Abstract
Over the past two decades, mass spectrometry (MS) has emerged as a bone fide approach for structural biology. MS can inform on all levels of protein organization, and enables quantitative assessments of their intrinsic dynamics. The key advantages of MS are that it is a sensitive, high-resolution separation technique with wide applicability, and thereby allows the interrogation of transient protein assemblies in the context of complex mixtures. Here we describe how molecular-level information is derived from MS experiments, and how it can be combined with spatial and dynamical restraints obtained from other structural biology approaches to allow hybrid studies of protein architecture and movements.
Introduction
The majority of proteins exist and operate in the cell as multimeric assemblies, held together by noncovalent interactions of varying strength and lifetime [1]. These protein complexes underpin virtually all cellular processes, and therefore understanding their interactions, structure, and dynamics is of critical importance for human health and medicine. Our knowledge of such molecular details is inexorably tied to the analytical approaches available, and their ability in overcoming the complexity of these macromolecules. However, many protein complexes of critical importance continue to confound individual structural biology techniques, often as a result of being present in low levels within mixtures, or displaying intrinsic dynamics. As such there is a growing interest in developing ‘hybrid’ strategies which combine the benefits of different technologies to characterize the most challenging protein assemblies [2].
Over the last two decades mass spectrometry (MS) has emerged as a key approach for structural biology. Generally associated with proteomics and systems biology, in which the proteins that comprise an interaction network are identified and quantified [3], MS can also be used to directly probe the structure and dynamics of protein assemblies intact in the gas phase [4]. Here we highlight important recent methodological advances in this field, and future areas of development. In parallel, we attempt to describe the ways in which MS can contribute to modern studies of protein assemblies, and make the case that this approach has now become an indispensable part of modern structural and dynamical biology.
Quantifying the Oligomeric Distribution of Protein Assemblies
In the early 1990s, studies were performed which demonstrated that protein assemblies could be transferred into the vacuum of the mass spectrometer without their dissociation [5], allowing the measurement of their mass with unprecedented precision and accuracy (Box 1). The information obtainable from just simple mass measurement of an intact protein complex is considerable, allowing the facile determination of oligomeric state, and the stoichiometry of ligand or cofactor binding. The unparalleled mass resolution of MS can however be confounded by the effects of multiple charging during nanolectrospray ionization (nESI, the preferred method for ionizing non-covalent complexes [6]) potentially leading to proteins of significantly different masses overlapping in mass-to-charge (m/z) space.
Box 1: Measuring the mass and abundance of protein oligomers.
Accuracy and precision
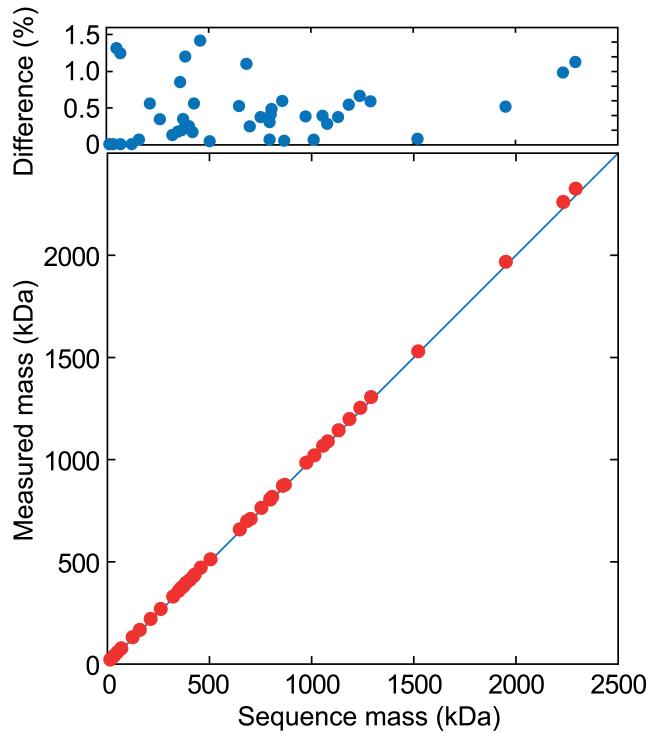
A comparison between the mass calculated from the primary sequence and that measured by means of nESI-MS is shown for a range of proteins and complexes (Chart 1). There is only a very small deviation between the experimental data (red points) and a 1:1 correlation between expected and measured mass (blue line). The small positive discrepancy results from the residual binding of solvent and buffer [6], and is less than 1.5 % for all the assemblies shown here (blue points). Such adduction is the primary determinant of the width of the peaks in the mass spectra [73], and leads to an ‘effective resolving power’ which is lower than the instrumental limits of modern mass spectrometers. Nevertheless, with mass differences of ≈1 % in 1 MDa routinely resolvable [73], the resolution remains far in excess of other mass-separative techniques.
Quantifying oligomeric distributions
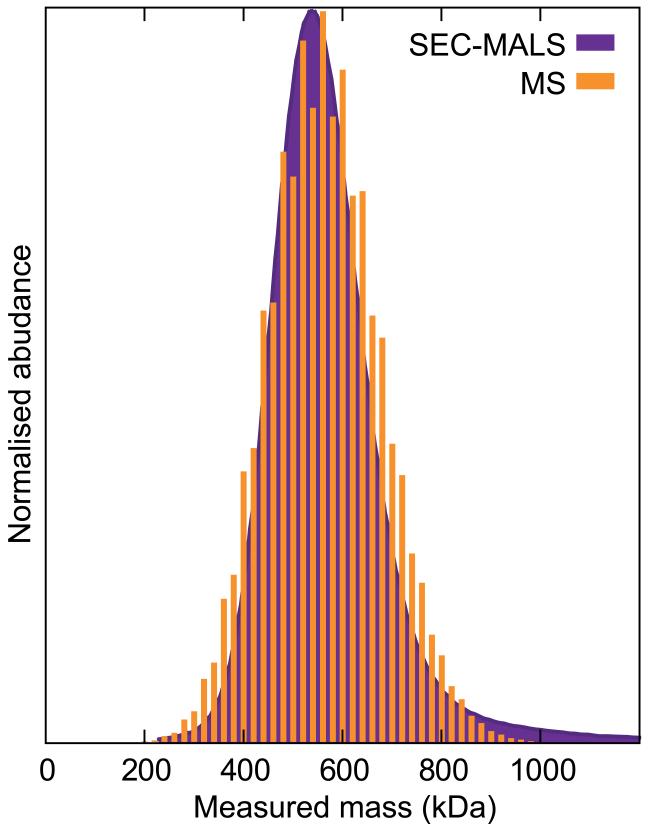
The unrivalled separative ability of MS allows for the identification of many different components within an mixture of complexes in solution. In cases where the species are of similar composition, the relative intensities recorded in MS data can be used to extract the relative populations of each oligomer. A comparison of the mass distribution of αB-crystallin obtained by multi-angle light scattering coupled to size-exclusion chromatography (SEC-MALS) (purple) [20] and MS (orange) [21] shows excellent agreement (Chart 2). This demonstrates how the solution phase distribution of oligomers is faithfully maintained in the gas phase, and can therefore be quantified by means of MS. MS offers dramatically improved mass accuracy and resolution of separation over SEC-MALS however, revealing that for αB-crystallin oligomers comprising an even number of subunits are more prevalent than those with an odd number (i.e. more 28- and 30mer than 29mer), a ~3% difference in mass not discernible by SEC-MALS (Chart 2).
One approach to overcome this challenge has been the development of user-guided software which facilitates the deconvolution of complex spectra [7,8]. As the peaks recorded for intact protein complexes are inherently broader than those obtained for small molecules and peptides, these approaches enable a more rigorous interpretation of such mass spectra [9,10]. Such software therefore represents an important step towards the fully automated MS analysis of proteins and protein assemblies.
Alternatively, an experimental strategy to simplify nESI data for complex mixtures is to reduce the charge states of the ionized components, and thereby increase the separation between adjacent peaks. Charge reduction can be achieved by using solution additives [11,12], or performing gas-phase chemistry [11,13,14], however currently the most commonly used strategy is tandem-MS with collision-induced dissociation (CID). In this approach, oligomer ions can be selected and activated such that monomers are removed, taking with them a disproportionate amount of charge [15]. This has the effect of both increasing the separation between charge states, and disrupting the congruence of peaks from species of different mass. This approach has allowed the separation of multiple stoichiometries of the ribosomal stalk complex which were overlapping in the original mass spectra [16], the confirmation of the accurate mass measurement of hepatitis B virus capids in the 3-4 MDa range [17], and elucidation of the stoichiometry of small-molecule binding to membrane protein assemblies [18].
As well as enabling the identification of different components within heterogeneous mixtures, MS can be used to extract the relative abundances of these components by quantifying their intensities in the mass and tandem mass spectra. This simple strategy is analogous to ‘spectrum counting’, a methodology widely used in quantitative proteomics [3]. In cases where the individual components are biophysically similar the oligomeric distributions derived by means of nESI-MS match those obtained using other approaches very well, demonstrating how the solution-phase distribution of oligomers can be faithfully maintained in the gas phase (Box 1).
Notably, however, MS offers dramatically improved mass accuracy and resolution of separation. In the case of the molecular chaperone HSP18.1 and its interaction with luciferase, over 300 complex stoichiometries were observed and their relative abundances quantified. Interestingly, complexes containing an even number of HSP18.1 subunits were found to be approximately 20% more abundant than those with an odd number [19], a property echoed in the distribution of oligomers populated by αB-crystallin [20,21]. In the latter case this ‘even preference’ was found to be regulated by post-translation modification or solution conditions [20,22], such that variations in the free energy of the dimeric interfaces could be determined [21]. Such subtleties in oligomeric distribution can currently only be resolved and quantified by MS, and demonstrate the utility of this approach for not only providing structural insight, but also simultaneously extracting the thermodynamics which govern protein assembly.
Blueprinting Multi-protein Assemblies
While studies of protein assemblies at equilibrium under native conditions can reveal their stoichiometries they populate, experiments employing solution conditions which perturb non-covalent interactions can provide complementary information on their composition, connectivity and architecture. Under ‘severe’ conditions, such as high concentrations of chemical denaturant or pH extremes, multi-protein complexes can be completely disassembled and their component chains unfolded. A mass spectrum under these conditions will allow the determination of accurate masses for the individual components. Such information is often vital for unambiguously assigning protein stoichiometry, especially in the case of low-abundance endogenous proteins for which sequence database entries are often lacking proper annotation [23].
Between these extremes of solution conditions, which result in either complete preservation or disruption of quaternary structure, variations in ionic strength, adjustment of pH, or the addition of small amounts of organic solvent can lead to partial destabilization of the oligomers [24]. Under such conditions, MS data can reveal ‘subcomplexes’, non-covalently bound building blocks of the assembly. When multiple such subcomplexes are identified they can be combined to elucidate the two-dimensional connectivity of subunits within the oligomer. This strategy was applied to elucidate the protein-protein interactions within two important molecular machines involved in ubiquitin-mediated proteolysis, the proteasome [25], and signalosome [26]. Furthermore, analysis of which subcomplexes are preferentially formed allows the establishment of a hierarchy of assembly, which correlates qualitatively with the size of the subunit interfaces and the evolutionary pathway of the protein complex [27]. The thermodynamic quantities governing interface strength and protein assembly can also be directly determined by examining the equilibrium distribution of complexes, subcomplexes and subunits as a function of temperature [19].
While of great utility for mapping inter-subunit connectivity, solution-phase disruption experiments are often complemented by studies which induce dissociation in the gas phase. Though the biophysical factors governing the release of subunits from heteromeric protein oligomers during CID remain incompletely understood, those that are readily expelled are unlikely to be located in the core of the assembly [15]. This observation has been used to help elucidate the topology of the proteasome lid [28], and RNA polymerase III [29]. Furthermore, close examination of the energy profile of CID can also be used to infer the protomers of protein assemblies [22,30].
The use of CID is however currently limited by its mechanistic underpinnings: in general exclusively monomers are expelled (irrespective of the oligomeric substructure), and only a small number thereof [15]. This pathway of gas-phase dissociation can be somewhat altered through the manipulation of the protein complex charge state selected for CID [12,31,32], or by depositing the activation energy on a much faster timescale. One means to achieve the latter is surface-induced dissociation (SID), which, in the case of the heterohexamer toyocamycin nitrile hydratase, resulted in disassembly into its component non-covalently bound trimers [33]. This is a particularly exciting result as it may prove SID to be a means for directly determining the “building blocks” of protein assemblies. Combined with the observation that gas-phase activation can lead to the fragmentation of individual monomers within a noncovalent assembly to give sequence information [12,31,34-36], the possibility therefore emerges of gleaning information spanning from primary sequence to quaternary architecture from a single, rapid gas-phase measurement.
Obtaining Three-Dimensional Spatial Restraints
Complementary to experiments aimed at obtaining two-dimensional maps of protein-protein connectivity, there has been much interest in developing MS-based approaches for obtaining three-dimensional shape information. Spatial restraints for protein modeling can be derived from MS experiments in a number of ways. Perhaps the most intuitive approach involves chemical cross-linking of the protein oligomer, followed by identification of any intra- and inter-molecular cross-links by proteolysis and tandem-MS. These can then be interpreted as a direct distance constraint to guide the modeling of both protein complexes and their constituent monomers [37,38]. Alternatively, oxidative foot-printing [39] and hydrogen/deuterium exchange (HDX) [40] experiments can be combined with MS to reveal the solvent accessibility of the protein chain. The former labels the side-chains at a resolution governed by the reactivity and accessibility of certain amino-acid side-chains [39], but recent developments in HDX-MS have led to the possibility of residue-level information. This can be achieved either through the use of a combination of proteases [41], or by electron-mediated cleavage of the protein backbone in the gas phase [42]. The latter option is particularly attractive as it allows for the injection of a mixture of proteins or conformers, their separation in m/z, and selective interrogation [43].
In addition to such experiments where spatial restraints are determined on a local level, MS experiments can also inform on a global oligomeric level. Since the charge states of globular proteins and assemblies are correlated with solvent-exposed surface area, this information can be used as general constraint to classify protein topology by MS [44], and to determine whether certain protein assemblies are in particularly extended or compact conformations [45]. A more explicit approach to assessing protein size is ion mobility spectrometry (IM), a technology that can be coupled directly to MS and separates protein assemblies according to their ability to traverse a pressurized ion guide under the influence of a weak electric field. The transit time of the ions is directly related to their size in terms of a rotationally averaged collision cross section (CCS) [46]. The correlation between the experimental CCSs and values calculated in silico from high-resolution structures of protein assemblies is excellent (Box 2). This demonstrates that proteins retain a ‘memory’ of their native quaternary structure in the gas phase, and thereby that IM measurements can be used to determine the native size of protein assemblies in solution.
Box 2: Measuring the size of protein oligomers.
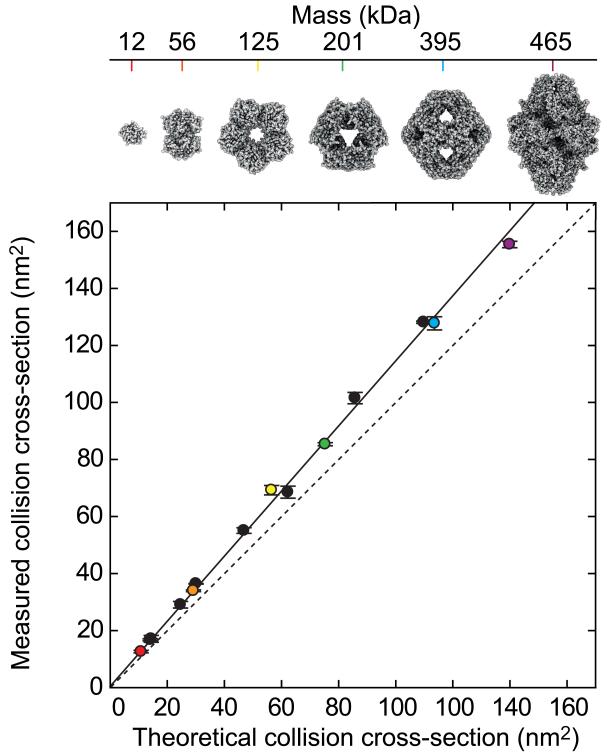
The use of IM-MS in structural biology is motivated by the general observation that the quaternary structure of proteins can be maintained in the gas phase, at least for the tens of milliseconds required for IM measurement [74], as evidenced by the correspondence between experimentally determined CCSs and those estimated from their atomic structures [75,76]. A comparison between theoretical CCSs, calculated in silico using a simple projection approximation (PA) algorithm [77], and CCS measurements for a range of proteins and complexes generated from native solution conditions [75] is shown in Chart 3. The error bars represent the variations in CCS over all charge states observed for the complexes (±2 standard deviations from the mean), and the data points are colour-coded with the structures shown in the top panel. The experimental and theoretical values are very well correlated, in this case by CCSExp = 1.14×CCSPA, with an RMSD of 3%. Though the precise relationship between theory and experiment depends on the specifics of how the theoretical CCS is determined, this data demonstrates that simple linear scaling can be used to relate measured and theoretical values, and therefore that IM-MS data can provide direct spatial restraints for protein topology models.
Alternative computational methods are available to estimate CCS, but are more computationally expensive and currently do not offer significantly greater accuracy for comparison with IM measurements of protein complexes than the scaled PA estimates shown in Chart 3 [78]. The PA approach has the additional advantage of supporting rapid coarse-grained protein complex representations that are of critical importance in cases where no atomic structures exist for comparison [53,79]. Protein complexes having flexible or labile structures can be difficult targets for CCS measurement, and on-going efforts are aimed at developing generalized methods for stabilizing such structures in the absence of bulk solvent [11,80]. Additionally, the generation of models for complexes of low symmetry is currently an obstacle for IM data interpretation in the absence of structural information from other approaches. Current commercial instrumentation relies on CCS calibration with known standards [75], and has both high accuracy and resolution [81,82]. For native proteins and assemblies it appears that the apparent resolution achieved in state-of-the-art IM instrumentation is largely governed by the conformational heterogeneity of the proteins under investigation [82]. Therefore, while subtle differences in CCS (<2%) remain challenging to resolve, careful analysis of IM peak widths can allow the probing of conformational states and fluctuations of proteins [83].
Though a CCS represents only a single spatial restraint, the fact that size information can be obtained on all species separable in m/z makes IM-MS very attractive for the study of heterogeneous systems. As a result there has been considerable effort in studying early aggregates associated with protein desposition diseases by means of IM-MS. Investigations of amyloid-forming protein fragments [47], Amyloid-β peptide [48,49], islet amyloid polypeptide [50], and β2-microglobulin [51] have shown the presence of not only multiple oligomeric states, but also different conformations of each. The ability of IM-MS to separate both these sources of heterogeneity allows for the detailed characterisation of oligomeric microstates, providing insight into the interplay between globular and extended oligomeric conformations on the fibrilogenesis and cytotoxicity pathways.
Similarly, while the trajectory of virus assembly has been investigated by a variety of MS-based approaches, the topology of oligomeric intermediates has remained elusive. IM-MS has recently been applied to assess the shape of capsid-protein oligomers, generated by destabilising intact norovirus and hepatitis B virus capsids [52]. By comparing CCS values with those calculated from atomic models, these oligomers were shown to be planar rather than globular, providing structural insight into disassembly and assembly of the capsid [52]. Measuring the size of oligomeric disassembly products can also be used to refine structural models of the corresponding intact complexes. For instance, IM measurements of two subcomplexes of the eukaryotic initiation factor 3 allowed for a two-dimensional blueprint of the complex to be refined into a partial topology model [53]. MS approaches therefore are capable of providing useful restraints, on both the local and global oligomeric levels, providing insight into the static structures of protein assemblies.
Monitoring dynamical motions of protein assemblies
The function of proteins is however not simply governed by their structures, but also by the motions they undergo. As these dynamics span secondary to quinary structure [54] and picoseconds to days [2], a variety of MS methods have been developed for their interrogation [55]. HDX-MS can reveal local fluctuations of individual protein chains [40], with amino-acid level information achievable on the minute timescale in real time [56]. Pulse-label HDX methods can achieve millisecond time resolution [40], and recent advances in pump/probe oxidative foot-printing have shown the capability of accessing microsecond regimes [57]. Online approaches, in which intact proteins and complexes (rather than peptides) are injected into the mass spectrometer have the benefit of enabling multiple species to be monitored in tandem [58]. The future combination of this with electron-mediated fragmentation raises the exciting possibility of obtaining dynamical information on co-populated, transient species at the residue level.
This separative capability of MS also renders it well suited to monitoring the assembly of protein complexes, simply by incubating components and obtaining mass spectra in real time. In this way the role of individual subunits and sections of sequence in governing the assembly of the 20S proteasome [59] and DNA clamp loader were elucidated [60]. A similar approach was employed to study the molecular chaperone action of HSP18.1, with the kinetics of target binding revealing a two-stage mechanism of protection [19].
Complementary to studying such assembly dynamics, the fluctuations in quaternary structure which proteins undergo at equilibrium can also be investigated by means of MS. Subunit exchange, the process in which monomers or other building blocks move between oligomers, can be monitored in considerable detail by means of MS through monitoring a sample containing ‘mass-labeled’ and unlabelled protein as a function of time [61]. The label can be of natural origins, such as the use of protein isoforms or homologues that differ in mass, or achieved through recombinant expression of the protein with heavy isotopes. In this way, the effect of small molecule binding on the rate of oligomeric dissociation in both transthyretin [62] and glucosamine-6-phosphate synthase [63] was elucidated. Furthermore, quantitative assessment of the quaternary dynamics suggested the presence of two conformations of HSP26 oligomers [64], revealed the pH dependence of the dissociation rates of the different interfaces in αB-crystallin [21], and provided a rationale for the specificity of subunit ordering in pilius assembly [65]. In the case of two plant molecular chaperones, subunit exchange was found to proceed via the movement of dimers, revealing them as the building block of the oligomers [61]. Experiments such as these can therefore simultaneously inform both on the architecture of protein assemblies and their inherent dynamics.
MS in Integrative Structural and Dynamical Biology
In this article we have detailed some of the ways in which MS-based approaches can be used to obtain information regarding the structure and dynamics of protein assemblies. MS can inform from the primary to quinary levels, and on the sub-millisecond to hour timescales (Fig 1). While other experimental and computational techniques can provide information of higher resolution, both in terms of space and time, MS has considerable benefits, three of which stand out as particularly important for structural biology. Firstly, MS is remarkably general in its applicability, allowing the study of complexes that range in terms of mass, size, solubility, flexibility, oligomeric composition, bound state, and dispersity. Additionally, it is a high-resolution separation technology that allows for the identification, quantification, and interrogation of different components within a mixture without ensemble averaging of species present in solution. Finally, MS has very low limits of detection and quantification, enabling not only the analysis of small amounts of dilute sample extracted directly from cells, but also the study of quaternary dynamics in real time.
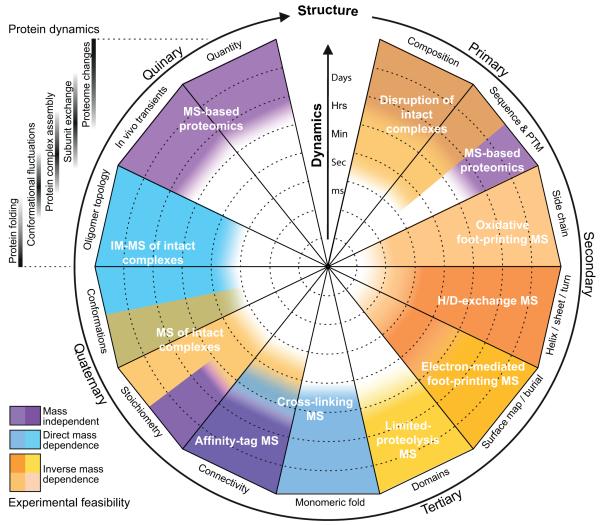
Figure 1. Structure and dynamics space accessible to MS-based approaches.
Proteins undergo a range of dynamical fluctuations, from folding of the polypeptide to changes in the composition of the proteome (indicated upper left). These processes span not only a wide range of timescales, but also all aspects of protein organisation, from primary to quinary (clockwise around wheel). A plethora of MS-based approaches can inform on many of these structural dynamics, and are indicated as overlapping coloured wedges, with their tractability in the temporal dimension indicated by shading and the radial scale bar (faster towards centre of wheel). These include ‘bottom-up’ experiments in which peptides, produced by proteolysis of cell extracts or purified components, are interrogated; and ‘top-down’ methodologies which rely on the examination of the proteins or assemblies intact in the gas-phase. Here we have grouped them according to their approximate feasibility as a function of mass (blue = easier as mass increases, orange = more difficult as mass increases, purple = independent of mass).
These valuable qualities of MS make it ideally placed for integration with other structural biology approaches. Most frequently, MS data is used to provide information as to oligomeric heterogeneity and stoichiometry, both as quality control or to directly guide high-resolution analysis [66,67]. Recently, MS has been used as a purification method, allowing the deposition of mass-selected ions for electron and atomic force microscopy investigation [68]. Spatial restraints from MS can also be directly integrated with those from other structural techniques to facilitate the building of topological models of protein assemblies [69,70]. Similarly measurements of hierarchical protein fluctuations can be correlated between techniques, providing ‘dynamical restraints’ as to the organization of the component species [71,72]. Such integrated measurement and correlation of spatial and dynamical information represents an emerging paradigm for modern structural biology, and one in which MS-based approaches are likely to play a central role.
Highlights for Benesch & Ruotolo.
Mass spectrometry is a sensitive, high-resolution means for determining the oligomeric distribution of proteins
The different oligomers and conformers comprising a heterogeneous ensemble of proteins can be individually interrogated
Intra- and inter-subunit connectivity, solvent accessibility, and oligomeric size can be elucidated
Pre-equilibrium and equilibrium dynamics spanning residue to oligomer levels can be measured on the μs to hour timescales
Mass spectrometry is an approach of wide applicability which can be integrated into hybrid structural biology approaches
Chart 1.
Chart 2.
Chart 3.
Acknowledgments
The authors thank Zoe Hall and Gillian Hilton (both University of Oxford); JLPB is a Royal Society University Research Fellow; and BTR acknowledges support from the National Institutes of Health (1-R01-GM-095832-01) and the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robinson CV, Sali A, Baumeister W. The molecular sociology of the cell. Nature. 2007;450:973–982. doi: 10.1038/nature06523. [DOI] [PubMed] [Google Scholar]
- 2.Russel D, Lasker K, Phillips J, Schneidman-Duhovny D, Velazquez-Muriel JA, Sali A. The structural dynamics of macromolecular processes. Curr Opin Cell Biol. 2009;21:97–108. doi: 10.1016/j.ceb.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson T, Mann M, Aebersold R, Yates JR, 3rd, Bairoch A, Bergeron JJ. Mass spectrometry in high-throughput proteomics: ready for the big time. Nat Methods. 2010;7:681–685. doi: 10.1038/nmeth0910-681. [DOI] [PubMed] [Google Scholar]
- 4.Heck AJ. Native mass spectrometry: a bridge between interactomics and structural biology. Nat Methods. 2008;5:927–933. doi: 10.1038/nmeth.1265. [DOI] [PubMed] [Google Scholar]
- 5.Loo JA. Studying noncovalent protein complexes by electrospray ionization mass spectrometry. Mass Spectrom Rev. 1997;16:1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Benesch JLP, Ruotolo BT, Simmons DA, Robinson CV. Protein complexes in the gas phase: technology for structural genomics and proteomics. Chem. Rev. 2007;107:3544–3567. doi: 10.1021/cr068289b. [DOI] [PubMed] [Google Scholar]
- 7.Hernández H, Makarova OV, Makarov EM, Morgner N, Muto Y, Krummel DP, Robinson CV. Isoforms of U1-70k control subunit dynamics in the human spliceosomal U1 snRNP. PLoS One. 2009;4:e7202. doi: 10.1371/journal.pone.0007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Breukelen B, Barendregt A, Heck AJ, van den Heuvel RH. Resolving stoichiometries and oligomeric states of glutamate synthase protein complexes with curve fitting and simulation of electrospray mass spectra. Rapid Commun Mass Spectrom. 2006;20:2490–2496. doi: 10.1002/rcm.2620. [DOI] [PubMed] [Google Scholar]
- 9.Lane LA, Fernandez-Tornero C, Zhou M, Morgner N, Ptchelkine D, Steuerwald U, Politis A, Lindner D, Gvozdenovic J, Gavin AC, et al. Mass spectrometry reveals stable modules in holo and apo RNA polymerases I and III. Structure. 2011;19:90–100. doi: 10.1016/j.str.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Poliakov A, van Duijn E, Lander G, Fu CY, Johnson JE, Prevelige PE, Jr., Heck AJ. Macromolecular mass spectrometry and electron microscopy as complementary tools for investigation of the heterogeneity of bacteriophage portal assemblies. J Struct Biol. 2007;157:371–383. doi: 10.1016/j.jsb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Bagal D, Kitova EN, Liu L, El-Hawiet A, Schnier PD, Klassen JS. Gas phase stabilization of noncovalent protein complexes formed by electrospray ionization. Anal Chem. 2009;81:7801–7806. doi: 10.1021/ac900611a. [DOI] [PubMed] [Google Scholar]
- 12.Pagel K, Hyung SJ, Ruotolo BT, Robinson CV. Alternate dissociation pathways identified in charge-reduced protein complex ions. Anal Chem. 2010;82:5363–5372. doi: 10.1021/ac101121r. • In depth study of how charge modulates the gas-phase unfolding and dissociation pathway of protein assemblies.
- 13.Abzalimov RR, Kaltashov IA. Electrospray ionization mass spectrometry of highly heterogeneous protein systems: protein ion charge state assignment via incomplete charge reduction. Anal Chem. 2010;82:7523–7526. doi: 10.1021/ac101848z. [DOI] [PubMed] [Google Scholar]
- 14.Bornschein RE, Hyung SJ, Ruotolo BT. Ion Mobility-Mass Spectrometry Reveals Conformational Changes in Charge Reduced Multiprotein Complexes. J Am Soc Mass Spectrom. 2011 doi: 10.1007/s13361-011-0204-y. in press. [DOI] [PubMed] [Google Scholar]
- 15.Benesch JLP. Collisional activation of protein complexes: picking up the pieces. J Am Soc Mass Spectrom. 2009;20:341–348. doi: 10.1016/j.jasms.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Gordiyenko Y, Deroo S, Zhou M, Videler H, Robinson CV. Acetylation of L12 increases interactions in the Escherichia coli ribosomal stalk complex. J Mol Biol. 2008;380:404–414. doi: 10.1016/j.jmb.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 17.Uetrecht C, Versluis C, Watts NR, Roos WH, Wuite GJ, Wingfield PT, Steven AC, Heck AJ. High-resolution mass spectrometry of viral assemblies: molecular composition and stability of dimorphic hepatitis B virus capsids. Proc Natl Acad Sci U S A. 2008;105:9216–9220. doi: 10.1073/pnas.0800406105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrera NP, Isaacson SC, Zhou M, Bavro VN, Welch A, Schaedler TA, Seeger MA, Miguel RN, Korkhov VM, van Veen HW, et al. Mass spectrometry of membrane transporters reveals subunit stoichiometry and interactions. Nat Methods. 2009;6:585–587. doi: 10.1038/nmeth.1347. •• Describes the ability of MS to determine the oligomeric state of, and stoichiometry of small molecule binding to, membrane protein assemblies, by transferring them into vacuum encapsulated within detergent micelles.
- 19.Stengel F, Baldwin AJ, Painter AJ, Jaya N, Basha E, Kay LE, Vierling E, Robinson CV, Benesch JLP. Quaternary dynamics and plasticity underlie small heat shock protein chaperone function. Proc Natl Acad Sci U S A. 2010;107:2007–2012. doi: 10.1073/pnas.0910126107. •• Demonstrates how tandem MS can be used to identify and quantify the >300 oligomers comprising an extremely polydisperse chaperone:target ensemble. Temperature- and time- dependent experiments also allow the extraction of thermodynamic and kinetic parameters determining oligomeric assembly.
- 20.Aquilina JA, Benesch JLP, Ding LL, Yaron O, Horwitz J, Robinson CV. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J. Biol. Chem. 2004;279:28675–28680. doi: 10.1074/jbc.M403348200. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin AJ, Lioe H, Robinson CV, Kay LE, Benesch JLP. aB-crystallin polydispersity is a conseqeunce of unbiased quaternary dynamics. J. Mol. Biol. 2011 doi: 10.1016/j.jmb.2011.07.016. in press. •• Back-to-back publications demonstrating how MS can be used to quantify oligomeric distributions and dynamics, and their interpretation in terms of interface free energies and microscopic rate constants. Correlation of these measurements with ones from NMR reveal a correlation between fluctuations in tertiary and quaternary structures, and thereby the regions of sequence responsible for protein assembly.
- 22.Benesch JLP, Ayoub M, Robinson CV, Aquilina JA. Small heat shock protein activity is regulated by variable oligomeric substructure. J Biol Chem. 2008;283:28513–28517. doi: 10.1074/jbc.M804729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou M, Robinson CV. When proteomics meets structural biology. Trends Biochem Sci. 2011;35:522–529. doi: 10.1016/j.tibs.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Hernández H, Robinson CV. Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nat Protoc. 2007;2:715–726. doi: 10.1038/nprot.2007.73. [DOI] [PubMed] [Google Scholar]
- 25.Sakata E, Stengel F, Fukunaga K, Zhou M, Saeki Y, Forster F, Baumeister W, Tanaka K, Robinson CV. The catalytic activity of ubp6 enhances maturation of the proteasomal regulatory particle. Mol Cell. 2011;42:637–649. doi: 10.1016/j.molcel.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Sharon M, Mao H, Boeri Erba E, Stephens E, Zheng N, Robinson CV. Symmetrical modularity of the COP9 signalosome complex suggests its multifunctionality. Structure. 2009;17:31–40. doi: 10.1016/j.str.2008.10.012. •• Multiplexed MS study on the signalosome, involving intact mass measurement of the complex, perturbation in solution, and dissociation in the gas phase, which culminates in a blueprint of this important assembly involved in proteolysis.
- 27.Levy ED, Boeri Erba E, Robinson CV, Teichmann SA. Assembly reflects evolution of protein complexes. Nature. 2008;453:1262–1265. doi: 10.1038/nature06942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharon M, Taverner T, Ambroggio XI, Deshaies RJ, Robinson CV. Structural organization of the 19S proteasome lid: insights from MS of intact complexes. PLoS Biol. 2006;4:e267. doi: 10.1371/journal.pbio.0040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzen K, Vannini A, Cramer P, Heck AJ. Structural biology of RNA polymerase III: mass spectrometry elucidates subcomplex architecture. Structure. 2007;15:1237–1245. doi: 10.1016/j.str.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Benesch JLP, Aquilina JA, Ruotolo BT, Sobott F, Robinson CV. Tandem mass spectrometry reveals the quaternary organization of macromolecular assemblies. Chem Biol. 2006;13:597–605. doi: 10.1016/j.chembiol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Benesch JLP, Ruotolo BT, Sobott F, Wildgoose J, Gilbert A, Bateman R, Robinson CV. Quadrupole-time-of-flight mass spectrometer modified for higher-energy dissociation reduces protein assemblies to peptide fragments. Anal Chem. 2009;81:1270–1274. doi: 10.1021/ac801950u. [DOI] [PubMed] [Google Scholar]
- 32.Lomeli SH, Peng IX, Yin S, Loo RR, Loo JA. New reagents for increasing ESI multiple charging of proteins and protein complexes. J Am Soc Mass Spectrom. 2010;21:127–131. doi: 10.1016/j.jasms.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackwell AE, Dodds ED, Bandarian V, Wysocki VH. Revealing the quaternary structure of a heterogeneous noncovalent protein complex through surface-induced dissociation. Anal Chem. 2011;83:2862–2865. doi: 10.1021/ac200452b. • Demonstrates how, by dissociating a hetero-hexamer into two non-covalently bound trimeric building blocks, SID has the potential to develop into a means for determining oligomeric architecture.
- 34.Wysocki VH, Jones CM, Galhena AS, Blackwell AE. Surface-induced dissociation shows potential to be more informative than collision-induced dissociation for structural studies of large systems. J Am Soc Mass Spectrom. 2008;19:903–913. doi: 10.1016/j.jasms.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin S, Loo JA. Top-Down Mass Spectrometry of Supercharged Native Protein-Ligand Complexes. Int J Mass Spectrom. 2011;300:118–122. doi: 10.1016/j.ijms.2010.06.032. • Two studies which describe the use of MS and electron-capture dissociation to first determine the stoichiometry of protein-protein and protein-ligand interactions, and then provide information on the sequence level as to protein identity or the site of ligand binding.
- 36.Zhang H, Cui W, Wen J, Blankenship RE, Gross ML. Native Electrospray and Electron-Capture Dissociation FTICR Mass Spectrometry for Top-down Studies of Protein Assemblies. Anal Chem. 2011 doi: 10.1021/ac200695d. DOI: 10.1021/ac200695d. • Two studies which describe the use of MS and electron-capture dissociation to first determine the stoichiometry of protein-protein and protein-ligand interactions, and then provide information on the sequence level as to protein identity or the site of ligand binding.
- 37.Leitner A, Walzthoeni T, Kahraman A, Herzog F, Rinner O, Beck M, Aebersold R. Probing native protein structures by chemical cross-linking, mass spectrometry, and bioinformatics. Mol Cell Proteomics. 2010;9:1634–1649. doi: 10.1074/mcp.R000001-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rappsilber J. The beginning of a beautiful friendship: cross-linking/mass spectrometry and modelling of proteins and multi-protein complexes. Journal of structural biology. 2011;173:530–540. doi: 10.1016/j.jsb.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiselar JG, Chance MR. Future directions of structural mass spectrometry using hydroxyl radical footprinting. J Mass Spectrom. 2010;45:1373–1382. doi: 10.1002/jms.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcsisin SR, Engen JR. Hydrogen exchange mass spectrometry: what is it and what can it tell us? Analytical and bioanalytical chemistry. 2010;397:967–972. doi: 10.1007/s00216-010-3556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcoux J, Man P, Petit-Haertlein I, Vives C, Forest E, Fieschi F. p47phox molecular activation for assembly of the neutrophil NADPH oxidase complex. J Biol Chem. 2010;285:28980–28990. doi: 10.1074/jbc.M110.139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rand KD, Zehl M, Jensen ON, Jorgensen TJ. Protein hydrogen exchange measured at single-residue resolution by electron transfer dissociation mass spectrometry. Anal Chem. 2009;81:5577–5584. doi: 10.1021/ac9008447. [DOI] [PubMed] [Google Scholar]
- 43.Pan J, Han J, Borchers CH, Konermann L. Conformer-Specific Hydrogen Exchange Analysis of Abeta(1-42) Oligomers by Top-Down Electron Capture Dissociation Mass Spectrometry. Anal Chem. 2011 doi: 10.1021/ac200906v. DOI: 10.1021/ac200906v. • HDX and electron-transfer dissociation MS study which demonstrates how residue-level solvent accessibility can be interrogated for different species separated in m/z, revealing insight into the fold of Amyloid-β oligomers.
- 44.Benesch JLP, Robinson CV. Mass spectrometry of macromolecular assemblies: preservation and dissociation. Curr. Opin. Struct. Biol. 2006;16:245–251. doi: 10.1016/j.sbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Lorenzen K, Olia AS, Uetrecht C, Cingolani G, Heck AJ. Determination of stoichiometry and conformational changes in the first step of the P22 tail assembly. J Mol Biol. 2008;379:385–396. doi: 10.1016/j.jmb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruotolo BT, Benesch JLP, Sandercock AM, Hyung SJ, Robinson CV. Ion mobility-mass spectrometry analysis of large protein complexes. Nat Protoc. 2008;3:1139–1152. doi: 10.1038/nprot.2008.78. [DOI] [PubMed] [Google Scholar]
- 47.Bleiholder C, Dupuis NF, Wyttenbach T, Bowers MT. Ion mobility-mass spectrometry reveals a conformational conversion from random assembly to beta-sheet in amyloid fibril formation. Nat Chem. 2011;3:172–177. doi: 10.1038/nchem.945. • Group of studies demonstrating the utility of IM-MS in assessing the size of the heterogeneous ensemble of oligomers formed by amyloidogenic peptides.
- 48.Bernstein SL, Dupuis NF, Lazo ND, Wyttenbach T, Condron MM, Bitan G, Teplow DB, Shea JE, Ruotolo BT, Robinson CV, et al. Amyloid-beta protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem. 2009;1:326–331. doi: 10.1038/nchem.247. • Group of studies demonstrating the utility of IM-MS in assessing the size of the heterogeneous ensemble of oligomers formed by amyloidogenic peptides.
- 49.Kloniecki M, Jablonowska A, Poznanski J, Langridge J, Hughes C, Campuzano I, Giles K, Dadlez M. Ion mobility separation coupled with MS detects two structural states of Alzheimer’s disease Abeta1-40 peptide oligomers. J Mol Biol. 2011;407:110–124. doi: 10.1016/j.jmb.2011.01.012. • Group of studies demonstrating the utility of IM-MS in assessing the size of the heterogeneous ensemble of oligomers formed by amyloidogenic peptides.
- 50.Salamekh S, Brender JR, Hyung SJ, Nanga RP, Vivekanandan S, Ruotolo BT, Ramamoorthy A. A Two-Site Mechanism for the Inhibition of IAPP Amyloidogenesis by Zinc. J Mol Biol. 2011 doi: 10.1016/j.jmb.2011.05.015. DOI:10.1016/j.jmb.2011.05.015. • Group of studies demonstrating the utility of IM-MS in assessing the size of the heterogeneous ensemble of oligomers formed by amyloidogenic peptides.
- 51.Smith DP, Radford SE, Ashcroft AE. Elongated oligomers in beta2-microglobulin amyloid assembly revealed by ion mobility spectrometry-mass spectrometry. Proc Natl Acad Sci U S A. 2010;107:6794–6798. doi: 10.1073/pnas.0913046107. •• IM-MS and real-time MS studies enable the study of the structure and quaternary dynamics of the heterogeneous ensemble of oligomers formed by β2-microglobulin, and provide insights into the early stages of fibrilogenesis.
- 52.Uetrecht C, Barbu IM, Shoemaker GK, van Duijn E, Heck AJ. Interrogating viral capsid assembly with ion mobility-mass spectrometry. Nat Chem. 2011;3:126–132. doi: 10.1038/nchem.947. •• Study that employs MS to accurately measure the mass of viral particles in excess 3 MDa, and, via destabilization of the capsids in solution, determine possible topologies of the oligomeric intermediates from IM-MS experiments.
- 53.Pukala TL, Ruotolo BT, Zhou M, Politis A, Stefanescu R, Leary JA, Robinson CV. Subunit architecture of multiprotein assemblies determined using restraints from gas-phase measurements. Structure. 2009;17:1235–1243. doi: 10.1016/j.str.2009.07.013. •• Demonstrates the use of IM-MS to individually address oligomers within a mixture and, by using coarse-grained modeling, determine the likely 3D arrangements of subunits.
- 54.McConkey EH. Molecular evolution, intracellular organization, and the quinary structure of proteins. Proc Natl Acad Sci U S A. 1982;79:3236–3240. doi: 10.1073/pnas.79.10.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-Nissan G, Sharon M. Capturing protein structural kinetics by mass spectrometry. Chem Soc Rev. 2011 doi: 10.1039/c1cs15052a. DOI: 10.1039/C1CS15052A. [DOI] [PubMed] [Google Scholar]
- 56.Sterling HJ, Williams ER. Real-Time Hydrogen/Deuterium Exchange Kinetics via Supercharged Electrospray Ionization Tandem Mass Spectrometry. Anal Chem. 2010;82:9050–9057. doi: 10.1021/ac101957x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J, Rempel DL, Gross ML. Temperature jump and fast photochemical oxidation probe submillisecond protein folding. J Am Chem Soc. 2010;132:15502–15504. doi: 10.1021/ja106518d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simmons DA, Konermann L. Characterization of transient protein folding intermediates during myoglobin reconstitution by time-resolved electrospray mass spectrometry with on-line isotopic pulse labeling. Biochemistry. 2002;41:1906–1914. doi: 10.1021/bi011697j. [DOI] [PubMed] [Google Scholar]
- 59.Sharon M, Witt S, Glasmacher E, Baumeister W, Robinson CV. Mass spectrometry reveals the missing links in the assembly pathway of the bacterial 20 S proteasome. J Biol Chem. 2007;282:18448–18457. doi: 10.1074/jbc.M701534200. [DOI] [PubMed] [Google Scholar]
- 60.Park AY, Jergic S, Politis A, Ruotolo BT, Hirshberg D, Jessop LL, Beck JL, Barsky D, O’Donnell M, Dixon NE, et al. A single subunit directs the assembly of the Escherichia coli DNA sliding clamp loader. Structure. 2010;18:285–292. doi: 10.1016/j.str.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Painter AJ, Jaya N, Basha E, Vierling E, Robinson CV, Benesch JLP. Real-time monitoring of protein complexes reveals their quaternary organization and dynamics. Chem Biol. 2008;15:246–253. doi: 10.1016/j.chembiol.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Hyung SJ, Deroo S, Robinson CV. Retinol and retinol-binding protein stabilize transthyretin via formation of retinol transport complex. ACS Chem Biol. 2010;5:1137–1146. doi: 10.1021/cb100144v. [DOI] [PubMed] [Google Scholar]
- 63.Chevreux G, Atmanene C, Lopez P, Ouazzani J, Van Dorsselaer A, Badet B, Badet-Denisot MA, Sanglier-Cianferani S. Monitoring the dynamics of monomer exchange using electrospray mass spectrometry: the case of the dimeric glucosamine-6-phosphate synthase. J Am Soc Mass Spectrom. 2011;22:431–439. doi: 10.1007/s13361-010-0054-z. [DOI] [PubMed] [Google Scholar]
- 64.Benesch JLP, Aquilina JA, Baldwin AJ, Rekas A, Stengel F, Lindner RA, Basha E, Devlin GL, Horwitz J, Vierling E, et al. The quaternary organization and dynamics of the molecular chaperone HSP26 are thermally regulated. Chem Biol. 2010;17:1008–1017. doi: 10.1016/j.chembiol.2010.06.016. • MS is combined with other biophysical techniques to quantify the oligomeric distribution and dynamics of this polydisperse molecular chaperone.
- 65.Rose RJ, Verger D, Daviter T, Remaut H, Paci E, Waksman G, Ashcroft AE, Radford SE. Unraveling the molecular basis of subunit specificity in P pilus assembly by mass spectrometry. Proc Natl Acad Sci U S A. 2008;105:12873–12878. doi: 10.1073/pnas.0802177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kintzer AF, Sterling HJ, Tang II, Williams ER, Krantz BA. Anthrax toxin receptor drives protective antigen oligomerization and stabilizes the heptameric and octameric oligomer by a similar mechanism. PLoS One. 2010;5:e13888. doi: 10.1371/journal.pone.0013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian Y, Simanshu DK, Ascano M, Diaz-Avalos R, Park AY, Juranek SA, Rice WJ, Yin Q, Robinson CV, Tuschl T, et al. Multimeric assembly and biochemical characterization of the Trax-translin endonuclease complex. Nat Struct Mol Biol. 2011;18:658–664. doi: 10.1038/nsmb.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benesch JLP, Ruotolo BT, Simmons DA, Barrera NP, Morgner N, Wang L, Saibil HR, Robinson CV. Separating and visualising protein assemblies by means of preparative mass spectrometry and microscopy. J Struct Biol. 2010;172:161–168. doi: 10.1016/j.jsb.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Jore MM, Lundgren M, van Duijn E, Bultema JB, Westra ER, Waghmare SP, Wiedenheft B, Pul U, Wurm R, Wagner R, et al. Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nat Struct Mol Biol. 2011;18:529–536. doi: 10.1038/nsmb.2019. •• Combined MS and electron microscopy study of Cascade, a protein assembly involved in the prokaryotic immune system, reveals its oligomeric shape and connectivity.
- 70.Zhou M, Sandercock AM, Fraser CS, Ridlova G, Stephens E, Schenauer MR, Yokoi-Fong T, Barsky D, Leary JA, Hershey JW, et al. Mass spectrometry reveals modularity and a complete subunit interaction map of the eukaryotic translation factor eIF3. Proc Natl Acad Sci U S A. 2008;105:18139–18144. doi: 10.1073/pnas.0801313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baldwin AJ, Hilton GR, Lioe H, Bagneris C, Benesch JLP, Kay LE. Quaternary dynamics of aB-crystallin as a direct conseqeunce of localised tertiary fluctuations in the C-terminus. J. Mol. Biol. 2011 doi: 10.1016/j.jmb.2011.07.017. in press. •• Back-to-back publications demonstrating how MS can be used to quantify oligomeric distributions and dynamics, and their interpretation in terms of interface free energies and microscopic rate constants. Correlation of these measurements with ones from NMR reveal a correlation between fluctuations in tertiary and quaternary structures, and thereby the regions of sequence responsible for protein assembly.
- 72.Carulla N, Zhou M, Arimon M, Gairi M, Giralt E, Robinson CV, Dobson CM. Experimental characterization of disordered and ordered aggregates populated during the process of amyloid fibril formation. Proc Natl Acad Sci U S A. 2009;106:7828–7833. doi: 10.1073/pnas.0812227106. • The kinetics of amyloid fibril formation monitored on the fibril, monomer, and residue levels by electron microscopy, MS, and nuclear magnetic resonance spectroscopy respectively. Temporal correlation of the experiments allows elucidation of the structural reorganizations which occur during fibrilogenesis.
- 73.McKay AR, Ruotolo BT, Ilag LL, Robinson CV. Mass measurements of increased accuracy resolve heterogeneous populations of intact ribosomes. J Am Chem Soc. 2006;128:11433–11442. doi: 10.1021/ja061468q. [DOI] [PubMed] [Google Scholar]
- 74.Ruotolo BT, Robinson CV. Aspects of native proteins are retained in vacuum. Curr. Opin. Chem. Biol. 2006;10:402–408. doi: 10.1016/j.cbpa.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 75.Bush MF, Hall Z, Giles K, Hoyes J, Robinson CV, Ruotolo BT. Collision cross sections of proteins and their complexes: a calibration framework and database for gas-phase structural biology. Anal Chem. 2010;82:9557–9565. doi: 10.1021/ac1022953. • Three papers describing figures of merit and best practices for state-of-the-art commercial IM-MS instrumentation.
- 76.Scarff CA, Thalassinos K, Hilton GR, Scrivens JH. Travelling wave ion mobility mass spectrometry studies of protein structure: biological significance and comparison with X-ray crystallography and nuclear magnetic resonance spectroscopy measurements. Rapid Commun Mass Spectrom. 2008;22:3297–3304. doi: 10.1002/rcm.3737. [DOI] [PubMed] [Google Scholar]
- 77.Williams JP, Lough JA, Campuzano I, Richardson K, Sadler PJ. Use of ion mobility mass spectrometry and a collision cross-section algorithm to study an organometallic ruthenium anticancer complex and its adducts with a DNA oligonucleotide. Rapid Commun Mass Spectrom. 2009;23:3563–3569. doi: 10.1002/rcm.4285. [DOI] [PubMed] [Google Scholar]
- 78.Jurneczko E, Barran PE. How useful is ion mobility mass spectrometry for structural biology? The relationship between protein crystal structures and their collision cross sections in the gas phase. Analyst. 2011;136:20–28. doi: 10.1039/c0an00373e. [DOI] [PubMed] [Google Scholar]
- 79.Politis A, Park AY, Hyung SJ, Barsky D, Ruotolo BT, Robinson CV. Integrating ion mobility mass spectrometry with molecular modelling to determine the architecture of multiprotein complexes. PLoS One. 2010;5:e12080. doi: 10.1371/journal.pone.0012080. • Demonstrates the use of IM-MS as a spatial restraint for atomic-resolution, coarse-grained, and hybrid molecular modeling strategies.
- 80.Han L, Hyung SJ, Mayers JJ, Ruotolo BT. Bound Anions Differentially Stabilize Multiprotein Complexes in the Absence of Bulk Solvent. J Am Chem Soc. 2011 doi: 10.1021/ja203527a. in press:DOI: 10.1021/ja203527a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giles K, Williams JP, Campuzano I. Enhancements in travelling wave ion mobility resolution. Rapid Commun Mass Spectrom. 2011;25:1559–1566. doi: 10.1002/rcm.5013. • Three papers describing figures of merit and best practices for state-of-the-art commercial IM-MS instrumentation.
- 82.Zhong Y, Hyung SJ, Ruotolo BT. Characterizing the resolution and accuracy of a second-generation traveling-wave ion mobility separator for biomolecular ions. Analyst. 2011 doi: 10.1039/c0an00987c. • Three papers describing figures of merit and best practices for state-of-the-art commercial IM-MS instrumentation.
- 83.Dupuis NF, Wu C, Shea JE, Bowers MT. Human islet amyloid polypeptide monomers form ordered beta-hairpins: a possible direct amyloidogenic precursor. J Am Chem Soc. 2009;131:18283–18292. doi: 10.1021/ja903814q. [DOI] [PMC free article] [PubMed] [Google Scholar]