Abstract
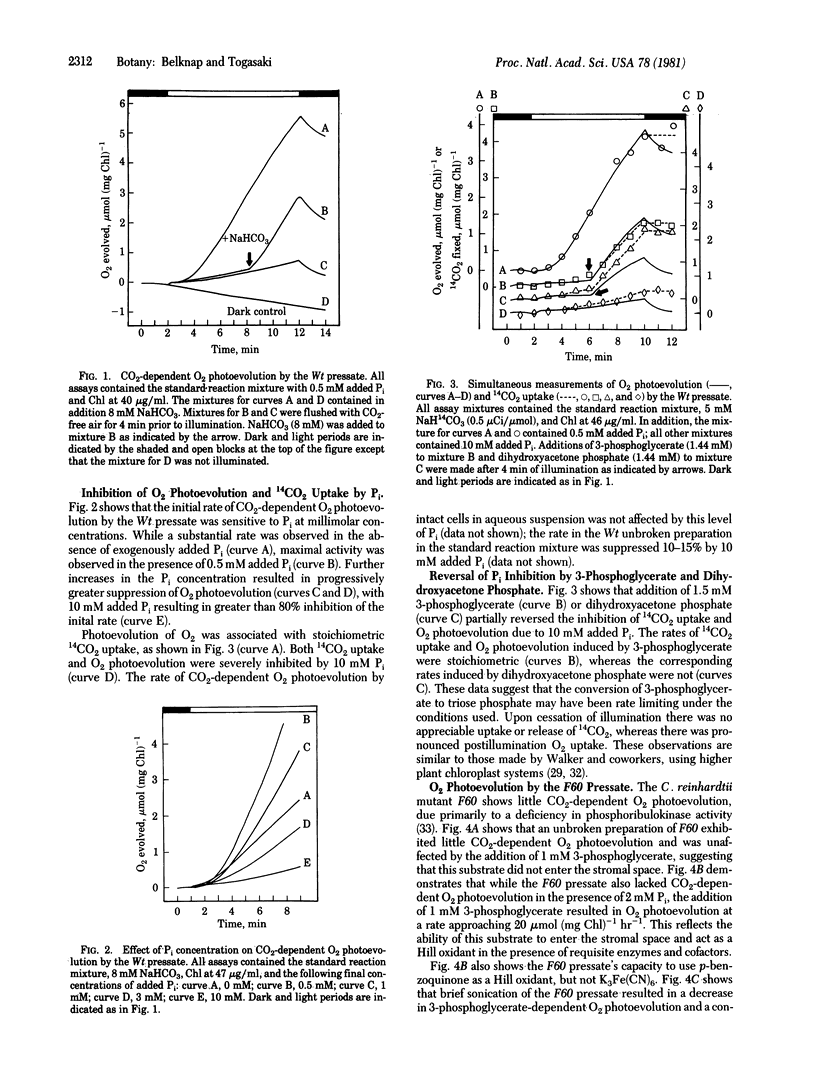
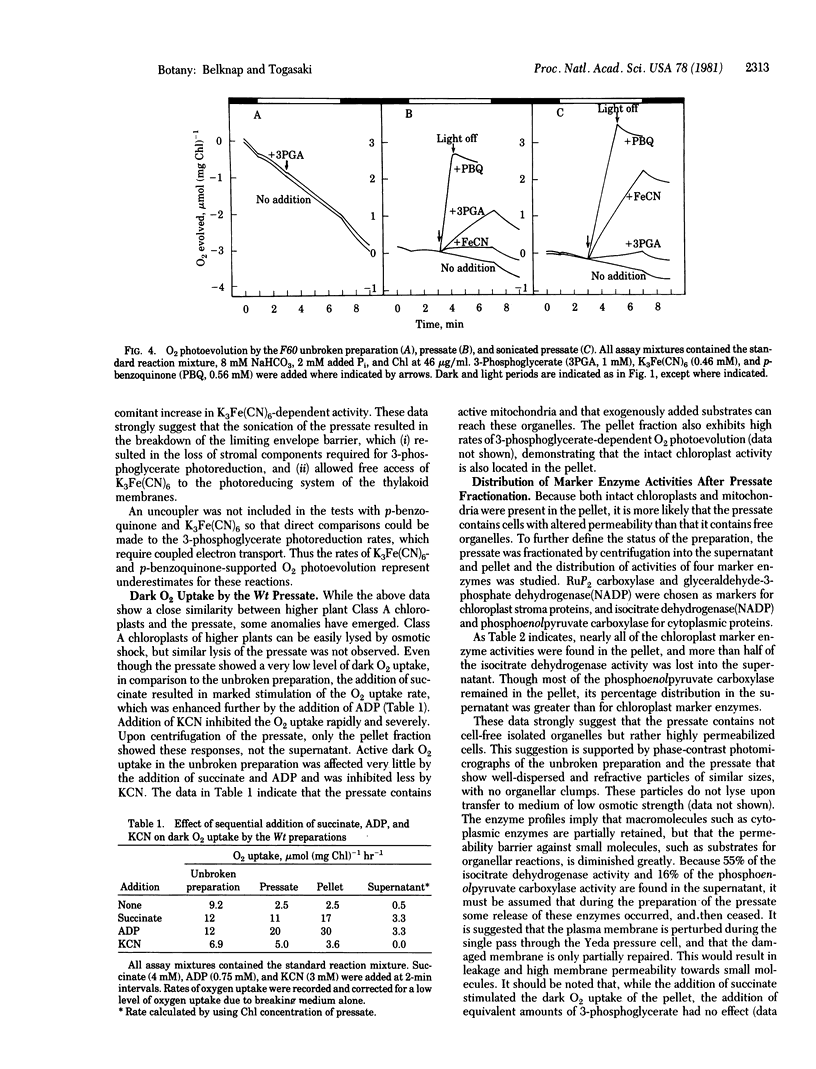
Chlamydomonas reinhardtii cells disrupted under low pressure in a Yeda press yielded a preparation ("presate") with high permeability toward substrates for Class A chloroplasts and intact mitochondria. The stoichiometric rates of CO2 uptake and O2 photoevolution by the wild-type cell pressate were severely suppressed by 10 mM exogenous phosphate, and this suppression could be reversed by the addition of either 3-phosphoglycerate or dihydroxyacetone phosphate. A mutant, F60, which lacks phosphoribulokinase activity and hence CO2-dependent O2 photoevolution, was studied by using intact cells, pressate, and sonicated pressate. In the pressate, the rate of 3-phosphoglycerate-dependent O2 photoevolution was high, whereas that dependent on K3Fe(CN)6 was low; the opposite was true of the sonicated pressate. p-Benzoquinone supported high rates of O2 evolution in both the pressate and the sonicated pressate. The slow O2 uptake in the dark by the dark-adapted wild-type pressate could be increased by the addition of succinate and further stimulated by ADP. Addition of KCN resulted in rapid but only partial suppression of this activity. Dark O2 uptake by the unpressed preparation did not show similar responses. The procedure described here opens the possibility of in situ analysis of Class A chloroplasts from wild-type and mutant strains of C. reinhardtii.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Amotz A., Avron M. Is nicotinamide adenine dinucleotide phosphate an obligatory intermediate in photosynthesis? Plant Physiol. 1972 Feb;49(2):244–248. doi: 10.1104/pp.49.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Avron M. Photosynthetic Activities of the Halophilic Alga Dunaliella parva. Plant Physiol. 1972 Feb;49(2):240–243. doi: 10.1104/pp.49.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell R. G., Levin W. B., Shephard D. C. Photosynthesis, Photorespiration and Respiration of Chloroplasts From Acetabularia mediterrania. Plant Physiol. 1969 Jul;44(7):946–954. doi: 10.1104/pp.44.7.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. I. Analysis of photosynthesis in green algae through mutation studies. Photophysiology. 1973;8:65–96. doi: 10.1016/b978-0-12-282608-5.50008-4. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Some effects of inorganic phosphate on O2 evolution by isolated chloroplasts. Biochim Biophys Acta. 1967;143(3):614–624. doi: 10.1016/0005-2728(67)90067-9. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. Photosynthesis by isolated chloroplasts. Reversal of orthophosphate inhibition by Calvin-cycle intermediates. Biochem J. 1968 Mar;107(1):89–95. doi: 10.1042/bj1070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsee W. T., Kahn J. S. Carbon dioxide fixation by isolated chloroplasts of Egulena gracilis. II. Inhibition of CO 2 fixation by AMP. Arch Biochem Biophys. 1972 May;150(1):302–309. doi: 10.1016/0003-9861(72)90039-2. [DOI] [PubMed] [Google Scholar]
- Forsee W. T., Kahn J. S. Carbon dioxide fixation by isolated chloroplasts of Euglena gracilis. I. Isolation of functionally intact chloroplasts and their characterization. Arch Biochem Biophys. 1972 May;150(1):296–301. doi: 10.1016/0003-9861(72)90038-0. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. O. Nomenclature for isolated chloroplasts. Nat New Biol. 1972 Jan 26;235(56):125–126. doi: 10.1038/newbio235125a0. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey S. W., Ulrich J., Allen M. B. Some photochemical properties of chloroplast preparations from the chrysomonad Hymenomonas sp. Biochim Biophys Acta. 1966 Jan 4;112(1):35–44. doi: 10.1016/s0926-6585(96)90005-9. [DOI] [PubMed] [Google Scholar]
- LEVINE R. P., EBERSOLD W. T. The genetics and cytology of Chlamydomonas. Annu Rev Microbiol. 1960;14:197–216. doi: 10.1146/annurev.mi.14.100160.001213. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Goodenough U. W. The genetics of photosynthesis and of the chloroplast in Chlamydomonas reinhardi. Annu Rev Genet. 1970;4:397–408. doi: 10.1146/annurev.ge.04.120170.002145. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975 Jun;55(6):1087–1092. doi: 10.1104/pp.55.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll B., Levine R. P. Characterization of a Photosynthetic Mutant Strain of Chlamydomonas reinhardi Deficient in Phosphoribulokinase Activity. Plant Physiol. 1970 Oct;46(4):576–580. doi: 10.1104/pp.46.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Walker D. A. Rapid separation of the chloroplast and cytoplasmic fractions from intact leaf protoplasts. Arch Biochem Biophys. 1979 Sep;196(2):319–323. doi: 10.1016/0003-9861(79)90584-8. [DOI] [PubMed] [Google Scholar]
- Saha S., Ouitrakul R., Izawa S., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. J Biol Chem. 1971 May 25;246(10):3204–3209. [PubMed] [Google Scholar]
- Schulman M. D., Gibbs M. D-glyceraldehyde 3-phosphate dehydrogenases of higher plants. Plant Physiol. 1968 Nov;43(11):1805–1812. doi: 10.1104/pp.43.11.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard D. C., Levin W. B., Bidwell R. G. Normal photosynthesis by isolated chloroplasts. Biochem Biophys Res Commun. 1968 Aug 13;32(3):413–420. doi: 10.1016/0006-291x(68)90677-3. [DOI] [PubMed] [Google Scholar]
- Shneyour A., Avron M. High biological activity in chloroplasts from Euglena gracilis prepared with a new gas pressure device. FEBS Lett. 1970 Jun 1;8(3):164–166. doi: 10.1016/0014-5793(70)80253-8. [DOI] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. F., Davies D. D. Regulation of phosphoenolpyruvate carboxylase of Zea mays by metabolites. Biochem J. 1973 Mar;131(3):451–458. doi: 10.1042/bj1310451. [DOI] [PMC free article] [PubMed] [Google Scholar]


