG3 responses, we proceeded with the construction of a VHH library on the premise that a careful selection process would allow us to isolate high affinity binders.
Figure 2. TCC competitive ELISA with individual IgG subclasses purified from llama 807 serum.
Representative curves showing the inhibition of binding of llama antibodies to TCC-BSA by increased amounts of free TCC. IgG1 (squares), IgG2 (circles), and IgG3 (triangles).
Library construction, panning and selection of TCC-specific VHH domains
Total RNA was extracted from 108 peripheral blood lymphocytes from llama 807 and retrotranscribed to cDNA, amplified and clone in pComb3X as described.. The phage displayed VH-VHH library, which had a size of 3.4 × 107 clones, was panned against TCC-BSA. Initial panning experiments using acidic elution proved to be unsuccessful because, despite the high rate of TCC-BSA-positive and BSA-negative phage clones, their binding was hardly inhibited by soluble TCC. On the basis of this result, we devised a new panning strategy by using decreasing concentration of the free hapten in the successive rounds of panning, and selected clones from the third round by differential screening on plates coated with limiting amounts of TCC-BSA in the presence or absence of serial dilutions of TCC in the 0–1000 ng/mL range. Clones showing the best different in the absence or presence of 20 ng/ml of TCC were selected.
Sequence alignment of antibody fragments to TCC
DNA sequencing revealed that five different clones were selected, all corresponding to sdAbs and not to conventional antibodies. All contain the hallmark substitutions in FR 2, which stabilize the structure of single domain antibodies and are characteristic of VHHs. These are residues F, E, R and F/G at positions 42, 49, 50 and 52, respectively (figure S-2, supporting information). Moreover, as frequently found in these antibodies, the variable domains possess long CDR3 regions (15–22 amino acids). Based on the classification of the VHH heavy-chain regions proposed by Harmsen et al. 20, clones T4-10 appear to belong to subfamily 1, while the VHH domain of clone T11 shows two additional cysteine residues, at positions 55 in FR2 and inside the CDR3, which are characteristic of subfamily 3. Except for the significant similarity between clones T9 and T4, all other clones present important differences in their CDRs. Interestingly, this observation suggests that they derived from independent B cell clones and recognized TCC in a different way.
Competitive ELISA for TCC
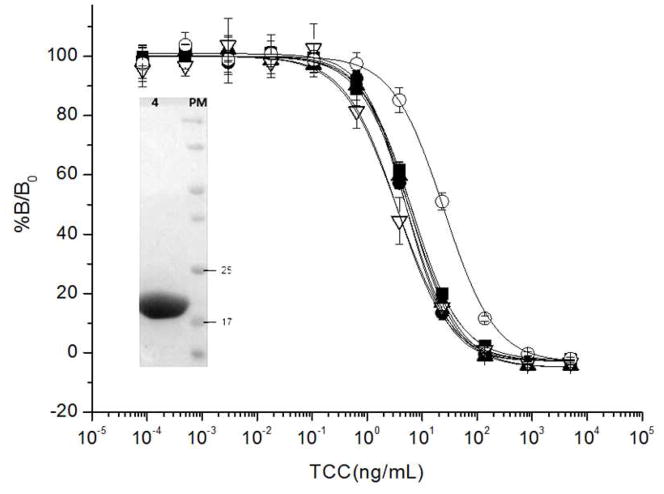
Each of the single domain antibodies fragment were cloned into the pET 28-a(+) vector using the OmpA signal peptide for periplasmic expression in E. coli BL21. Typically, about 30 mg of homogeneous recombinant VHHs per liter of culture were obtained after purification of the periplasmic extract on Ni-NTA affinity columns (insert in figure 3). The purified VHHs were then tested for their performance in competitive assays using serial dilutions of the free hapten, after checkerboard optimization of the coating antigen (TCC-BSA) and VHH concentration (figure 3). All clones showed a similar behavior, with IC50 values between 3.5 and 6.4 ng/mL (11–20 nM), except for clone T10, which had a higher IC50 (25 ng/mL). In all cases, the IC50 was notably lower that the average value attained with the llama serum, indicating that the strategy used for phage selection was appropriate to isolate the TCC-high-affinity VHH subpopulation. Actually, these values are about one hundred times better than any of the previously reported values for anti-hapten VHHs, table 1.
Figure 3. Competition ELISA with VHH clones.
Representative curves showing the inhibition of the VHH binding to TCC-BSA with increasing amounts of TCC. T4 (black squares), T7 (black circles), T9 (black triangles), T10 (open circles) and T11 (open triangles). Each point represents the average of seven replicates. The concentrations of coating antigen - purified VHH used in the assays were, 125-31, 125-125, 125-63, 250-125, 250-31 ng/mL for T4, T7, T9, T10 and T11, respectively. The insert shows a typical PAGE analysis of a VHH (clone T9) purified by Ni-NTA and run on a 12% SDS-gel.
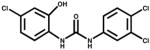
The specificity of the assay was tested for clone T9, which exhibited one of the best IC50 values, using TCC-related compounds (Table 2). Overall, insignificant cross reactivity was observed, except for some modest reactivity with structurally highly similar compounds such as 2′-OH TCC (CR% = 16.9%), one of the major metabolites of TCC differing only by the presence of a single hydroxyl group at position 2′, followed by the dichloro TCC variant with (CR% = 13.3%).
Table 2.
Cross-Reactivity (CR) of the TCC competitive ELISA set up with VHH T9
| Compound | Structure | CR (%) | Remarks |
|---|---|---|---|
| TCC |
|
100 | Antimicrobial |
| 2′-OH TCC |
|
16.90 | Metabolite of TCC |
| 4,4′-Dichlorocarbanilide |
|
13.3 | Impurity during TCC synthesis |
| (3-Trifluoromethyl- 4,4′-dichlorocarbanilide) |
|
6.97 | Antimicrobial |
| 3,3′,4,4′-Tetrachloro- carbanilide |
|
2.46 | Impurity during TCC synthesis |
| Carbanilide |
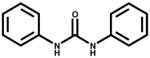
|
1.78 | Analog |
| Sulfate of 2′-OH TCC |
|
<0.1 | Metabolite of TCC |
| Triclosan |
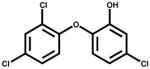
|
<0.1 | Antimicrobial |
| Dinuron |
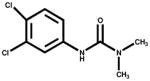
|
<0.1 | Herbicide |
| sEHi, #1555 (*) |
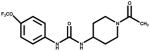
|
<0.1 | sEH inhibitor |
| sEHi, #1709 (*) |
|
<0.1 | sEH inhibitor |
CR% = IC50 (TCC) ÷ IC50 (cross-reacting compound) × 100.
Soluble epoxy hydrolase inhibitors.
Surface Plasmon Resonance characterization
The affinity of three anti-TCC VHH clones was determined by SPR analysis on a Biacore 3000 instrument (Biacore AB, Uppsala, Sweden) using sensor chip coated with TCC-BSA (see supporting information for details). The sensorgrams of T4, T7 and T9 VHH clones are shown in supporting information figure S-3, panels A-C, respectively. All VHHs bound to TCC–BSA covalently immobilized on the CM5 sensor chip, which was not affected by addition of BSA, showing the specificity of interaction. The VHH equilibrium dissociation constants (KD) were obtained from the sensorgrams globally fitted with a heterogeneous ligand model related to TCC site accessibility, table 3. This produced two affinity values (lowest and best) for each VHH, which correspond to the average less- and more-favored oriented interaction of the VHH with the immobilized hapten, denoted in table 3 by the subscripts a and b, respectively. Both KD values obtained for each VHH with the heterogeneous ligand model fitting approach were very similar. The three VHHs tested reacted with high affinity with the immobilized hapten, with KD values in the nanomolar range (0.99 to 3.77 nM).
Table 3.
Kinetics and affinity constants of the binding of anti-TCC VHH clones to immobilized TCC–BSA
| VHH | ka1 (M−1 s−1) | kd1 (s−1) | KD1 (nM) | ka2 (M−1 s−1) | kd2 (s−1) | KD2 (nM) |
|---|---|---|---|---|---|---|
| T4 | 1.88 E+05 | 7.08 E-04 | 3.77 | 1.78 E+04 | 1.75 E-05 | 0.98 |
| T7 | 2.62 E+04 | 6.49 E-05 | 2.48 | 3.29 E+05 | 4.52 E-04 | 1.37 |
| T9 | 3.69 E+05 | 5.92 E-04 | 1.60 | 4.21 E+04 | 4.64 E-05 | 1.10 |
Kinetics and affinity constants were obtained from fitting data shown in Figure S-3, panels A- C in the Supporting Information, with a two heterogeneous ligand parallel reaction model (BIAevaluation software).
We also used SPR to study the interaction of the free analyte with the immobilized antibody fragments. To this end, VHH T9 was immobilized at 1500 RU onto one flow cell of a CM5 sensor chip and different concentrations of TCC in the 50 to 800 nM range were injected (supporting information figure S-3, D). Due to the low molecular weight of TCC (315 Da) and the high drifts of the Req parameter, it was difficult to obtain a reliable KD value. An estimation of the KD using a 1:1 Langmuir model indicated that the KD for the interaction of VHH T9 with the free hapten is about one order of magnitude higher than the KD value obtained with the immobilized hapten, which is in agreement with the IC50 (11 nM) obtained in the competitive ELISA with VHH T9.
Thermal Stability of VHH anti TCC clones
The endurance of antibodies to physical and chemical perturbation is a key element to warrant their prolonged functionality in any application. In order to analyze this matter, we studied the thermal stability of our VHHs (1 mg/mL in PBS) at different temperatures and incubation times. After heating in a thermocycler, the samples were cooled down to room temperature and their reactivity with TCC-BSA analyzed by ELISA, supporting information figure S-4, All VHHs were more stable than conventional antibodies, which were inactivated immediately. Short time exposure of VHHs revealed that not all of them withstand heat in the same way. At 85°C, VHH T10 rapidly lost its reactivity while VHH T7 retained 60% of its reactivity after one hour; at 100°C, both of these antibodies were quickly inactivated. All other VHHs remained active, even after one hour at 100°C. VHHs T4 and T11, belonging to VHH subfamilies 1 and 3, respectively, were further heated at 100°C for 9 h. Both were inactivated at the end of the incubation period, but VHH T4 was inactivated at a lower rate. This was somehow unexpected because VHH T11 has and additional disulfide bridge between CDR3 and FR2, which has been suggested as a further stabilization element of the VHH structure.
Conclusions
Most antecedents in the literature, and our own results, show that llamas respond to haptens mainly by producing conventional IgG1 antibodies. This is an expected result because they are more fitted to accommodate the hapten in deep binding pockets at the interface of the light and heavy chains. In spite of that, and due to the versatility of the antibody response, anti-hapten sdAbs are also produced, though with lower average affinity. Most of the reported examples of anti-hapten VHHs summarized in table 1 utilized unspecific elution conditions during the panning steps. These strategies tend to select VHHs with affinity for the immobilized hapten, regardless of their cross-reactivity with the free hapten, which is a critical feature for analytical applications of these antibodies. Here we report a selection process that combines the use of decreasing amounts of free hapten during the rounds of panning with a competitive screening. This has two major advantages; competitive elution promotes cross-reactivity with the actual target compound (free hapten), while the use of limiting amounts of the competitor fosters the selection of high-affinity binders. In this way we selected VHHs against free TCC that allowed us to set up competitive assays with IC50 values that are a hundred-fold better than those previously reported for other haptens. The isolated VHHs showed the characteristic thermal stability of VHHs and fine specificity when clone VHH T9 was tested against a large panel of TCC related compounds. The strategy described here demonstrated that is feasible to isolate high-affinity VHHs against small analytes, and due to its general applicability it may help to advance the isolation of VHHs for immunodetection of this important group of compounds.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Fogarty International Center Grant TW05718; NIEHS Superfund Basic Research program, P42 ES04699; and the NIEHS Center, P30 ES05707. BDH is a George and Judy Marcus Senior Fellow of the American Asthma Foundation
Footnotes
Supporting Information Available: Three figures showing the llama response to the carrier and hapten, sequence alignment of VHH clones, and SPR sensorgrams, and details of primers and the SPR analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363(6428):446–8. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 2.van der Linden RH, Frenken LG, de Geus B, Harmsen MM, Ruuls RC, Stok W, de Ron L, Wilson S, Davis P, Verrips CT. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim Biophys Acta. 1999;1431(1):37–46. doi: 10.1016/s0167-4838(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 3.Muyldermans S. Single domain camel antibodies: current status. J Biotechnol. 2001;74(4):277–302. doi: 10.1016/s1389-0352(01)00021-6. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, MacKenzie R, Durocher Y. Production of chimeric heavy-chain antibodies. Methods Mol Biol. 2009;525:323–36. xv. doi: 10.1007/978-1-59745-554-1_17. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Tanha J, Hirama T, Khieu NH, To R, Tong-Sevinc H, Stone E, Brisson JR, MacKenzie CR. Pentamerization of single-domain antibodies from phage libraries: a novel strategy for the rapid generation of high-avidity antibody reagents. J Mol Biol. 2004;335(1):49–56. doi: 10.1016/j.jmb.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Perez JM, Renisio JG, Prompers JJ, van Platerink CJ, Cambillau C, Darbon H, Frenken LG. Thermal unfolding of a llama antibody fragment: a two-state reversible process. Biochemistry. 2001;40(1):74–83. doi: 10.1021/bi0009082. [DOI] [PubMed] [Google Scholar]
- 7.Sheedy C, Yau KY, Hirama T, MacKenzie CR, Hall JC. Selection, characterization, and CDR shuffling of naive llama single-domain antibodies selected against auxin and their cross-reactivity with auxinic herbicides from four chemical families. J Agric Food Chem. 2006;54(10):3668–78. doi: 10.1021/jf060219i. [DOI] [PubMed] [Google Scholar]
- 8.Spinelli S, Frenken LG, Hermans P, Verrips T, Brown K, Tegoni M, Cambillau C. Camelid heavy-chain variable domains provide efficient combining sites to haptens. Biochemistry. 2000;39(6):1217–22. doi: 10.1021/bi991830w. [DOI] [PubMed] [Google Scholar]
- 9.Ladenson RC, Crimmins DL, Landt Y, Ladenson JH. Isolation and characterization of a thermally stable recombinant anti-caffeine heavy-chain antibody fragment. Anal Chem. 2006;78(13):4501–8. doi: 10.1021/ac058044j. [DOI] [PubMed] [Google Scholar]
- 10.Anderson GP, Goldman ER. TNT detection using llama antibodies and a two-step competitive fluid array immunoassay. J Immunol Methods. 2008;339(1):47–54. doi: 10.1016/j.jim.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Yau KY, Groves MA, Li S, Sheedy C, Lee H, Tanha J, MacKenzie CR, Jermutus L, Hall JC. Selection of hapten-specific single-domain antibodies from a non-immunized llama ribosome display library. J Immunol Methods. 2003;281(1–2):161–75. doi: 10.1016/j.jim.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Rueda N, Behar G, Ferre V, Pugniere M, Roquet F, Gastinel L, Jacquot C, Aubry J, Baty D, Barbet J, Birkle S. Generation of llama single-domain antibodies against methotrexate, a prototypical hapten. Mol Immunol. 2007;44(7):1680–90. doi: 10.1016/j.molimm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Lange IG, Daxenberger A, Meyer HH. Studies on the antibody response of Lama glama--evaluation of the binding capacity of different IgG subtypes in ELISAs for clenbuterol and BSA. Vet Immunol Immunopathol. 2001;83(1–2):1–9. doi: 10.1016/s0165-2427(01)00376-2. [DOI] [PubMed] [Google Scholar]
- 14.(a) Lamminmaki U, Kankare JA. Crystal structure of a recombinant anti-estradiol Fab fragment in complex with 17beta -estradiol. J Biol Chem. 2001;276(39):36687–94. doi: 10.1074/jbc.M102367200. [DOI] [PubMed] [Google Scholar]; (b) Monnet C, Bettsworth F, Stura EA, Le Du MH, Menez R, Derrien L, Zinn-Justin S, Gilquin B, Sibai G, Battail-Poirot N, Jolivet M, Menez A, Arnaud M, Ducancel F, Charbonnier JB. Highly specific anti-estradiol antibodies: structural characterisation and binding diversity. J Mol Biol. 2002;315(4):699–712. doi: 10.1006/jmbi.2001.5284. [DOI] [PubMed] [Google Scholar]
- 15.Spinelli S, Tegoni M, Frenken L, van Vliet C, Cambillau C. Lateral recognition of a dye hapten by a llama VHH domain. J Mol Biol. 2001;311(1):123–9. doi: 10.1006/jmbi.2001.4856. [DOI] [PubMed] [Google Scholar]
- 16.Doyle PJ, Arbabi-Ghahroudi M, Gaudette N, Furzer G, Savard ME, Gleddie S, McLean MD, Mackenzie CR, Hall JC. Cloning, expression, and characterization of a single-domain antibody fragment with affinity for 15-acetyl-deoxynivalenol. Mol Immunol. 2008;45(14):3703–13. doi: 10.1016/j.molimm.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Franco EJ, Sonneson GJ, DeLegge TJ, Hofstetter H, Horn JR, Hofstetter O. Production and characterization of a genetically engineered anti-caffeine camelid antibody and its use in immunoaffinity chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(2):177–86. doi: 10.1016/j.jchromb.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Frenken LG, van der Linden RH, Hermans PW, Bos JW, Ruuls RC, de Geus B, Verrips CT. Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. J Biotechnol. 2000;78(1):11–21. doi: 10.1016/s0168-1656(99)00228-x. [DOI] [PubMed] [Google Scholar]
- 19.Lorimer IA, Keppler-Hafkemeyer A, Beers RA, Pegram CN, Bigner DD, Pastan I. Recombinant immunotoxins specific for a mutant epidermal growth factor receptor: targeting with a single chain antibody variable domain isolated by phage display. Proc Natl Acad Sci U S A. 1996;93(25):14815–20. doi: 10.1073/pnas.93.25.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmsen MM, Ruuls RC, Nijman IJ, Niewold TA, Frenken LG, de Geus B. Llama heavy-chain V regions consist of at least four distinct subfamilies revealing novel sequence features. Mol Immunol. 2000;37(10):579–90. doi: 10.1016/s0161-5890(00)00081-x. Table of content graphic. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.