Abstract
Toll-like receptor (TLR) ligands that signal via TIR-domain-containing adapter-inducing IFNβ (TRIF) activate the IκB kinase (IKK)-related kinases, TRAF associated NFκB activator (TANK)-binding kinase-1 (TBK1) and IKKε, which then phosphorylate IRF3 and induce the production of IFNβ. Here we show that TBK1 and IKKε are also activated by TLR ligands that signal via MyD88. Notably, the activation of IKKε is rapid, transient, and it precedes a more prolonged activation of TBK1. The MyD88- and TRIF-dependent signaling pathways activate the IKK-related kinases by two signaling pathways. One is mediated by the canonical IKKs, whereas the other culminates in the autoactivation of the IKK-related kinases. Once activated, TBK1/IKKε then phosphorylate and inhibit the canonical IKKs. The negative regulation of the canonical IKKs by the IKK-related kinases occurs in both the TRIF- and MyD88-dependent TLR pathways, whereas IRF3 phosphorylation is restricted to the TRIF-dependent signaling pathway. We have discovered that the activation of IKKε is abolished, the activation of TBK1 is reduced, and the interaction between the IKK-related kinases and the canonical IKKs is suppressed in TANK−/− macrophages, preventing the IKK-related kinases from negatively regulating the canonical IKKs. In contrast, IRF3 phosphorylation and IFNβ production was normal in TANK−/− macrophages. Our results demonstrate a key role for TANK in enabling the canonical IKKs and the IKK-related kinases to regulate each other, which is required to limit the strength of TLR signaling and ultimately, prevent autoimmunity.
Toll-like receptors (TLRs) respond to components of pathogens leading to the activation of several signaling pathways that culminate in the production of inflammatory mediators to combat the invading pathogen. However, there is mounting evidence that disregulation of TLR signaling pathways can lead to the overproduction of inflammatory mediators resulting in the development of inflammatory and autoimmune diseases (e.g., refs. 1, 2). Understanding the mechanisms that prevent the excessive production of inflammatory mediators may identify novel drug targets for treating these diseases.
TLRs initiate signal transduction pathways using two receptor-proximal protein adaptors, called MyD88 and TIR-domain-containing adapter-inducing IFNβ (TRIF) (3). TLRs 1, 2, and 5–9 signal via MyD88; TLR3 signals via TRIF; and TLR4 signals via both MyD88 and TRIF (3). In the MyD88-dependent pathway, an E3 ubiquitin ligase TRAF6, generates K63-linked polyubiquitin (K63-pUb) chains, which activate the protein kinase TAK1 (4, 5). These K63-pUb chains also interact with NFκB essential modulator (NEMO), a component of the canonical IKK complex (6, 7), inducing a conformational change that facilitates the activation of this complex by TAK1 and by autophosphorylation (8, 9). Subsequently, the canonical IKKs (IKKα and IKKβ) activate downstream targets, which include the protein kinase Tpl2 (10, 11) and the transcription factor NFκB (12) stimulating the production and secretion of inflammatory mediators, such as TNFα, IL-6, and IL-12. Engagement of the TRIF-dependent pathway also leads to the activation of two other members of the IKK subfamily of protein kinases, TRAF associated NFκB activator (TANK)-binding kinase-1 (TBK1) and IKKε, termed the IKK-related kinases (3, 12). TBK1/IKKε then phosphorylate the transcription factor IRF3, leading to its dimerization and nuclear translocation where it stimulates the transcription of genes, such as IFNβ, to set up an antiviral state (13–16). For these reasons, the IKK-related kinases have been thought to play separate roles from the canonical IKKs during innate immunity.
Recently, we found that in pathways triggered by TLR3 and TLR4 in macrophages, the IKK-related kinases and the canonical IKKs are interconnected in a signaling network required to balance their respective catalytic activities (9). The activation of the IKK-related protein kinases requires their phosphorylation at Ser172 and occurs by two distinct signaling pathways. One involves the direct phosphorylation of TBK1 and IKKε by the canonical IKKs, whereas the second culminates in the autoactivation of the IKK-related kinases (9). Once activated, the IKK-related kinases phosphorylate the catalytic and regulatory subunits of the canonical IKK complex on sites that inhibit their catalytic activity (9). This network prevents the hyperactivation of the IKK-family members and raises the question of how they interact with one another to control innate immunity.
The regulatory proteins that control the activation of the IKK-related kinases have yet to be fully defined. TBK1 and IKKε interact via their C termini with TANK, NFκB activating kinase (NAK)-associated protein 1 (NAP1) and similar to NAP1 TBK1 adaptor (Sintbad) (17–19), but only TBK1 interacts with optineurin (20). It has therefore been suggested that distinct forms of the IKK-related kinases may be present in cells, each comprising a catalytic subunit and a different regulatory subunit, which are involved in regulating different aspects of innate immunity (21). The recent characterization of the TANK−/− mouse led to the unexpected finding that TANK is not required for the production of IFNβ in response to viral infection, but instead is required to limit MyD88-dependent TLR signaling and so prevent the development of autoimmune nephritis (1). How TANK negatively regulates TLR signaling remains poorly defined. Notably, macrophages from the TANK−/− mice displayed increased NFκB signaling in response to TLR ligands (1). Because TANK, but not the TBK1 and IKKε catalytic subunits, is reported to interact with NEMO (22, 23), we reasoned that TANK may represent a key component of a molecular bridge that facilitates the “cross-talk” between the IKK-related kinases and the canonical IKKs. In this paper, we have therefore investigated this hypothesis by studying the activation of, and cross-talk between, the IKK family members in TANK−/− macrophages in response to MyD88- and TRIF-dependent agonists.
Results
Activation of TBK1 and IKKε by MyD88- and TRIF-Dependent TLR Agonists.
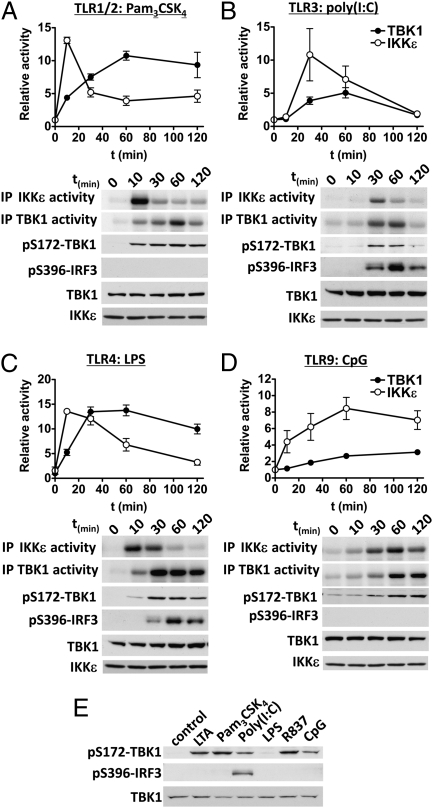
Recently, we discovered that TBK1 and IKKε are activated by the MyD88-dependent cytokine IL-1α in fibroblasts, which signals exclusively via MyD88 (24). We therefore investigated whether the IKK-related kinases were also activated in bone marrow-derived macrophages (BMDMs) by MyD88-dependent TLR agonists (Fig. 1 A–D and Fig. S1). Ligands that signal via MyD88, namely Pam3CSK4 (TLR1/2), lipoteichoic acid (LTA; TLR2/6), R837 (TLR7), and CpG (TLR9) activated TBK1 and IKKε at least as robustly as the synthetic dsRNA mimetic poly(I:C) (TLR3), which signals via TRIF, or LPS (TLR4), which signals via TRIF and MyD88. However, the kinetics of activation differed between agonists and between the IKK-related kinases themselves. In general, the peak of IKKε activity preceded that of TBK1, a rapid and transient activation of IKKε being followed by a slower and more sustained activation of TBK1. Importantly, LTA, Pam3CSK4, poly(I:C), R837 and CpG induced the activation of TBK1 and IKKε in BMDMs from the LPS-insensitive mouse strain C3H/HeJ, demonstrating that the ability of the different MyD88-dependent TLR agonists to activate the IKK-related kinases was not due to contamination with endotoxin (Fig. 1E).
Fig. 1.
Kinetics of activation of TBK1 and IKKε by different TLR agonists. BMDMs were stimulated for the times indicated with (A) 1 μg/mL Pam3CSK4, (B) 10 μg/mL poly(I:C), (C) 100 ng/mL LPS, or (D) 2 μM CpG. TBK1 and IKKε were immunoprecipitated (IP) and their catalytic activities were measured by incubating the immunoprecipitated kinases with GST-IRF3 and Mg[γ-32P]-ATP. Reactions were terminated in SDS, the proteins resolved by SDS/PAGE, and the gel autoradiographed (A–D, Upper two panels). Kinase activity was quantified by phosphorimager analysis (mean ± SEM, n = 3–6). An aliquot of each immunoprecipitation was also immunoblotted for TBK1 and IKKε as a loading control (Lower). Cell extract (20 μg protein) was additionally immunoblotted with antibodies that recognize TBK1 phosphorylated at Ser172 (to monitor activation by a second independent method) and for the phosphorylation of IRF3 at Ser396 (A–D, Lower three panels). (E) BMDMs from the LPS-resistant mouse strain C3H/HeJ were stimulated for 60 min with 2 μg/mL LTA, 1 μg/mL Pam3CSK4, 10 μg/mL poly(I:C), 100 ng/mL LPS, 2 μg/mL R837 or 2 μM CpG. Cell extracts (20 μg protein) were immunoblotted with the same antibodies used in A–D.
IKKε is also called “IKK-inducible” (IKKi), because its expression is enhanced by prolonged exposure to proinflammatory stimuli (25) through an NFκB-dependent pathway driven by the canonical IKKs (9). We showed that the LPS-stimulated activation of IKKε peaks at 10 min and then decreases to a low level by 60 min, which is sustained for the next 24 h, even though LPS (Fig. S2A) and other TLR agonists (Fig. S2B) greatly increase IKKε protein expression over this period. Thus, a high level of IKKε protein expression does not correlate with high IKKε activity in mouse macrophages.
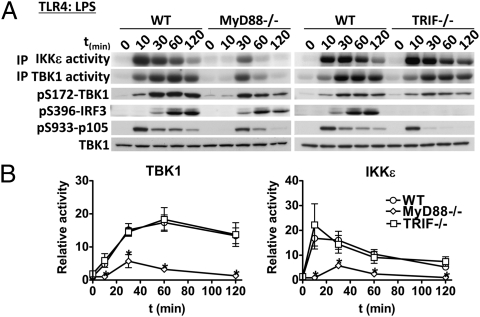
We next studied the activation of the IKK-related kinases in MyD88−/− and TRIF−/− macrophages. As expected, the activation of TBK1 and IKKε by Pam3CSK4, R837, and CpG was abolished in MyD88−/− macrophages but was normal in TRIF−/− macrophages (Fig. S3 A, C, and D), whereas the poly(I:C)-mediated activation of TBK1 and IKKε was normal in MyD88−/− macrophages but absent in TRIF−/− macrophages (Fig. S3B). However, of particular note was the finding that LPS was able to induce the activation of TBK1 and IKKε via the MyD88-dependent and the TRIF-dependent pathway, although with different kinetics. In MyD88−/− BMDMs, the LPS-induced activation of the IKK-related kinases was reduced, delayed until 30 min, and transient, returning to near basal levels after 1–2 h (Fig. 2), whereas in TRIF−/− macrophages, the activation of TBK1 and IKKε by LPS was similar to wild-type (WT) macrophages (Fig. 2). Thus, the IKK-related kinases are activated by both the MyD88- and TRIF-dependent signaling pathways in macrophages. The LPS-stimulated activation of the canonical IKKs (monitored by the phosphorylation of p105, an established substrate of IKKβ (26)) paralleled that of IKKε, becoming maximal after 10 min in TRIF−/− BMDMs and after 30 min in MyD88−/− BMDMs (Fig. 2).
Fig. 2.
Activation of TBK1 and IKKε in response to LPS occurs via both the MyD88- and the TRIF-dependent pathways, but only the latter leads to the phosphorylation of IRF3. (A) BMDMs from MyD88−/− or TRIF−/− mice or WT littermates were stimulated for the times indicated with 100 ng/mL LPS. The catalytic activities of TBK1 and IKKε (A, Upper two panels) were measured as described in Fig. 1 and Materials and Methods. Cell extracts (20 μg protein) were immunoblotted with the antibodies indicated (A, Lower four panels). (B) Quantitation of TBK1 and IKKε activities by phosphorimager analysis (mean ± SEM, n = 3).
Phosphorylation of IRF3 by the IKK-Related Kinases Occurs Only via the TRIF-Dependent Signaling Pathway.
It is well established that the IKK-related kinases phosphorylate IRF3 in a TRIF-dependent pathway that is activated by TLR3 and TLR4 ligands (in the Introduction) and a strict correlation between the induction of IRF3 phosphorylation at Ser396 and coupling to TRIF was observed in the present study (Fig. 1 B, C, and E and Fig. 2). Poly(I:C) and LPS stimulated the phosphorylation of IRF3 (Fig. 1 B and C), but all of the MyD88-dependent TLR agonists tested failed to induce any phosphorylation of IRF3 (Fig. 1E), although they activated TBK1 and IKKε at least as robustly (Fig. 1). Moreover, the LPS-stimulated phosphorylation of IRF3 was not impaired in MyD88-deficient macrophages (Fig. 2A), even though far lower TBK1/IKKε activity was generated in these cells. Furthermore, LPS failed to induce IRF3 phosphorylation in TRIF-deficient macrophages, even though the activation of the IKK-related kinases was similar to WT macrophages (Fig. 2B).
Cross-Talk Between the Canonical IKKs and IKK-Related Kinases During MyD88-Dependent Signaling in Macrophages.
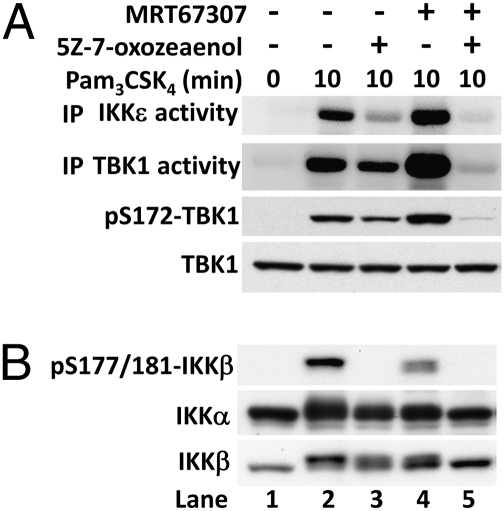
We have shown previously that the activation of the IKK-related kinases by IL-1α in fibroblasts or LPS and poly(I:C) in RAW264.7 cells requires two signaling pathways. One of these is IKKα/β dependent, whereas the other appears to culminate in the autoactivation of the IKK-related kinases, because it can be prevented by pharmacological inhibition of TBK1/IKKε (9). In the present study, we found that Pam3CSK4, a MyD88-dependent agonist, also activated TBK1 and IKKε by these two pathways in primary BMDMs (Fig. 3A). Thus, incubation of BMDMs with the TAK1 inhibitor 5Z-7-oxozeaenol, which prevents the activation of IKKβ and the phosphorylation of its substrate p105 (Fig. 3B), partially suppressed the Pam3CSK4-stimulated activation of TBK1 and IKKε (Fig. 3A). In contrast, incubation with the TBK1/IKKε inhibitor MRT67307 (9) increased the activation of these IKK-related kinases, but incubation with both inhibitors abolished activation (Fig. 3A). The enhanced activation of TBK1 and IKKε when cells are incubated with MRT67307, and which is abolished by inhibition of TAK1 (Fig. 3A), has been noted previously in IL-1–stimulated fibroblasts and LPS-stimulated RAW264.7 cells (24), and indicates that TBK1/IKKε control a negative feedback loop that suppresses their activation by the canonical IKKs.
Fig. 3.
Cross-talk between the canonical IKKs and the IKK-related kinases during MyD88 signaling in macrophages. (A) Activation of the IKK-related kinases. BMDMs were treated for 1 h with either 1 μM 5Z-7-oxozeaenol, 2 μM MRT67307, or both kinase inhibitors before stimulation for 10 min with 1 μg/mL Pam3CSK4. The catalytic activities of TBK1 and IKKε (Upper two panels) were measured as in Fig. 1 and Materials and Methods. In these experiments, TBK1 and IKKε were immunoprecipitated from the cell extracts and the immunoprecipitates washed to remove MRT67307 before assaying in the absence of this compound, because MRT67307 is a reversible inhibitor of the IKK-related kinases. Cell extracts (20 μg protein) were also immunoblotted with the antibodies indicated (Lower two panels). (B) The cell extracts in A were immunoblotted with the antibodies indicated. The phospho-specific antibody that recognizes IKKβ phosphorylated at Ser177 and Ser181 also recognizes IKKα phosphorylated at Ser176 and Ser180. However, the bands shown correspond to phosphorylated IKKβ only. Phosphorylated IKKα migrates more rapidly than IKKβ and is recognized very poorly by the antibody (figure S1A in ref. 9), which may be explained by lower levels of expression and/or activation of IKKα compared with IKKβ.
The TAK1-dependent activation of the canonical IKKs can be monitored by the phosphorylation of two serine residues in their activation loops (Ser177 and Ser181 of IKKβ). Once activated, the canonical IKKs are subsequently inhibited by the phosphorylation of amino acid residues distinct from those located in the activation loops, and which are targeted by the canonical IKKs themselves (27) and by the IKK-related kinases (9). The phosphorylation of these inhibitory sites can be monitored by a retardation in the electrophoretic mobility of IKKα and IKKβ, which is reversed by phosphatase treatment (9). In the present study, we found that Pam3CSK4 induced a decrease in the electrophoretic mobility of IKKα and IKKβ (Fig. 3B, lane 2), which was partially suppressed by inhibition of TAK1 (Fig. 3B, compare lanes 2 and 3) or the IKK-related kinases (Fig. 3B, compare lanes 2 and 4), and prevented by the combined inhibition of all four IKKs (Fig. 3B, compare lanes 2 and 5). Pharmacological inhibition of the IKK-related kinases increased the activity of IKKα and IKKβ as shown by the increased phosphorylation of TBK1 and IKKε, which was blocked by inhibition of TAK1 (Fig. 3A). Thus, the cross-talk between the different members of the IKK family is conserved among the different TLR signaling pathways in macrophages.
To verify that MRT67307 exerts its effect by inhibition of the IKK-related kinases, and not by an off-target effect, we carried out further experiments with mouse embryonic fibroblasts (MEFs) from mice that do not express TBK1 and IKKε. The IL-1α stimulated phosphorylation of the IKKβ substrates p105 (26) and RelA (9) was enhanced in MEFs that do not express the IKK-related kinases (Fig. S4A). Moreover, the IL-1–induced retardation in the electrophoretic mobility of IKKβ was much less pronounced in MEFs lacking the IKK-related kinases (Fig. S4B). It should be noted that the more striking enhancement in the phosphorylation of p105 seen in IL-1α–stimulated TBK1/IKKε double knockout (DKO) MEFs in Fig. S4A is explained by the use of 10-fold lower concentrations of IL-1α than those used in Fig. S4B.
Cross-Talk Within the IKK Family Requires the Adaptor Protein TANK.
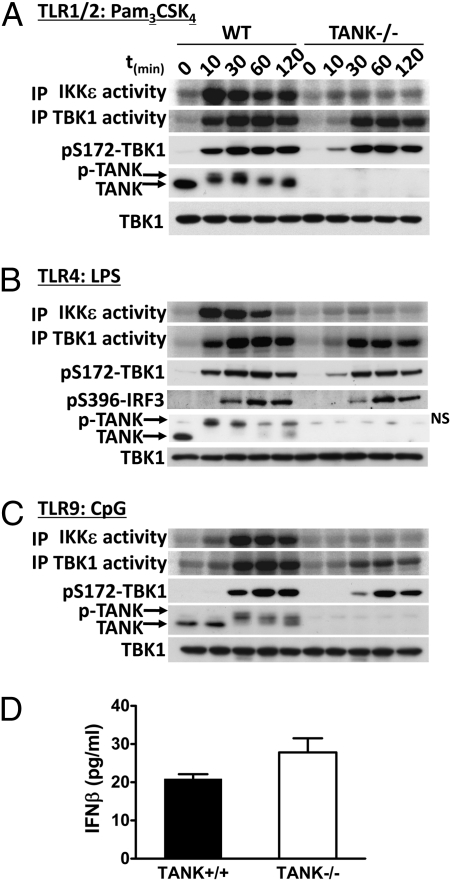
The finding that the IKK-related kinases are activated by the MyD88-dependent signaling pathway raised the question of which binding partners of TBK1 and IKKε might be important for their activation via this pathway. Because TBK1 was originally discovered as a protein that interacts with TANK (17), we studied the catalytic activity of TBK1 and IKKε in BMDMs from WT and TANK−/− mice in response to Pam3CSK4, LPS, and CpG (Fig. 4). Strikingly, the catalytic activity of IKKε was not stimulated by any TLR agonist tested in the TANK−/− macrophages. The phosphorylation and activation of TBK1 was also reduced at the earliest time points examined, but not abolished (Fig. 4). These results demonstrate that TANK is absolutely required for the activation of IKKε activation by TLR ligands but not for the activation of TBK1 in mouse macrophages. In contrast, the LPS-stimulated phosphorylation of IRF3 and production of IFNβ was similar in BMDMs from TANK−/− and WT mice (Fig. 4 B and D). This is consistent with our previous observation that TANK was not required for IFNα production in dendritic cells after infection by Newcastle disease virus (1).
Fig. 4.
TANK is required for efficient activation of the IKK-related kinases by TLR agonists. (A–C) BMDMs from TANK−/− mice or WT littermates were stimulated for the times indicated with (A) 1 μg/mL Pam3CSK4, (B) 100 ng/mL LPS, or (C) 2 μM CpG. The catalytic activities of TBK1 and IKKε (A–C, Upper two panels) were measured as described in Fig. 1 and Materials and Methods. Cell extracts (20 μg protein) were also immunoblotted with the antibodies indicated (A and C, Lower three panels and B, Lower four panels). NS in B denotes a nonspecific band detected by the TANK antibody. (D) BMDMs from TANK−/− mice or WT littermates were stimulated for 6 h with 100 ng/mL LPS and the concentration of IFNβ in the culture supernatant measured with the ELISA kit from R&D Systems (mean ± SEM, n = 3).
The TLR ligands also induced a striking decrease in the electrophoretic mobility of TANK, which is caused by phosphorylation (28). The phosphorylation of TANK was partially suppressed by pharmacological inhibition of the IKK-related kinases, partially suppressed by inhibition of the canonical IKKs, and completely prevented by inhibition of all of the IKK family members (9). Thus, TANK is phosphorylated by all of the IKK family members, but the role of phosphorylation has yet to be elucidated.
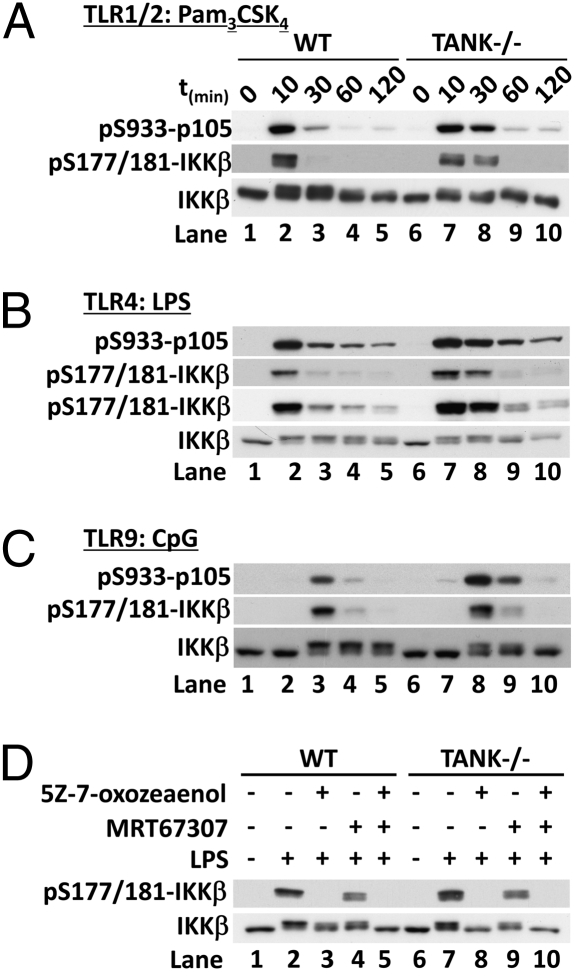
We next investigated how the absence of TANK affected the activity of IKKβ. Stimulation of TANK−/− BMDMs with TLR ligands led to a more prolonged and/or enhanced activation of IKKβ, as judged by the phosphorylation of the serine residues positioned in the activation loop and by the phosphorylation of its substrate p105 (Fig. 5 A–C), suggesting that TANK is required for the negative regulation of the canonical IKKs. There was also a smaller reduction in the electrophoretic mobility (phosphorylation) of IKKβ in the TANK−/− macrophages than in the WT macrophages (Fig. 5 A and B, compare lanes 2 and 7 or Fig. 5C, compare lanes 3–5 and 8–10). Because the activation of IKKε was abolished and the activation of TBK1 was reduced in the TANK−/− macrophages (Fig. 4), the simplest explanation for these results is that the activity of the IKK-related kinases is insufficient to mediate the negative regulation of the canonical IKKs in these cells.
Fig. 5.
Negative regulation of the canonical IKKs by the IKK-related kinases is impaired in TANK−/− macrophages. BMDMs from TANK−/− mice or WT littermates were stimulated for the times indicated with (A) 1 μg/mL Pam3CSK4, (B) 100 ng/mL LPS, or (C) 2 μM CpG. Cell extracts (20 μg protein) were immunoblotted with the antibodies indicated. (D) BMDMs from TANK−/− mice or WT (TANK+/+) littermates were treated for 1 h with 1 μM 5Z-7-oxozeaenol, and/or 2 μM MRT67307, before stimulation for 10 min with 100 ng/mL LPS. Cell extracts were immunoblotted as described above.
If the negative regulation of IKKβ by the IKK-related kinases is lost in BMDMs from TANK−/− mice, then the decrease in the electrophoretic mobility of IKKβ induced by TLR ligands should result solely from phosphorylation by TAK1 and subsequent autophosphorylation by IKKβ and therefore be prevented by the inhibition of TAK1 alone. This prediction was verified by the finding that the TAK1 inhibitor 5Z-7-oxozeaenol blocked the LPS-stimulated decrease in the mobility of IKKβ in TANK−/− macrophages (Fig. 5D, Lower, lanes 6–8), but the combination of 5Z-7-oxozeaenol and the TBK1/IKKε inhibitor MRT67307 was required to obtain a similar effect in WT macrophages (Fig. 5D, lanes 1–5). These studies establish that TANK is required for the activation of IKKε and contributes to the activation of TBK1, and that the cross-talk between the canonical IKKs and the IKK-related kinases during TLR signaling is disrupted in TANK−/− macrophages.
TANK Is Required for the Interaction of the IKK-Related Kinases with the Canonical IKK Complex.
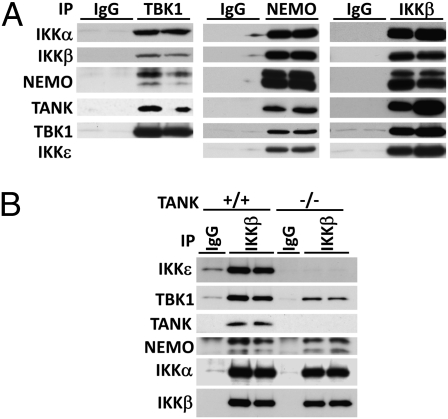
TANK has been reported to bind to NEMO (23), suggesting that TANK might be important for interaction between the IKK-related kinases and the canonical IKKs to facilitate cross-talk between the different IKK family members. We therefore initially investigated whether an interaction between the canonical IKKs and the IKK-related kinases could be detected in macrophages. We immunoprecipitated TBK1 from the cell extracts and immunoblotted for the presence of components of the canonical IKK complex or, conversely, immunoprecipitated NEMO or IKKβ and immunoblotted for the presence of components of the IKK-related kinases. These experiments showed that IKKα, IKKβ, and NEMO could be immunoprecipitated with anti-TBK1, whereas TANK, TBK1, and IKKε could be immunoprecipitated with either anti-NEMO or anti-IKKβ, but not by control IgG (Fig. 6A). Strikingly, the IKKβ antibody was unable to immunoprecipitate IKKε from extracts of TANK−/− macrophages, whereas the amount of TBK1 that could be immunoprecipitated was reduced considerably. In contrast, the ability of anti-IKKβ to immunoprecipitate IKKα and NEMO was unaffected (Fig. 6B). These experiments demonstrate that the IKK-related kinases and the canonical IKKs interact in macrophages and that the presence of TANK is critical for the formation of these complexes.
Fig. 6.
Association of the canonical IKKs and IKK-related kinases is dependent on TANK. (A) Lysates of RAW264.7 macrophages were incubated with antibodies from nonimmunized sheep (IgG) or antibodies against TBK1, NEMO, or IKKβ. The immunoprecipitates were washed, separated by SDS/PAGE, and immunoblotted with the antibodies indicated. (B) Cell extracts from TANK+/+ and TANK−/− macrophages were used to immunoprecipitate IKKβ and coimmunoprecipitating proteins were monitored by immunoblotting.
Discussion
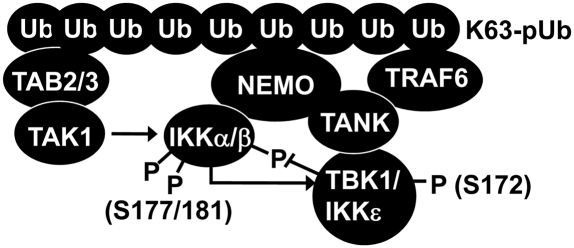
In this paper we show that TANK is required for the activation of IKKε, that it contributes to the early phase of activation of TBK1, and that it is required for the interaction of the IKK-related kinases with the canonical IKKs. These findings can explain why the negative regulation of the canonical IKKs by the IKK-related kinases is lost in TANK−/− macrophages leading to enhanced activation of the canonical IKKs. Thus, an important role for TANK is to facilitate the activation of the IKK-related kinases and couple them to the canonical IKKs allowing for efficient cross-talk between them during TLR signaling. We also establish that, similar to the IL-1–stimulated MyD88-dependent pathway that we described recently in MEFs (9), the activation of the IKK-related kinases by TLR ligands occurs via two pathways in BMDMs, one mediated by the canonical IKKs and the other by a pathway that culminates in the autoactivation of the IKK-related kinases (9). On the basis of these findings and other information in the literature, we propose the following model for the regulation of the IKK family of protein kinases (Fig. 7). When TLR signaling pathways are activated by the MyD88-dependent pathway, K63-pUb chains generated by the action of TRAF6 bind to the TAB2 and TAB3 regulatory components of the TAK1 complex, inducing conformational changes that trigger the autoactivation of TAK1 (8). The K63-pUb chains also interact with NEMO, a component of the canonical IKK complex. This induces conformational changes that allow TAK1 to initiate activation of the canonical IKK complex (29). Because the canonical IKK complex interacts with the IKK-related kinases (Fig. 6A) via TANK (Fig. 6B), which has itself been reported to interact with NEMO (23), the binding of K63-pUb chains to NEMO may induce conformational changes in the TANK–IKKε and TANK–TBK1 complexes that allow them to be activated by the canonical IKKs and by autophosphorylation. This proposal can explain why NEMO is required for both the activation of the IKK-related kinases by the pathway that is independent of the canonical IKKs and for direct activation by the canonical IKKs (9). The TANK-dependent interaction between the IKK-related kinases and the canonical IKK complex also permits the IKK-related kinases to phosphorylate inhibitory sites on IKKα/β and so dampen their catalytic activity and the production of inflammatory mediators (Fig. 7). The IKK-related kinases and the canonical IKKs additionally phosphorylate NEMO and TANK (9), but the contribution that these phosphorylation events make to cross-talk between the different members of the IKK subfamily has yet to be determined.
Fig. 7.
Model for the cross-talk between IKK family members in the MyD88 signaling pathway. K63-pUb chains formed by TRAF6 in response to IL-1 or TLR stimulation bind to TAB2/3–TAK1 and IKKα/β–NEMO–TANK–TBK1/IKKε complexes enabling the sequential activation of the canonical IKKs by TAK1 and then the activation of the IKK-related kinases by the canonical IKKs and by autoactivation, as discussed in the text. The IKK-related kinases then negatively regulate the canonical IKKs by phosphorylating sites that inhibit activity.
One of our laboratories has reported that macrophages from TANK−/− mice show increased TRAF6 polyubiquitylation in response to TLR7 stimulation, implying that the E3 ligase activity of TRAF6 is enhanced (1). Interestingly, TANK was originally identified as a protein that interacts with different members of the TRAF family (30, 31) and TANK may be able to bind simultaneously to NEMO and TRAF6 because these two proteins bind to distinct regions of TANK (23, 30). An intriguing possibility is that the interaction of TRAF6 with TANK may enable the IKK-related kinases to phosphorylate and negatively regulate TRAF6, explaining why TRAF6 E3 ligase activity is enhanced on TANK−/− macrophages. Alternatively, or in addition, the IKK-related kinases may negatively regulate one or more “upstream” components of the signaling pathway that leads to the activation of TRAF6. Thus, the IKK-related kinases may negatively regulate TLR–MyD88 signaling pathway in several ways. This may explain why TANK−/− mice overproduce IL-6 and other inflammatory mediators and develop autoimmune nephritis, which can be prevented by crossing to MyD88-deficient mice (1). It would therefore be more appropriate if the acronym TANK stood for “TRAF-associated NFκB inhibitor” and not “TRAF-associated NFκB activator.”
We also found that TANK was dispensable for IRF3 phosphorylation and IFNβ production in response to LPS (Fig. 4 B and D), even though the LPS-stimulated activation of IKKε was abolished. Similarly, LPS-stimulated TBK1 activity was greatly decreased in MyD88−/− macrophages without affecting the phosphorylation of IRF3 (Fig. 2). These observations suggest that a form of TBK1, distinct from the TBK1–TANK complex, and which accounts for only a minor proportion of the total TBK1 activity that we measure in BMDM extracts, mediates the phosphorylation of IRF3 in vivo.
A further unresolved question is why TLR agonists that signal via MyD88 activate the IKK-related kinases robustly without inducing any phosphorylation of IRF3 (Fig. 1 and Fig. S1), even though the activated IKK-related kinases can phosphorylate IRF3 in vitro. These findings indicate that, although the IKK-related kinases are required for the phosphorylation of IRF3 in vivo, their activation is not sufficient and an additional molecular determinant(s) is (are) required to couple TBK1 to the phosphorylation of IRF3. One determinant could be the formation of a TLR3/4–TRIF–TRAF3 complex at the endosomal membrane (32), because the phosphorylation of IRF3 and production of IFNβ by this pathway does not occur in TRAF3−/− macrophages (33, 34). The requirement to colocalize a TLR with TRAF3 at a membrane to produce IFNβ has been elegantly demonstrated by targeting TRAF3 to the plasma membranes of macrophages, which permitted TLR2 agonists that signal via MyD88 to induce high levels of IFNβ, which does not happen if TRAF3 is not targeted to these membranes (32). For these reasons, TRAF3 has been widely believed to operate upstream of the IKK-related kinases in the signaling pathway leading to the activation of IRF3 (3). However, we found that TRAF3 is not required for IL-1 to activate TBK1 and IKKε by the MyD88 pathway in fibroblasts (9), suggesting that TRAF3 may instead function “downstream” of, or in parallel with, the IKK-related kinases to couple TBK1 and IKKε to the phosphorylation of IRF3 by an unknown mechanism.
Materials and Methods
To measure the activity of the IKK-related kinases, endogenous TBK1 and IKKε were immunoprecipitated from 50 μg cell lysate protein using 1 μg of the corresponding sheep antibodies (TBK1-S041C; IKKε-S277C). Immunocomplexes were washed three times in lysis buffer, twice in 50 mM Tris-HCl pH 7.5, 0.1% (vol/vol) 2-mercaptoethanol, 0.1 mM EGTA, 10 mM magnesium acetate, and then resuspended in 25 μL of the same buffer containing 2 μM of recombinant GST-IRF3, 0.1 mM [γ-32P]ATP (2,500 cpm/pmol). After incubation at 30 °C, reactions were terminated by the addition of 25 μL of 2% (wt/vol) SDS containing 40 mM EDTA pH 7.0, heated for 5 min at 100 °C, separated by SDS/PAGE, and the phosphorylated IRF3 was detected by autoradiography and quantitated by scanning using a phosphorimager. We validated this assay by demonstrating that activity in the TBK1 and IKKε immunoprecipitates was completely suppressed by MRT67307, a relatively specific inhibitor of the IKK-related kinases that does not affect the canonical IKKs (Fig. S1A) (9). This result shows that the activity being measured is TBK1 and IKKε and not another protein kinase(s) present in the immunoprecipitates as a contaminant. The activation of TBK1 and IKKε was also monitored in parallel by immunoblotting using a phospho-specific antibody that recognizes the activation loop residue Ser172 (9, 24).
Supplementary Material
Acknowledgments
We thank Julia Carr and Gail Fraser for genotyping the mice, the University of Dundee Resource Centre (coordinated by Don Tennant and Lorraine Malone) for housing the mice, and the protein and antibody production teams of the Division of Signal Transduction Therapy, Medical Research Council (MRC) Protein Phosphorylation Unit, University of Dundee (coordinated by Hilary McLauchlan and James Hastie) for proteins and antibodies. K.C. was the recipient of a long-term fellowship from the European Molecular Biology Organization during part of this work. The work was supported by the UK MRC, AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck-Serono, and Pfizer.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114194108/-/DCSupplemental.
References
- 1.Kawagoe T, et al. TANK is a negative regulator of Toll-like receptor signaling and is critical for the prevention of autoimmune nephritis. Nat Immunol. 2009;10:965–972. doi: 10.1038/ni.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nanda SK, et al. Polyubiquitin binding to ABIN1 is required to prevent autoimmunity. J Exp Med. 2011;208:1215–1228. doi: 10.1084/jem.20102177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 4.Kanayama A, et al. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 6.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 8.Xia ZP, et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature. 2009;461:114–119. doi: 10.1038/nature08247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark K, et al. Novel cross-talk within the IKK family controls innate immunity. Biochem J. 2011;434:93–104. doi: 10.1042/BJ20101701. [DOI] [PubMed] [Google Scholar]
- 10.Beinke S, Robinson MJ, Hugunin M, Ley SC. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105. Mol Cell Biol. 2004;24:9658–9667. doi: 10.1128/MCB.24.21.9658-9667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waterfield M, Jin W, Reiley W, Zhang M, Sun SC. IkappaB kinase is an essential component of the Tpl2 signaling pathway. Mol Cell Biol. 2004;24:6040–6048. doi: 10.1128/MCB.24.13.6040-6048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, et al. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 15.Hemmi H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry AK, Chow EK, Goodnough JB, Yeh WC, Cheng G. Differential requirement for TANK-binding kinase-1 in type I interferon responses to toll-like receptor activation and viral infection. J Exp Med. 2004;199:1651–1658. doi: 10.1084/jem.20040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999;18:6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita F, et al. Identification of NAP1, a regulatory subunit of IkappaB kinase-related kinases that potentiates NF-kappaB signaling. Mol Cell Biol. 2003;23:7780–7793. doi: 10.1128/MCB.23.21.7780-7793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryzhakov G, Randow F. SINTBAD, a novel component of innate antiviral immunity, shares a TBK1-binding domain with NAP1 and TANK. EMBO J. 2007;26:3180–3190. doi: 10.1038/sj.emboj.7601743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton S, Hesson L, Peggie M, Cohen P. Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS Lett. 2008;582:997–1002. doi: 10.1016/j.febslet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 21.Chau TL, et al. Are the IKKs and IKK-related kinases TBK1 and IKK-epsilon similarly activated? Trends Biochem Sci. 2008;33:171–180. doi: 10.1016/j.tibs.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhao T, et al. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat Immunol. 2007;8:592–600. doi: 10.1038/ni1465. [DOI] [PubMed] [Google Scholar]
- 23.Chariot A, et al. Association of the adaptor TANK with the I kappa B kinase (IKK) regulator NEMO connects IKK complexes with IKK epsilon and TBK1 kinases. J Biol Chem. 2002;277:37029–37036. doi: 10.1074/jbc.M205069200. [DOI] [PubMed] [Google Scholar]
- 24.Clark K, Plater L, Peggie M, Cohen P. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: A distinct upstream kinase mediates Ser-172 phosphorylation and activation. J Biol Chem. 2009;284:14136–14146. doi: 10.1074/jbc.M109.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimada T, et al. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- 26.Lang V, et al. betaTrCP-mediated proteolysis of NF-kappaB1 p105 requires phosphorylation of p105 serines 927 and 932. Mol Cell Biol. 2003;23:402–413. doi: 10.1128/MCB.23.1.402-413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 28.Gatot JS, et al. Lipopolysaccharide-mediated interferon regulatory factor activation involves TBK1-IKKepsilon-dependent Lys(63)-linked polyubiquitination and phosphorylation of TANK/I-TRAF. J Biol Chem. 2007;282:31131–31146. doi: 10.1074/jbc.M701690200. [DOI] [PubMed] [Google Scholar]
- 29.Bloor S, et al. Signal processing by its coil zipper domain activates IKK gamma. Proc Natl Acad Sci USA. 2008;105:1279–1284. doi: 10.1073/pnas.0706552105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng G, Baltimore D. TANK, a co-inducer with TRAF2 of TNF- and CD 40L-mediated NF-kappaB activation. Genes Dev. 1996;10:963–973. doi: 10.1101/gad.10.8.963. [DOI] [PubMed] [Google Scholar]
- 31.Rothe M, et al. I-TRAF is a novel TRAF-interacting protein that regulates TRAF-mediated signal transduction. Proc Natl Acad Sci USA. 1996;93:8241–8246. doi: 10.1073/pnas.93.16.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Häcker H, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 34.Oganesyan G, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.