Abstract
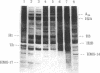
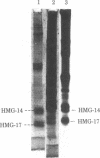
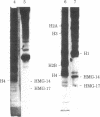
The protein composition of nucleosome fractions differing in their sensitivity to micrococcal nuclease and derived from butyrate-treated or untreated HeLa cells has been compared. Most of the high-mobility-group-14 (HMG-14) and HMG-17 content of HeLa cell chromatin is associated with those nucleosomes that are preferentially sensitive to micrococcal nuclease. Furthermore, electrophoresis of these two HMG proteins from the transcriptionally active chromatin fraction MN1 of butyrate-treated cells resolves them into a series of bands. The multiple band pattern of HMG-14 and -17 from butyrate-treated cells results from hyperphosphorylation rather than hyperacetylation. Phosphorylation of these two small nonhistone proteins may play some role in the modulation of the structure of transcriptionally active chromatin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boffa L. C., Vidali G., Mann R. S., Allfrey V. G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem. 1978 May 25;253(10):3364–3366. [PubMed] [Google Scholar]
- D'Anna J. A., Tobey R. A., Gurley L. R. Concentration-dependent effects of sodium butyrate in Chinese hamster cells: cell-cycle progression, inner-histone acetylation, histone H1 dephosphorylation, and induction of an H1-like protein. Biochemistry. 1980 Jun 10;19(12):2656–2671. doi: 10.1021/bi00553a019. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977 Mar;74(3):864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Kuehl L., Lyness T., Dixon G. H., Levy-Wilson B. Distribution of high mobility group proteins among domains of trout testis chromatin differing in their susceptibility to micrococcal nuclease. J Biol Chem. 1980 Feb 10;255(3):1090–1095. [PubMed] [Google Scholar]
- Langan T. A. Histone phosphorylation: stimulation by adenosine 3',5'-monophosphate. Science. 1968 Nov 1;162(3853):579–580. doi: 10.1126/science.162.3853.579. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Phosphorylation of liver histone following the administration of glucagon and insulin. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1276–1283. doi: 10.1073/pnas.64.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Wilson B., Dixon G. H. Limited action of micrococcal nuclease on trout testis nuclei generates two mononucleosome subsets enriched in transcribed DNA sequences. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1682–1686. doi: 10.1073/pnas.76.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Nelson D., Covault J., Chalkley R. Segregation of rapidly acetylated histones into a chromatin fraction released from intact nuclei by the action of micrococcal nuclease. Nucleic Acids Res. 1980 Apr 25;8(8):1745–1763. doi: 10.1093/nar/8.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N., Sinha P. K. Effect of sodium butyrate on mammalian cells in culture: a review. In Vitro. 1976 Feb;12(2):125–132. doi: 10.1007/BF02796360. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Saffer J. D., Glazer R. I. The phosphorylation of high mobility group proteins 14 and 17 from Ehrlich ascites and L1210 in vitro. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1280–1285. doi: 10.1016/0006-291x(80)90628-2. [DOI] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. DNA associated with hyperacetylated histone is preferentially digested by DNase I. Nucleic Acids Res. 1978 Jun;5(6):1863–1876. doi: 10.1093/nar/5.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978 May;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978 May;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Sterner R., Vidali G., Heinrikson R. L., Allfrey V. G. Postsynthetic modification of high mobility group proteins. Evidence that high mobility group proteins are acetylated. J Biol Chem. 1978 Nov 10;253(21):7601–7604. [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. C., Levy W. B., Dixon G. H. Free ubiquitin is a non-histone protein of trout testis chromatin. Nature. 1978 Nov 9;276(5684):196–198. doi: 10.1038/276196a0. [DOI] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Augustine R., Schulman H. Calcium-dependent phosphorylation of histone H3 in butyrate-treated HeLa cells. Nature. 1980 Sep 4;287(5777):74–76. doi: 10.1038/287074a0. [DOI] [PubMed] [Google Scholar]
- Wong N. C., Poirier G. G., Dixon G. H. Adenosine diphosphoribosylation of certain basic chromosomal proteins in isolated trout testis nuclei. Eur J Biochem. 1977 Jul 1;77(1):11–21. doi: 10.1111/j.1432-1033.1977.tb11635.x. [DOI] [PubMed] [Google Scholar]