Abstract
The IκB kinase (IKK) complex plays a well-documented role in innate and adaptive immunity. This function has been widely attributed to its role as the central activator of the NF-κB family of transcription factors. However, another important consequence of IKK activation is the regulation of TPL-2, a MEK kinase that is required for activation of ERK-1/2 MAP kinases in myeloid cells following Toll-like receptor and TNF receptor stimulation. In unstimulated cells, TPL-2 is stoichiometrically complexed with the NF-κB inhibitory protein NF-κB1 p105, which blocks TPL-2 access to its substrate MEK, and the ubiquitin-binding protein ABIN-2 (A20-binding inhibitor of NF-κB 2), both of which are required to maintain TPL-2 protein stability. Following agonist stimulation, the IKK complex phosphorylates p105, triggering its K48-linked ubiquitination and degradation by the proteasome. This releases TPL-2 from p105-mediated inhibition, facilitating activation of MEK, in addition to modulating NF-κB activation by liberating associated Rel subunits for translocation into the nucleus. IKK-induced proteolysis of p105, therefore, can directly regulate both NF-κB and ERK MAP kinase activation via NF-κB1 p105. TPL-2 is critical for production of the proinflammatory cytokine TNF during inflammatory responses. Consequently, there has been considerable interest in the pharmaceutical industry to develop selective TPL-2 inhibitors as drugs for the treatment of TNF-dependent inflammatory diseases, such as rheumatoid arthritis and inflammatory bowel disease. This review summarizes our current understanding of the regulation of TPL-2 signaling function, and also the complex positive and negative roles of TPL-2 in immune and inflammatory responses.
Keywords: ABIN-2, COT, IKK, MAP3K8, MAP kinase, TLR, TPL-2, NF-κB, p105
Discovery of Tpl2 oncogene and initial characterization
The serine/threonine kinase TPL-2, also known as COT and MAP3K8, was independently discovered in three different laboratories in the early 1990s. Initially, Miyoshi et al. 1 identified Cot (cancer Osaka thyroid) as an oncogene, using DNA isolated from a human thyroid carcinoma cell line, to transform the SHOK hamster embryonic cell line in vitro. The rat homolog of Cot, called Tpl2 (tumor progression locus-2), was subsequently identified as a target for provirus integration in Moloney murine leukemia virus (MoMuLV)-induced T-cell lymphomas and demonstrated to transform NIH 3T3 fibroblasts in vitro 2. More recently, MoMuLV insertion into the murine Tpl2 locus was also found in two genome-wide screens for oncogenes using genetically sensitized mouse strains 3, 4. It has also been reported that the murine Tpl2 locus is a site of Mouse Mammary Tumor Virus (MMTV) proviral integration associated with the induction of mammary carcinomas in mice 5. (For simplicity, the different mammalian homologs will be referred to as Tpl2 in this review.) Proviral activation of Tpl2 oncogenicity consistently results in production of TPL-2 proteins truncated at the C terminus compared to the wild-type protein (generically termed TPL-2ΔC in this review), suggesting important roles of the C terminus in regulation of TPL-2 oncogenic activity (Figure 1). Consistent with this hypothesis, generation of transgenic mice expressing rat TPL-2 or TPL-2ΔC in their T cells has revealed that C-terminal deletion is essential for TPL-2 to induce the formation of T cell lymphoblastic lymphomas 6.
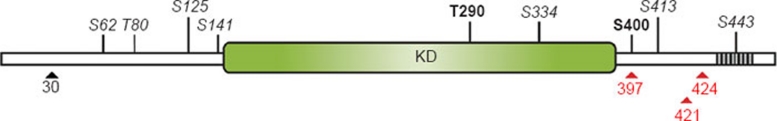
Figure 1.
TPL-2 structure and phosphorylation sites. The Tpl2 gene encodes two proteins, full-length M1-TPL-2 (p58) and M30-TPL-2 (p52). M30-TPL-2 is translated from the same mRNA transcript as M1-TPL-2 by alternative translational initiation at methionine 30 (M30, black arrowhead). The TPL-2 kinase domain (KD) is located in the centre of the protein, flanked by N-terminal and C-terminal regions with largely unknown functions. C-terminal truncation, however, results in a protein (TPL-2ΔC) with increased kinase-specific activity, suggesting that this region may inhibit TPL-2 kinase activity 6. Furthermore, a proposed degron sequence (435-457, shaded box) is located within the C terminus and confers destabilizing properties to full-length TPL-2 8. Consequently, TPL-2ΔC has increased protein stability and is expressed at higher levels. The positions of oncogenic truncations in TPL-2 identified in MoMuLV- and MMTV-induced murine tumors (424), human TPL-2 (COT) in transformed SHOK cells (397) and in a human lung adenocarcinoma (421) are indicated by red arrowheads. Several phosphorylation sites in TPL-2 have been identified by mass spectrometry 54. Two of these sites, T290 and S400, are known to regulate TPL-2 MEK kinase activity in vivo. T290 phosphorylation may also regulate TPL-2 release from its binding partner p105 (see Figure 4). The physiological significance of the sites shown in italics is not yet known.
Tpl2 is expressed in cells as 58 and 52 kDa protein isoforms due to alternative translational initiation at methionine 1 (M1) or methionine 30 (M30) 7. Both M1- and M30-TPL-2 proteins are predominantly localized in the cytoplasm 1. The weak transforming activity associated with full-length TPL-2 (i.e., not C-terminally truncated) in SHOK cells is predominantly due to M1-TPL-2 7. Thus, the presence of an intact N terminus and the absence of the C terminus appear to be necessary for optimal oncogenic transformation by TPL-2. The precise role of the N-terminal 29 amino acids in TPL-2 transformation is unknown. However, removal of the C-terminal domain appears to activate transforming potential of TPL-2 by two mechanisms. First, C-terminal truncation increases the specific kinase activity of TPL-2, and it has been suggested that the C terminus may modulate TPL-2 catalytic activity by folding back onto the kinase domain 6, 8. Second, C-terminal truncation removes a degron sequence (amino acids 435-457) that promotes proteolysis of TPL-2 by the proteasome 8. Consequently, TPL-2ΔC is expressed at higher levels in cells than TPL-2.
Surprisingly, although multiple studies have demonstrated that Tpl2 can function as an oncogene in rodent cells, in some situations, Tpl2 appears to act as a tumor suppressor. For example, Tpl2−/− mice bred onto an MHC Class I-restricted T-cell antigen receptor (TCR) transgenic background develop T-cell lymphomas due to hyper-responsiveness of CD8+ T cells following TCR stimulation 9. Furthermore, a recent study demonstrated that Tpl2−/− mice have a significantly increased incidence of skin tumors in a two-stage skin carcinogenesis model, which correlated with increased inflammation and production of proinflammatory cytokines 10.
Tpl2 can clearly function as an experimental oncogene in mice and rats, and in some conditions as a tumor suppressor. However, it is less clear whether TPL-2 is important in etiology of human cancers. DNA sequencing analyses have demonstrated that TPL-2 is not mutated in the majority of human cancers. Amongst 477 unique primary tumor samples in the Sanger Institute COSMIC database that have been analyzed for somatic mutations in Tpl2, only a single brain tumor sample from a patient with glioblastoma multiforme was found to have a somatic A to T mutation at residue 387 (Parsons et al. 11 and http://www.sanger.ac.uk/genetics/CGP/cosmic/). It is not known whether this mutation contributed to tumor development or progression. In addition, one study reported a Tpl2 point mutation in a primary human lung adenocarcinoma, which results in the production of a C-terminally truncated protein 12. However, analyses of other lung cancer cells suggested that this is a very rare event. Tpl2 overexpression may be important in oncogenesis, and has been reported both in breast tumors 13 and in a small panel of large granular lymphocyte proliferative disorders 14. Thus, while somatic mutation of Tpl2 does not appear to be an important event in the development of human cancer, increased TPL-2 protein expression may sometimes contribute to oncogenesis or tumor progression.
Regulation of signaling pathways by TPL-2
While the potential of truncated TPL-2 to behave as a transforming oncoprotein kinase was clear, the physiological function of wild-type TPL-2 was initially less evident. Early northern blot analyses of rat tissues demonstrated that Tpl2 mRNA is expressed at highest levels in the spleen, thymus and lungs, with low levels in the brain, testis and liver 2, 15. A later study also detected Tpl2 mRNA in the brain, intestine, kidney and skeletal muscles, suggesting that Tpl2 may be widely expressed 16. Interestingly, in situ hybridization of adult mouse tissues could only identify Tpl2 mRNA in granular duct cells in the submandibular glands, serous cells in the parotid gland, peptic cells in gastric glands and goblet cells in colonic glands 17. (The failure of in situ hybridization to detect Tpl2 mRNA expression in other tissues suggests this may be a less sensitive technique than northern blotting.) These latter results raise the possibility that TPL-2 might regulate secretory pathways and/or innate immune responses in the gastrointestinal tract.
In the mid 1990s, DNA sequence homology comparisons revealed that TPL-2 kinase domain is related to the MAP3 kinases STE11 and MEK kinase 18, 19, suggesting that TPL-2 might regulate MAP kinase signaling pathways. Consistent with this hypothesis, TPL-2 overexpression in COS-7 and 3T3 cells activates ERK, JNK, p38γ and ERK5 MAP kinases 18, 19, 20. Immunoprecipitated TPL-2 phosphorylates MEK-1, MKK-4 (also known as SEK-1), MEK-5 and MKK-6, suggesting that TPL-2 functions directly as a MAP3 kinase 19, 20. This was subsequently confirmed using recombinant TPL-2 purified from lysates of baculovirus-infected insect cells and recombinant MEK protein as a substrate 21. Overexpression of TPL-2 also activates NFAT (nuclear factor of activated T cells) in Jurkat T cells and induces IL-2 (interleukin-2) production 22. IL-2 induction by TPL-2 has also been suggested to involve activation of NF-κB transcription factors via the related MAP3 kinase NIK 23, 24. Transfection experiments in Jurkat T cells indicate that TPL-2 may control NF-κB activity by modulating the transactivation potential of the RelA transcription factor 25, 26. In addition, overexpression experiments in COS-7 cells demonstrate that TPL-2, which is complexed with the NF-κB inhibitory protein NF-κB1 p105 27 (see section 3), may regulate NF-κB activation by inducing p105 proteolysis, releasing associated Rel subunits for translocation into the nucleus 28.
Overexpression of kinases can result in artifactual phosphorylation of cellular proteins, and it remained unclear for several years whether TPL-2 really regulated the activation of so many downstream signaling pathways under physiological conditions. This was clarified through the generation of a Tpl2−/− mouse strain by the Tsichlis laboratory. Tpl2−/− mice display no overt phenotype, are of normal size and weight and have a normal lifespan under pathogen-free conditions 29. The development of immune cells (T cells, B cells, dendritic cells (DC), natural killer cells and macrophages) occurs normally in the absence of TPL-2. Surprisingly, despite the results obtained with overexpressed TPL-2 in T-cell lines, Tpl2−/− CD4+ T cells produce similar amounts of IL-2 to WT cells after TCR stimulation 29, 30, indicating that TPL-2 is not important for the physiological regulation of IL-2 production. LPS-induced activation of NF-κB and proteolysis of p105 is also normal in Tpl2−/− macrophages, suggesting that TPL-2 may not be critical for TLR4 activation of NF-κB. However, TPL-2 is only associated with a small fraction of total cellular p105. It therefore remains possible that TPL-2 regulates the proteolysis of this pool of p105, which is likely to contribute to only a fraction of total NF-κB activity. In addition, phosphorylation of RelA on S276 (a critical regulatory site 31) is dependent on TPL-2 in primary fibroblasts stimulated with TNF 32. It is therefore possible that TPL-2 contributes to NF-κB activation in a cell type- and stimulus-specific fashion. The mechanism by which endogenous TPL-2 regulates RelA phosphorylation has not yet been established.
Analysis of Tpl2−/− macrophages revealed an essential function for TPL-2 in LPS activation of MEK-1/2 and ERK-1/2, but not of p38, JNK or NF-κB. Furthermore, LPS stimulation activates the MEK kinase activity of TPL-2 33, 34. TPL-2 is also required for TLR2, TLR9 and TNF activation of ERK in macrophages 35, 36, 37 and CD40 activation of ERK in B cells 35. In other cell types, the function of TPL-2 may not be restricted to the regulation of the ERK MAP kinase pathway. Consistent with this idea, TPL-2 is required for optimal activation of ERK and JNK in embryonic fibroblasts after TNF or IL-1β stimulation, and for maximal p38 activation in LPS- and CpG-stimulated DC 32, 36. These phenotypes may result directly from TPL-2 signaling, which can phosphorylate and activate MKK-4 and MKK-6, the activators of JNK and p38 MAP kinases, respectively 19, 20.
Together, analyses of primary cells from Tpl2−/− mice indicate that the major physiological function of TPL-2 is to regulate ERK MAP kinase activation in immune responses following stimulation of receptors of the TLR and TNF-R families. However, TPL-2 may also regulate the activation of other MAP kinases and NF-κB in a cell type- and stimulus-specific fashion.
Regulation of TPL-2 signaling by NF-κB1 p105
An important step toward our current understanding of the regulation of TPL-2 function was the identification of NF-κB1 p105 as a TPL-2-interacting protein in a yeast two-hybrid screen 28. TPL-2 binds stoichiometrically with the C-terminal half of NF-κB1 p105 via two distinct interactions. The TPL-2 C terminus (residues 398-467) binds to a region adjacent to the ankyrin repeat region on p105, while the TPL-2 kinase domain interacts with the p105 death domain (Figure 2) 38. The binding site on p105 for the TPL-2 C terminus is a conserved helical domain (residues 497-539) that is required for p105 dimerization 38. This region is within a “processing inhibiting domain” (PID; residues 474-544), which regulates processing of p105 to p50 by the proteasome 39. It has been proposed that p105 dimerization via this domain blocks processing, possibly due to the increased size of the dimer preventing entry into the proteasome 40. Therefore, efficient binding of TPL-2 to p105 may require p105 dimerization, and be restricted to a pool of p105 that cannot be processed to p50. While all detectable cellular TPL-2 in unstimulated cells is associated with p105, it is important to note that the majority of total cellular p105 (> 95% in macrophages) is not complexed with TPL-2, but presumably is associated with Rel proteins 28, 41.
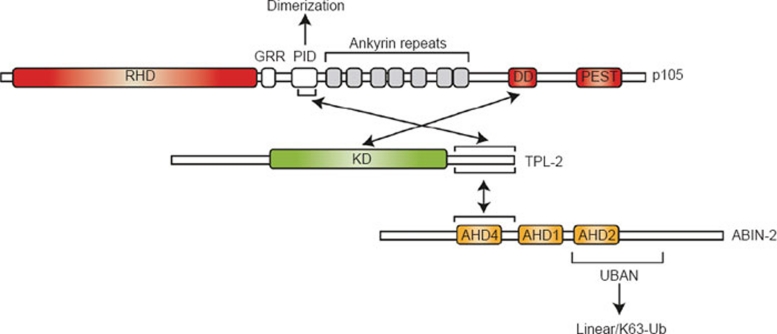
Figure 2.
TPL-2 interactions with NF-κB1 p105 and ABIN-2. In unstimulated cells, all detectable TPL-2 is complexed with NF-κB1 p105 and ABIN-2. The TPL-2 kinase domain (KD) directly interacts with the death domain (DD) of p105. This regulates TPL-2 MEK kinase activity by blocking access of the substrate to the active site. The TPL-2 C terminus (398-467) interacts with a region (497-539) of p105 within the “processing inhibitory domain” (PID; 474-544) 39, which also mediates p105 dimerization 38. These two distinct interactions contribute to a very strong association between TPL-2 and p105, and dissociation of recombinant TPL-2/p105 complex produced in insect cells requires high concentrations of urea (8 M) 21. The importance of TPL-2 regulation by p105 is highlighted by the dysregulated TPL-2 MEK kinase activity and tumorigenic potential of C-terminal truncated TPL-2, which lacks one of the binding sites for p105 (see text for details). The same region of TPL-2 that mediates binding to the PID of p105 is also the principal interaction site with ABIN-2. The binding site on ABIN-2 (194-250) contains ABIN-homology domain (AHD) 4 (203-220), which is also present in ABIN-1 and ABIN-3 100. ABIN-2 interaction is critical to maintain TPL-2 protein stability, and steady-state levels of TPL-2 are dramatically reduced in cells deficient in ABIN-2. RHD: Rel homology domain, GRR: glycine-rich region, PEST: Domain rich in proline (P), glutamate (E), serine (S) and threonine (T), UBAN: ubiquitin-binding in ABIN and NEMO.
NF-κB1 p105 regulates two aspects of TPL-2 function. First, p105 is essential to maintain TPL-2 stability. Steady-state levels of TPL-2 are very low in p105-deficient Nfκb1−/− cells 34, 38, and consequently LPS activation of MEK and ERK is substantially reduced in bone-marrow-derived macrophages (BMDM) generated from Nfκb1−/− mice 34. Since the proposed degron sequence of TPL-2 is located within one of the binding sites for p105, it is possible that this interaction stabilizes TPL-2 by covering the degron 8. Second, direct interaction of p105 with the kinase domain of TPL-2 inhibits TPL-2 MEK kinase activity by preventing access to MEK 34, 38. TPL-2 is actively prevented from phosphorylating MEK in unstimulated BMDM, since it is all associated with p105 41. However, following LPS stimulation, TPL-2 MEK kinase activity increases, indicating that TPL-2 is no longer subject to p105 inhibition 34. Indeed, depleting p105 from cell lysates by immunoprecipitation reveals that LPS stimulation induces the release of a fraction of TPL-2 from p105. TPL-2 MEK kinase activity is restricted to this p105-free pool 34, 41.
Like other IκB proteins 42, 43, the signal-induced proteolysis of NF-κB1 p105 is regulated by the IκB kinase complex (IKK), which phosphorylates S927 and S932 in the p105 PEST region (see Figures 3 and 4). These phosphorylations induce the binding of SCFβTrCP E3 ligase, which catalyzes the subsequent K48-linked polyubiqitination and proteolysis of p105 by the proteasome 42, 44, 45. This predominantly leads to the complete degradation of p105, rather than processing to p50 42, 44. As a consequence of signal-induced p105 degradation, associated Rel subunits are released to regulate NF-κB target genes in the nucleus. A recent study has demonstrated that IKK-induced p105 proteolysis is necessary for optimal TCR-induced NF-κB activation in CD4+ T cells and mature CD4+ T-cell helper function 30.
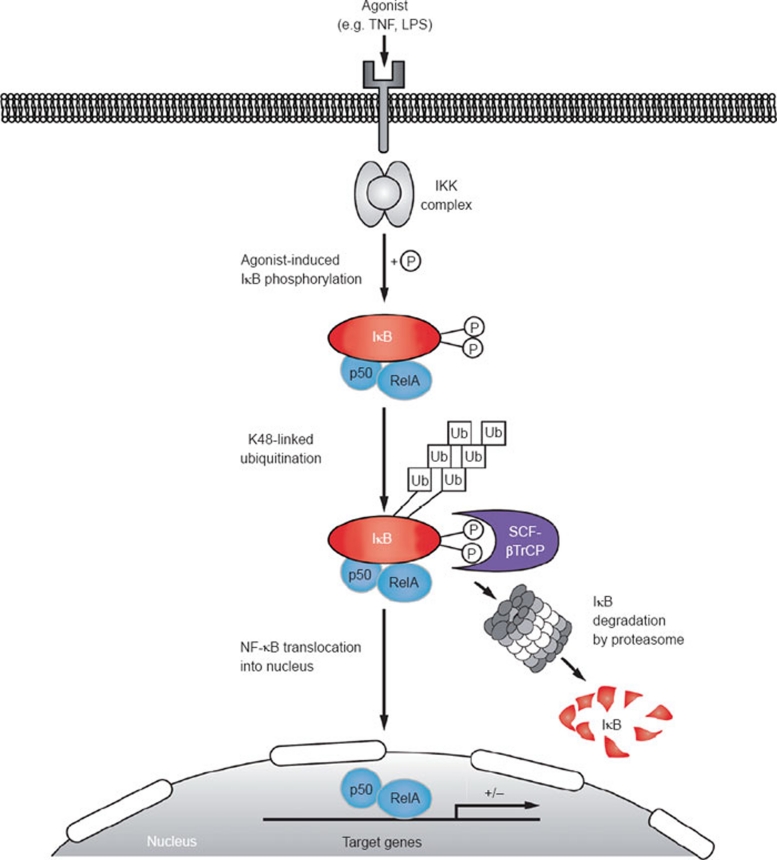
Figure 3.
Canonical pathway of NF-κB activation. Various stimuli activate the classical NF-κB pathway. Agonist stimulation induces adapter recruitment to the cognate receptor, which then activates the IKK complex by a process that involves linear and K63-linked ubiquitination, as well as phosphorylation (reviewed in Skaug et al. 101, Vallabhapurapu and Karin 102). IKK phosphorylates two N-terminal residues in IκBα, creating a binding site for the SCFβTrCP ubiquitin E3 ligase complex, which attaches K48-linked ubiquitin chains to two adjacent lysine residues, targeting IκBα for proteasomal degradation. Associated NF-κB dimers are consequently liberated to translocate to the nucleus and modulate target gene expression.
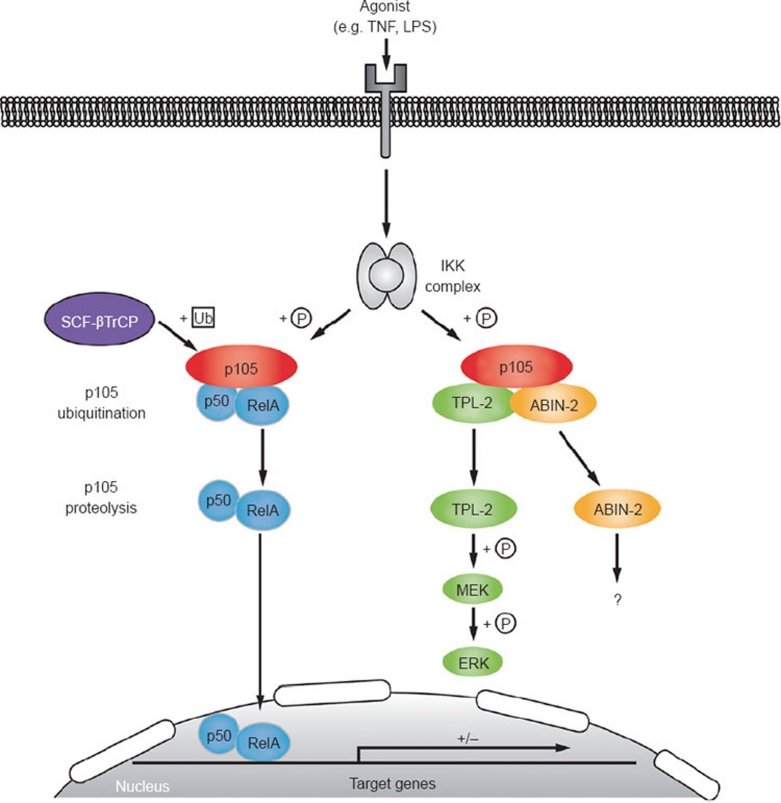
Figure 4.
Regulation of TPL-2 through IKK-induced p105 proteolysis. TPL-2 is confined to a cytoplasmic complex with the NF-κB1 precursor protein p105, and the ubiquitin-binding protein ABIN-2. NF-κB1 p105 has multiple functions. First, it serves as a precursor molecule for the NF-κB1 p50 subunit, which is generated by limited proteolysis (processing) of p105 by the proteasome, which removes p105's C-terminal half 33. Second, p105 functions as a classical IκB (see Figure 2), retaining associated NF-κB subunits in the cytoplasm. Cellular stimulation with various agonists, such as TLR ligands, IL-1β, TNF and CD40L, induces the formation of receptor proximal complexes that trigger activation of the MAP3 kinase TAK1 101. Activation loop phosphorylation of IKK2 by TAK1, in turn, activates IKK2 to phosphorylate the target residues S927 and S932 in p105, creating a binding site for the SCFβTrCP ubiquitin E3 ligase complex. K48-linked ubiquitination of p105 by SCFβTrCP triggers its complete degradation by the 26S proteasome. IKK-induced proteolysis of p105 releases associated NF-κB dimers, which then translocate to the nucleus and modulate expression of target genes, similar to activation of NF-κB dimers in the classical pathway (see Figure 3). Stimulus-induced p105 proteolysis also couples activation of NF-κB pathways to MAP kinase signaling by releasing TPL-2 from p105 inhibition. After liberation from p105, TPL-2 directly phosphorylates MEK and thereby activates downstream ERK MAP kinase signaling. Based on immunoblotting of total cell lysates, it has been suggested that LPS stimulation results in the selective dissociation of M1-TPL-2 from p105 34. However, immunoblotting of p105-depleted lysates indicates that both M1 and M30 TPL-2 are actually released from p105 after LPS stimulation 41, although the relative contribution of M1 and M30 TPL-2 to MEK phosphorylation in LPS-stimulated macrophages is not known. Activation of TPL-2 MEK kinase activity additionally requires trans-/autophosphorylation of S400 by an unknown kinase and autophosphorylation at T290 in the TPL-2 activation loop. Proteasomal degradation of p105 also releases ABIN-2 from association with p105 and TPL-2. The function and downstream targets of ABIN-2 are not known.
In macrophages, pharmacological inhibition of the proteasome blocks both TPL-2 release from p105 and ERK activation following LPS stimulation 41, 46, raising the possibility that IKK-induced p105 proteolysis might also regulate these processes. Consistent with this hypothesis, LPS-induced release of TPL-2 from p105, and its subsequent activation of MEK and ERK, are blocked by expression of p105SSAA, which is resistant to IKK-induced proteolysis due to mutation of the IKK target serines to alanine. Furthermore, LPS activation of ERK in macrophages is dependent on IKK2 catalytic activity. Thus, IKK-induced p105 proteolysis is essential for LPS activation of TPL-2, and for TPL-2-dependent activation of ERK following TNF stimulation 46. It is likely that this IKK-induced p105 proteolysis also facilitates TPL-2 phosphorylation of MEK following stimulation with other TLR ligands, IL-1β and CD40, which each activate ERK via TPL-2.
Regulation of TPL-2 signaling by phosphorylation
Similar to other MAP3 kinases 47, TPL-2 signaling function is regulated by phosphorylation. Activation of TPL-2 requires phosphorylation of T290 in the activation loop of its kinase domain (Figure 1) 48, which may also regulate the association of TPL-2 with p105 49. Based on experiments testing the effect of high concentrations of the IKK2 inhibitor PS-1145 in RAW264.7 macrophages, it was originally suggested that TPL-2 T290 phosphorylation is controlled by IKK2 50. However, lower concentrations of PS-1145 that effectively block IL-1β-induced IκBα degradation do not affect TPL-2 T290 phosphorylation, suggesting that this is an “off-target” effect of the inhibitor 51. Indeed, experiments with a small-molecule inhibitor of TPL-2 have recently suggested that T290 is autophosphosphorylated by TPL-2 itself after IL-1β stimulation of IL-1R-expressing 293T cells 52. TPL-2 must also be phosphorylated on S400 in its C-terminal tail to activate MEK following LPS stimulation of macrophages 53. Different experimental systems have suggested that S400 is either autophosphosphorylated by TPL-2 (IL-1β-stimulated IL-1R-293T cells) or transphosphorylated by an unknown kinase (LPS-stimulated RAW264.7 macrophages) 52, 53. Interestingly, a recent study has shown that the extracellular nutrient arginine positively regulates the phosphorylation of TPL-2 on both T290 and S400, and consequently TPL-2 activation following LPS stimulation 54. Although the mechanism underlying this effect has not yet been worked out, these data imply that nutritional status may directly influence ERK activation in innate immune responses via regulation of TPL-2 phosphorylation.
In summary, current data indicate that activation of TPL-2 MEK kinase activity involves at least two regulatory steps: auto- and/or transphosphorylation on two residues (T290 and S400), and release from p105-mediated inhibition, which is triggered by IKK-induced p105 proteolysis by the proteasome. The IKK complex, therefore, directly regulates both NF-κB and the ERK MAP kinase activation in innate immune responses via NF-κB1 p105. However, although p105 inhibits TPL-2 phosphorylation of MEK, it does not directly regulate TPL-2 catalytic activity 53, 55. Therefore, it is possible that TPL-2 phosphorylates substrates other than MEK when complexed with p105, and independently of regulation by the IKK complex.
Regulation of TPL-2 stability by ABIN-2
Tandem-affinity purification and peptide mass fingerprinting identified A20-binding inhibitor of NF-κB 2 (ABIN-2) as an NF-κB1 p105-interacting protein 56, 57. Cotransfection experiments indicate that ABIN-2 can also interact directly with TPL-2, but preferentially forms a ternary complex with p105 and TPL-2. Consistently, a substantial fraction of endogenous ABIN-2 is associated with p105 and TPL-2 in primary macrophages 57. ABIN-2 was originally identified in a two-hybrid screen, with the NF-κB inhibitory protein A20 as bait, and overexpression experiments indicate that ABIN-2 may be a downstream effector of A20, mediating its NF-κB inhibitory function 58. ABIN-2 contains an UBAN (ubiquitin binding in ABIN and NEMO) ubiquitin-binding domain, which is essential for its ability to inhibit NF-κB in overexpression experiments 59. Binding analyses with recombinant ABIN-2 have indicated interaction with linear ubiquitin chains, and also weak binding to K63-linked ubiquitin chains 60.
Initial experiments to investigate the significance of ABIN-2 association with p105 and TPL-2 utilized siRNA knockdown in 293 cells. These experiments demonstrated that ABIN-2 is required to maintain TPL-2 protein stability 57, which was subsequently confirmed by analysis of various types of primary cells isolated from Abin2−/− (also termed Tnip2−/−) mice 61. Consequently, LPS and TNF activation of ERK is reduced, but not absent, in Abin2−/− macrophages. Since LPS activation of ERK in macrophages is completely dependent on TPL-2 29, this implies that the small amount of TPL-2 protein present in Abin2−/− macrophages is still activated in the absence of ABIN-2. Consistent with this hypothesis, retroviral expression of TPL-2 in Abin2−/− macrophages substantially increases LPS- and TNF-induced ERK activation 61. ABIN-2, therefore, does not seem to be important for activation of TPL-2. In addition, ABIN-2 is released from p105 and TPL-2 after LPS stimulation, and is not associated with the pool of TPL-2 that can activate MEK 57.
It is unlikely that the sole function of ABIN-2 is to stabilize TPL-2, since this does not require the conserved UBAN domain of ABIN-2 61. Retroviral expression of ABIN-2 deletion mutants has also demonstrated that ABIN-2 binding to A20 is not required for stabilization of TPL-2 in macrophages. The importance of ABIN-2 ubiquitin-binding activity and interaction with A20 in immune responses remains unclear. Interestingly, ABIN-2 has been reported to interact with SMRT (silencing mediator of retinoic acid and thyroid hormone receptors) 62, a core component of corepressor complexes that actively repress expression of a fraction of inflammatory response genes in macrophages 63. TLR activation of these genes requires the active removal of SMRT, in addition to the recruitment of activators and coactivators. ABIN-2 has also been shown to interact with Baf60a, a subunit of the SWI/SNF chromatin-remodeling complex 64, which positively regulates a subset of TLR4-induced genes in macrophages 65. Tandem-affinity purification has demonstrated that ABIN-2 complexes with Rel subunits 56, although it is not known whether these are direct interactions or are mediated via NF-κB1 p105. Since ABIN-2 is released from p105 following LPS stimulation of macrophages 57 and can enter the nucleus 64, it is possible that ABIN-2 regulates gene expression by controlling the interaction of SMRT, Baf60a and/or Rel subunits with target genes.
Regulation of immune and inflammatory responses by TPL-2
Analyses of Tpl2−/− mice have suggested critical functions for TPL-2 in immune responses, which are summarized in Table 1. Initial experiments investigated the role of TPL-2 in inflammatory responses. Tpl2−/− mice were found to produce very low levels of TNF after intraperitoneal LPS injection and to be resistant to septic shock induced by LPS and 𝒟-galactosamine 29. TPL-2 is also required for optimal TNF production by LPS-stimulated macrophages, the major cellular source of TNF during inflammatory responses, while LPS-induced TNF production by DC is partially dependent on TPL-2 29, 37. However, TPL-2 is not universally involved in induction of TNF in macrophages, since curdlan stimulation of dectin-1, which activates ERK via Raf, induces TNF independently of TPL-2 expression 37, 66. The requirement for TPL-2 in TNF-induced innate immune responses is, therefore, both cell- and stimulus specific.
Table 1. Effects of TPL-2 deficiency on immune and inflammatory responses.
| Model | Phenotype | Expression of effectors | References |
|---|---|---|---|
| Septic shock (LPS/𝒟-gal) | Resistant | Serum: TNF ↓, IL-1β ↓ | 29, 37 |
| IBD | Delayed onset (TnfΔARE/ΔARE/Map3k8−/−) Spleen: T cells ↑ (CD4+CD44lo ↑, CD8+ and CD4+ CD44hi ↓) | 73 | |
| Zymosan injection | Inflammation ↓ | 37, 104 | |
| Intraperitoneal Neutrophil recruitment ↓ Macrophage recruitment ↓ | Intraperitoneal Serum: TNF ↓ | ||
| Intraplantar Neutrophil recruitment ↓ Hypernociception ↓ | Intraplantar TNF ↓, IL-1β ↓, IL-6 ↓ G-CSF ↓, GM-CSF ↓, NGF ↓ MCP-1 ↓, MIP-α ↔, MIP-1β ↓, KC ↓ PGE2, LTB4 ↑ (5 h), PGE2, LTB4 ↓ (24 h) | ||
| Bronchoalveolar inflammation (OVA-induced) | Inflammation ↑ | 77 | |
| Bronchoalveolar exudates: Lymphocytes ↑ Eosinophils ↑ | Bronchoalveolar exudates: IL-4 ↑, IL-5 ↑ Eotaxin ↑ IgE ↑ | ||
| Caerulein-induced pancreatitis | Inflammation ↓ | 85 | |
| Pancreas: Neutrophil activity/recruitment ↓ | Pancreas: IL-6 ↓ MCP-1 ↓, MIP-2 ↓ | ||
| L. monocytogenes infection | Lethality ↑ Bacterial burdens in liver and spleen ↑ | Serum: TNF ↔, IL-12 (p70) ↑, IL-27 ↑ (In vitro infected BMDM and splenocytes: IL-1β ↓) | 37 |
| L. major infection | Parasite load ↔ | LN CD4+ T cells (Ag-restimulated): IFN-γ ↑, IL-4 ↓ | 79 |
| T. gondii infection | Susceptibility ↑ Lethal upon Tpl2−/− T cell → Rag2−/− % of infected PECs ↑ Spleen and PECs: NK and T cell numbers ↔ | Serum: IFN-γ ↓, IL-12 ↑, IL-6 ↔ MCP-1 ↓ LN (Ag-restimulated): IFN-γ ↓ | 77 |
| Ag-specific responses | Serum: OVA + CFA: IgG2a ↑ OVA + alum: IgG1 ↓ IgE ↑ (Ag-specific and total) 77 IgE ↓ 79 | 77, 79 |
Abbreviations and symbols used: ↑, increase; ↓, decrease; ↔, unaffected; Ag, antigen; IBD, inflammatory bowel disease; LN, lymph node; PECs, peritoneal exudate cells.
Pharmacological inhibition of ERK activation with MEK inhibitors impairs LPS induction of TNF by macrophages, implying that TPL-2 regulates TNF production by controlling ERK activation 29, 67. Initial experiments suggested that TPL-2/ERK might regulate TNF production at a post-transcriptional level by promoting the export of Tnf mRNA from the nucleus 29. In apparent support of this hypothesis, a recent study using TPL-2 and MEK inhibitors showed that TPL-2/ERK controls, at the post-transcriptional level, the expression of the nuclear mRNA export receptor Tip-associated protein (TAP) 68. However, pre-TNF protein levels in LPS-stimulated primary macrophages are minimally affected by pharmacological MEK inhibition or TPL-2 deficiency, indicating that defective intracellular TNF mRNA transport is unlikely to explain the failure of Tpl2−/− macrophages to produce TNF 67. Rather, TPL-2/ERK signaling is required for the appearance of pre-TNF at the cell surface and processing of pre-TNF to soluble TNF, which may both be controlled by ERK-mediated phosphorylation of TNFα-converting enzyme (TACE) on T735 69. TNF maturation, but not pre-TNF production, has also recently been shown to be blocked in LPS-stimulated macrophages isolated from a new Tpl2 mutant mouse strain, called Sluggish, in which the TPL-2 kinase domain is partially deleted. This study confirms the importance of TPL-2 in the posttranslational control of TNF production 70.
TPL-2 also positively regulates mRNA and protein induction of IL-1β and IL-10 following stimulation of macrophages and DC with LPS or CpG (TLR9 ligand) 36, 37. MEK inhibitor experiments suggest that TPL-2 controls TLR-induction of IL-10 and IL-1β via ERK 36, 61, while positive regulation of IL-1β production by TPL-2 following stimulation with the dectin-1 ligand, curdlan, appears to be ERK independent 37. TLR induction of IL-12 p70 and IFN (interferon)-β is inhibited by TPL-2/ERK signaling, and this occurs, at least in part, independently of any effects on IL-10 induction 36, 71. TPL-2 signaling therefore has complex pro- and anti-inflammatory effects on the production of cytokines in innate immune responses. In addition, TPL-2 not only controls the production of cytokines but also the cellular response to TNF and IL-1β, since TPL-2 expression is required for activation of ERK by both of these proinflammatory cytokines 32, 52, 72. Indeed, the development of TNF-induced Crohn's-like inflammatory bowel disease in TnfΔARE/ΔARE mice, which constitutively overproduce TNF, is reduced in the absence of TPL-2 expression 73, 74. TPL-2 is therefore important in driving TNF-induced inflammation.
It will be important in the future to determine how the complex effects of TPL-2 on the production and response to cytokines regulate immune responses to pathogens, in which multiple pattern recognition receptors are activated and a complex interplay of cytokines is required to mount an effective immune response. One recent study demonstrated that Tpl2−/− mice have increased pathogen burdens compared to wild-type controls after infection with Listeria monocytogenes, an intracellular Gram-positive bacterium 37. The increased susceptibility of Tpl2−/− mice to listeriosis correlates with a substantial reduction of IL-1β production following infection. However, while IL-1β is required for optimal anti-listerial responses 75, 76, it remains to be established whether defective IL-1β induction explains the increased susceptibility of Tpl2−/− mice to L. monocytogenes infection.
Tpl2−/− mice also have an impaired immune response to the intracellular parasite Toxoplasma gondii. Surprisingly, transfer experiments with purified T cells suggest that this is due to a T-cell intrinsic defect, rather than an altered innate immune response 77. Resistance to T. gondii critically depends on IFN-γ production 78, and in vitro experiments revealed that TPL-2 is required for optimal differentiation of naïve T cells to Th1 effector cells producing IFN-γ 77. Interestingly, in an ovalbumin-induced model of asthma that is driven by Th2 effector cells, lung inflammation is exacerbated in Tpl2−/− mice compared to wild-type controls 77. Negative feedback regulation between Th subsets may explain this phenotype, whereby decreased Th1 differentiation in absence of TPL-2 results in increased Th2 polarization. It has been suggested that defective TCR-mediated activation of ERK in Tpl2−/− CD4+ T cells results in reduced T-bet and Stat4 expression, and consequently in reduced Th1 differentiation 77. However, the reduction in TCR-induced ERK phosphorylation in TPL-2-deficient cells is relatively modest and it is possible that TPL-2 has other signaling functions in Th1 differentiation.
In contrast to the above-mentioned studies suggesting a requirement for TPL-2 in Th1 differentiation, an independently generated Tpl2−/− mouse strain has been reported to mount a Th1-skewed immune response following Leishmania major infection 79, possibly due to the increased production of IL-12 by TPL-2-deficient innate immune cells. These conflicting results may be explained by differences in how the two Tpl2−/− mouse strains were generated, genetic background differences of the mice or by differential responses elicited by the two pathogens used in the studies. If the latter explanation is correct, this would suggest that the net effect of TPL-2 deficiency on effector T-cell development may be dependent on the specific type of immune response involved. In addition, TPL-2 may not only directly influence the differentiation of the Th1 cell subset, as it has recently been reported that production of the Th17-promoting cytokine IL-23 is impaired in LPS-stimulated Tpl2−/− macrophages 80. This suggests that TPL-2 may positively regulate Th17 cell differentiation. It will be very interesting to investigate this possibility in vivo using Tpl2−/− mice, given the importance of Th17 cells in autoimmune diseases 81.
TPL-2 expression is required for CD40 activation of ERK in B cells 35. In vitro experiments suggest that IgE switching of purified splenic B cells induced by CD40 plus IL-4 requires TPL-2 activation of ERK 35. However, IgE antibody production is not defective in Tpl2−/− mice, possibly due to enhanced Th2 cell differentiation (see above), increasing the available concentration of IL-4, which promotes switching to IgE 77. Analysis of B cells purified from TPL-2-deficient Nfκb1−/− mice has also suggested that TLR4 utilizes TPL-2 to activate ERK in B cells 82. This pathway is proposed to positively regulate B cell survival by promoting the degradation of the pro-apoptotic protein Bim 83, which is directly phosphorylated by ERK 84. However, the importance of TLR4 activation of ERK via TPL-2 in B cell antibody responses is not known.
Several studies indicate that TPL-2 can also control inflammatory responses by signaling in non-hematopoietic cells. For example, TPL-2 positively regulates the development of pancreatic and lung inflammation during caerulein-induced acute pancreatitis 85. By generating bone marrow chimeras, pancreatic inflammation was shown to be controlled by TPL-2 in non-myeloid cells. Analyses of pancreas homogenates demonstrate that caerulein activation of ERK, JNK and AP-1 is dependent on TPL-2 expression, as is the induction of MCP-1 (monocyte chemotactic protein-1), MIP-2 (macrophage-inflammatory protein-2) and IL-6 85. Thus, TPL-2 may regulate pancreatic inflammation during pancreatitis by mediating proinflammatory signals and the generation of neutrophil chemoattracting factors. In vitro experiments also show that TPL-2 is involved in inflammatory cytokine activation of ERK and lipolysis in adipocytes, and is upregulated in adipose tissue in obese mice and humans 86. TPL-2 therefore may play a role in adipose tissue dysfunction in obesity and type-2 diabetes.
TPL-2 kinase as an anti-inflammatory drug target
The proinflammatory cytokine TNF plays an important role in the pathogenesis of rheumatoid arthritis (RA) and Crohn's disease, and biological agents that block its activity have been used to treat these diseases 87, 88. However, only a fraction of RA and Crohn's sufferers respond well to anti-TNF antibodies. Consequently, there is still a need for more effective, less expensive and orally active drugs for RA and Crohn's treatment. One approach is to target the signaling pathways that regulate the production of TNF 89. TPL-2 is critical for production of TNF during TLR-induced inflammatory responses, and is an attractive drug target since TPL-2 is not required for normal development or viability. Furthermore, TPL-2 only regulates MEK activation in response to inflammatory stimuli, since activation of MEK by growth factors and antigen receptors is mediated by Raf isoforms 90. Amino acid sequence comparisons also demonstrate that the TPL-2 kinase domain has relatively low homology to other kinases and is also the only human kinase domain with a unique proline (P145) rather than a conserved glycine on the Gly-rich loop 48. Therefore, it may be possible to identify inhibitors that are selective to TPL-2, which do not affect other kinases.
Using in vitro assays in which recombinant truncated TPL-2 (M30-TPL-2ΔC) phosphorylates the MEK activation loop 21, high-throughput screening by the pharmaceutical companies Abbott and Wyeth/Pfizer have identified different classes of small-molecule TPL-2 inhibitors (see George and Salmeron 91 for a comprehensive review). Several of these appear to be relatively specific and block LPS-induced ERK activation and TNF production in primary macrophages at low micromolar concentrations 92, 93. In addition, three of Wyeth/Pfizer compounds have been reported to have efficacy in vivo, blocking TNF production in mice after intraperitoneal LPS injection 94.
Although there has been considerable progress toward the generation of specific TPL-2 inhibitors, more work is needed to develop compounds suitable for in vivo use, which should be of sufficient potency and have the appropriate physicochemical properties. TPL-2 remains a difficult drug target to develop further, since the lack of a crystal structure has prevented structure-based drug design. Indeed, it is unlikely that it will be technically possible to crystallize isolated M30-TPL-2ΔC, since this is associated with heat shock proteins and has a tendency to form insoluble aggregates, suggesting that it is not correctly folded 21. Furthermore, full-length recombinant TPL-2 has an even greater tendency to aggregate. Analyses of Nfκb1−/− and Abin2−/− cells suggest that production of correctly folded and stable recombinant TPL-2 requires its association with p105 and ABIN-2 34, 38, 61. Coexpression of p105 and ABIN-2 may eventually allow structural determination of TPL-2, which could be used in structure-based drug design. However, it is unclear whether drugs developed from the structure of complexed TPL-2 would also target the physiologically relevant form of TPL-2, which is released from p105 41. Nevertheless, the pharmaceutical industry is likely to remain interested in developing TPL-2 as a drug target, given its critical role in regulating TNF production in inflammation.
Conclusions and future research directions
Since its initial discovery in 1991, much progress has been made in our understanding of the regulation and functions of TPL-2 MAP3 kinase. The discovery by the Tsichlis laboratory of the critical role played by TPL-2 in the induction of TNF in inflammatory responses using Tpl2−/− mice was particularly important 29. This result identified TPL-2 as a potential anti-inflammatory drug target, and continues to stimulate both academic and industrial research on this protein. However, TPL-2 also has anti-inflammatory functions, such as the negative regulation of IL-12 and IFN-β production in myeloid cells 36. It will therefore be important to test Tpl2−/− mice in a variety of autoimmune disease models to establish whether TPL-2 inhibition is likely to have an overall anti-inflammatory effect. It will also be important to test the effect of TPL-2 deficiency on immune responses against various pathogens to determine whether long-term pharmacological inhibition of TPL-2 is likely to significantly increase susceptibility to opportunistic infection.
Dysregulation of the ERK MAP kinase pathway is common in many human cancers, often arising because of mutations in the MEK kinase B-Raf 95. Targeted inhibitors of BRAF and its downstream target MEK are already undergoing clinical testing 96. Although Tpl2 was originally identified as an oncogene, DNA sequence analyses have failed to identify Tpl2 mutations in primary human cancer cells. However, activation of ERK signaling by TPL-2 could still contribute to the transformation of cells in which IKK is constitutively switched on, such as in diffuse large cell lymphoma 97, since IKK-induced p105 proteolysis facilitates TPL-2 activation. This may be an interesting question to investigate in the future, since specific inhibitors of TPL-2 are currently under development and could potentially be used to treat such tumors.
The role of p105 in regulating the MEK kinase activity of TPL-2 is well established 41, 46. However, our understanding of how phosphorylation regulates TPL-2 activation is less clear. For example, it is not known why S400 phosphorylation is required for TPL-2 activation of MEK after LPS stimulation in macrophages 53, especially since TPL-2S400A has normal catalytic activity when expressed in Jurkat or 293T cells 52, 98. In addition, the functional importance of several other known phosphorylation sites on TPL-2 has yet to be investigated 54. The identity of the kinases that phosphorylate TPL-2 also remains unknown, as is the role of phosphatases in regulating TPL-2 signaling activity.
One key outstanding question is whether TPL-2 only controls MAP kinase signaling via phosphorylation of MAP2 kinases or whether TPL-2 can phosphorylate other classes of proteins and consequently regulate other signaling pathways. The function of the TPL-2-associated ubiquitin-binding protein, ABIN-2, in immunity is also completely unclear. Since ABIN-2 expression is required to maintain steady-state TPL-2 levels 61, progress in this area will require the generation of more sophisticated Abin2-mutant strains, which block ABIN-2 signaling function (e.g., ubiquitin-binding function) without affecting TPL-2 stability.
The consequences of linking NF-κB and ERK activation via IKK-induced p105 proteolysis are also unknown. One interesting question is whether these two p105-dependent signaling pathways regulate the transcription of the same target genes in TLR-stimulated macrophages. If this is the case, at least for some TLR-induced genes, p105 proteolysis could potentially promote transcription via synergistic interactions between NF-κB and AP-1 transcription factors on the promoters of target genes 99. What is clear, however, is that care has to be taken in attributing the phenotypes of Nemo-, Ikk2- and Nfκb1-deficient mice solely to impaired NF-κB activation, as TPL-2 signaling function will also be affected.
References
- Miyoshi J, Higashi T, Mukai H, Ohuchi T, Kakunaga T. Structure and transforming potential of the human cot oncogene encoding a putative protein kinase. Mol Cell Biol. 1991;11:4088–4096. doi: 10.1128/mcb.11.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriotis C, Makris A, Bear SE, Tsichlis PN. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T cell lymphomas and in T cell activation. Proc Natl Acad Sci USA. 1993;90:2251–2255. doi: 10.1073/pnas.90.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AH, Turner G, Trubetskoy A, et al. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nat Genet. 2002;32:160–165. doi: 10.1038/ng956. [DOI] [PubMed] [Google Scholar]
- Mikkers H, Allen J, Knipscheer P, et al. High throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat Genet. 2002;32:153–159. doi: 10.1038/ng950. [DOI] [PubMed] [Google Scholar]
- Erny KM, Peli J, Lambert JF, Muller V, Diggelmann H. Involvement of the TPL-2/COT oncogene in MMTV tumorigenesis. Oncogene. 1996;13:2015–2020. [PubMed] [Google Scholar]
- Ceci JD, Patriotis CP, Tsatsanis C, et al. TPL-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes Dev. 1997;11:688–700. doi: 10.1101/gad.11.6.688. [DOI] [PubMed] [Google Scholar]
- Aoki M, Hamada F, Sugimoto T, Sumida S, Akiyama T, Toyoshima K. The human cot proto-oncogene encodes two protein serine/threonine kinases with different transforming activities by alternative initiation of translation. J Biol Chem. 1993;268:22723–22732. [PubMed] [Google Scholar]
- Gandara ML, Lopez P, Hernando R, Castano JG, Alemany S. The COOH-terminal domain of wild-type Cot regulates its stability and kinase specific activity. Mol Cell Biol. 2003;23:7377–7390. doi: 10.1128/MCB.23.20.7377-7390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis C, Vaporidi K, Zacharioudaki V, et al. Tpl2 and ERK transduce anti-proliferative T cell receptor signals and inhibit transformation of chronically stimulated T cells. Proc Natl Acad Sci USA. 2008;105:2987–2992. doi: 10.1073/pnas.0708381104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCicco-Skinner KL, Trovato EL, Simmons JK, Lepage PK, Wiest JS.Loss of tumor progression locus 2 (tpl2) enhances tumorgenesis and inflammation in two-stage skin carcinogenesis Oncogene11 October 2010; doi: 10.1038/onc.2010.447 [DOI] [PMC free article] [PubMed]
- Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AM, Reynolds SH, Anderson M, Wiest JS. Mutational activation of the MAP3K8 proto-oncogene in lung cancer. Genes Chromosomes Cancer. 2004;41:99–108. doi: 10.1002/gcc.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourvinos G, Tsichlis PN, Spandidos DA. Overexpression of the Tpl-2/Cot oncogene in human breast cancer. Oncogene. 1999;18:4968–4973. doi: 10.1038/sj.onc.1202891. [DOI] [PubMed] [Google Scholar]
- Christoforidou AV, Papadaki HA, Margioris AN, Eliopoulos AG, Tsatsanis C. Expression of the Tpl2/Cot oncogene in human T-cell neoplasias. Mol Cancer. 2004;3:34. doi: 10.1186/1476-4598-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris A, Patriotis C, Bear SE, Tsichlis PN. Genomic organization and expression of Tpl-2 in normal cells and moloney murine leukemia virus-induced rat T-cell lymphomas: activation by provirus insertion. J Virol. 1993;67:4283–4289. doi: 10.1128/jvi.67.7.4283-4289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SO, Irwin P, Katz S, Pelech SL. Expression of mitogen-activated protein kinase pathways during postnatal development of rat heart. J Cell Biochem. 1998;71:286–301. doi: 10.1002/(sici)1097-4644(19981101)71:2<286::aid-jcb13>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Ohara R, Hirota S, Onoue H, Nomura S, Kitamura Y, Toyoshima K. Identification of the cells expressing cot proto-oncogene mRNA. J Cell Sci. 1995;108:97–103. doi: 10.1242/jcs.108.1.97. [DOI] [PubMed] [Google Scholar]
- Patriotis C, Makris A, Chernoff J, Tsichlis PN. Tpl-2 acts in concert with Ras and Raf-1 to activate mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1994;91:9755–9759. doi: 10.1073/pnas.91.21.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron A, Ahmad TB, Carlile GW, Pappin D, Narsimhan RP, Ley SC. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 1996;15:817–826. [PMC free article] [PubMed] [Google Scholar]
- Chiariello M, Marinissen MJ, Gutkind JS. Multiple mitogen-activated protein kinase signaling pathways connect the Cot oncoprotein to the c-Jun promoter and to cellular transformation. Mol Cell Biol. 2000;20:1747–1758. doi: 10.1128/mcb.20.5.1747-1758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Quinn CM, Bump NJ, et al. Purification and kinetic characterization of recombinant human mitogen-activated protein kinase kinase kinase COT and the complexes with its cellular partner NF-κB1 p105. Arch Biochem Biophys. 2005;441:64–74. doi: 10.1016/j.abb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Patriotis C, Bear SE, Tsichlis PN. The TPL-2 proto-oncoprotein activates the nuclear factor of activated T cells and induces interleukin 2 expression in T cell lines. Proc Natl Acad Sci USA. 1998;95:3827–3832. doi: 10.1073/pnas.95.7.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Cunningham ET, Mu Y, Geleziunas R, Greene WC. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-κB acting through the NF-κB-inducing kinase and IκB kinases. Immunity. 1999;10:271–280. doi: 10.1016/s1074-7613(00)80027-8. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Patriotis C, Tsichlis PN. Tpl-2 induces IL-2 expression in T-cell lines by triggering multiple signaling pathways that activate NFAT and NF-κB. Oncogene. 1998;17:2609–2618. doi: 10.1038/sj.onc.1202460. [DOI] [PubMed] [Google Scholar]
- Babu G, Waterfield M, Chang M, Wu X, Sun SC. Deregulated activation of oncoprotein kinase Tpl2/Cot in HTLV-1-transformed T cells. J Biol Chem. 2006;281:14041–14047. doi: 10.1074/jbc.M512375200. [DOI] [PubMed] [Google Scholar]
- Sanchez-Valdepenas C, Punzon C, San-Antonio B, Martin AG, Fresno M. Differential regulation of p65 and c-Rel NF-κB transactivating activity by Cot, protein kinase C ζ and NIK protein kinases in CD3/CD28 activated T cells. Cell Signal. 2007;19:528–537. doi: 10.1016/j.cellsig.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Belich MP, Salmeron A, Johnston LH, Ley SC. TPL-2 kinase regulates the proteolysis of the NF-κB inhibitory protein NF-κB1 p105. Nature. 1999;397:363–368. doi: 10.1038/16946. [DOI] [PubMed] [Google Scholar]
- Dumitru CD, Ceci JD, Tsatsanis C, et al. TNFα induction by LPS is regulated post-transcriptionally via a TPL2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Sriskantharajah S, Belich MP, Papoutsopoulou S, et al. Proteolysis of NF-κB1 p105 is essential for T cell antigen receptor-induced proliferation. Nat Immunol. 2009;10:38–47. doi: 10.1038/ni.1685. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nat Immunol Rev. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Das S, Cho J, Lambertz I, et al. Tpl2/Cot signals activate ERK, JNK and NF-κB in a cell-type and stimulus-specific manner. J Biol Chem. 2005;280:23748–23757. doi: 10.1074/jbc.M412837200. [DOI] [PubMed] [Google Scholar]
- Beinke S, Ley SC. Functions of NF-κB1 and NF-κB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield MR, Zhang M, Norman LP, Sun SC. NF-κB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the TPL-2 kinase. Mol Cell. 2003;11:685–694. doi: 10.1016/s1097-2765(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Eliopoulos AG, Wang CC, Dumitru CD, Tsichlis PN. TPL-2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 2003;22:3855–3864. doi: 10.1093/emboj/cdg386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser F, Cook D, Papoutsopoulou S, et al. TPL-2 negatively regulates interferon-beta production in macrophages and myeloid dendritic cells. J Exp Med. 2009;206:1863–1871. doi: 10.1084/jem.20091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke LA, Elkins KL, Wei L, et al. Tumor progression locus 2 (Map3k8) is critical for host defense against Listeria monocytogenes and IL-1 production. J Immunol. 2009;183:7984–7993. doi: 10.4049/jimmunol.0901336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinke S, Deka J, Lang V, et al. NF-κB p105 negatively regulates TPL-2 MEK kinase activity. Mol Cell Biol. 2003;23:4739–4752. doi: 10.1128/MCB.23.14.4739-4752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Lahav-Baratz S, Ciechanover A. Two distinct ubiquitin-dependent mechanisms are involved in NF-κB p105 proteolysis. Biochem Biophys Res Commun. 2006;345:7–13. doi: 10.1016/j.bbrc.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Savinova OV, Hoffmann A, Ghosh G. The Nfκb1 and Nfκb2 proteins p105 and p100 function as the core of high-molecular-weight heterogeneous complexes. Mol Cell. 2009;34:591–602. doi: 10.1016/j.molcel.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinke S, Robinson MJ, Salmeron A, Hugunin M, Allen H, Ley SC. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IκB kinase-induced proteolysis of NF-κB1 p105. Mol Cell Biol. 2004;24:9658–9667. doi: 10.1128/MCB.24.21.9658-9667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang V, Janzen J, Fischer GZ, et al. βTrCP-mediated proteolysis of NF-κB1 p105 requires phosphorylation of p105 serines 927 and 932. Mol Cell Biol. 2003;23:402–413. doi: 10.1128/MCB.23.1.402-413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron A, Janzen J, Soneji Y, et al. Direct phosphorylation of NF-κB p105 by the IκB kinase complex on serine 927 is essential for signal-induced p105 proteolysis. J Biol Chem. 2001;276:22215–22222. doi: 10.1074/jbc.M101754200. [DOI] [PubMed] [Google Scholar]
- Heissmeyer V, Krappmann D, Hatada EN, Scheidereit C. Shared pathways of IκB kinase-induced SCFβTrCP-mediated ubiquitination and degradation for the NF-κB precursor p105 and IκBα. Mol Cell Biol. 2001;21:1024–1035. doi: 10.1128/MCB.21.4.1024-1035.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian A, Gonen H, Bercovich B, et al. SCFβTrCP ubiquitin ligase-mediated processing of NF-κB p105 requires phosphorylation of its C-terminus by IκB kinase. EMBO J. 2000;19:2580–2591. doi: 10.1093/emboj/19.11.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M, Jin W, Reiley W, Zhang M, Sun SC. IkappaB kinase is an essential component of the Tpl2 signaling pathway. Mol Cell Biol. 2004;24:6040–6048. doi: 10.1128/MCB.24.13.6040-6048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons A, Beinke S, Ley SC. MAP kinase kinase kinases and innate immunity. Trends Immunol. 2006;27:40–48. doi: 10.1016/j.it.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Luciano BS, Hsu S, Channavajhala PL, Lin LL, Cuozzo JW. Phoshorylation of threonine 290 in the activation loop of Tpl2/Cot is necessary but not sufficient for kinase activity. J Biol Chem. 2004;279:52117–52123. doi: 10.1074/jbc.M403716200. [DOI] [PubMed] [Google Scholar]
- Cho J, Tsichlis PN. Phosphorylation at T290 regulates Tpl2 binding to NF-κB1/p105 and Tpl2 activation and degradation by lipopolysaccharide. Proc Natl Acad Sci USA. 2005;102:2350–2355. doi: 10.1073/pnas.0409856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Melnick M, Solidakis GP, Tsichlis PN. Tpl2 (tumor progression locus 2) phosphorylation at Thr290 is induced by lipopolysaccharide via an IκB kinase-β-dependent pathway and is required for Tpl2 activation by external signals. J Biol Chem. 2005;280:20442–20448. doi: 10.1074/jbc.M413554200. [DOI] [PubMed] [Google Scholar]
- Stafford MJ, Morrice N, Peggie MW, Cohen P. Interleukin-1 stimulated activation of the COT catalytic subunit through the phosphorylation of Thr290 and Ser62. FEBS Lett. 2006;580:4010–4014. doi: 10.1016/j.febslet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Handoyo H, Stafford MJ, McManus E, Baltzis D, Peggie M, Cohen P. IRAK1-independent pathways required for the interleukin 1-stimulated activation of the TPL-2 catalytic subunit and its dissociation from ABIN-2. Biochem J. 2009;424:109–118. doi: 10.1042/BJ20091271. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Beinke S, Kouroumalis A, Tsichlis PN, Ley SC. Phosphorylation of TPL-2 on serine 400 is essential for lipopolysaccharide activation of extracellular signal-regulated kinase in macrophages. Mol Cell Biol. 2007;27:7355–7364. doi: 10.1128/MCB.00301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black TM, Andrews CL, Kilili G, Ivan M, Tsichlis PN, Vouros P. Characterization of phosphorylation sites on Tpl2 using IMAC enrichment and a linear ion trap mass spectrometer. J Proteome Res. 2007;6:2269–2276. doi: 10.1021/pr0700293. [DOI] [PubMed] [Google Scholar]
- Babu GR, Jin W, Norman L, et al. Phosphorylation of NF-κB1/p105 by oncoprotein kinase Tpl2: implications for a novel mechanism of Tpl2 regulation. Biochim Biophys Acta. 2006;1763:174–181. doi: 10.1016/j.bbamcr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Bauch A, Ruffner H, et al. A physical and functional map of the human TNFα/NF-κB signal transduction pathway. Nat Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- Lang V, Symons A, Watton SJ, et al. ABIN-2 forms a ternary complex with TPL-2 and NF-κB1 p105 and is essential for TPL-2 protein stability. Mol Cell Biol. 2004;24:5235–5248. doi: 10.1128/MCB.24.12.5235-5248.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huffel S, Delaei F, Heyninck K, de Valck D, Beyaert R. Identification of a novel A20-binding inhibitor of nuclear factor-κB activation termed ABIN-2. J Biol Chem. 2001;276:30216–30223. doi: 10.1074/jbc.M100048200. [DOI] [PubMed] [Google Scholar]
- Wagner S, Carpentier I, Rogov V, et al. Ubiquitin binding mediates the NF-κB inhibitory potential of ABIN proteins. Oncogene. 2008;27:3739–3745. doi: 10.1038/sj.onc.1211042. [DOI] [PubMed] [Google Scholar]
- Komander D, Reyes-Turcu F, Licchesi JD, Odenwaelder P, Wilkinson KD, Barford D. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10:466–473. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsopoulou S, Symons A, Tharmalingham T, et al. ABIN-2 is required for optimal activation of the TPL-2/ERK MAP kinase pathway in innate immune responses. Nat Immunol. 2006;7:606–615. doi: 10.1038/ni1334. [DOI] [PubMed] [Google Scholar]
- Albers M, Kranz H, Kober I, et al. Automated yeast two-hybrid screening for nuclear receptor-interacting proteins. Mol Cell Proteomics. 2005;4:205–213. doi: 10.1074/mcp.M400169-MCP200. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lozach J, Jepsen K, et al. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci USA. 2004;101:14461–14466. doi: 10.1073/pnas.0405786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CY, Liu WK, Chou CK, Su JY. The A20-binding protein ABIN-2 exerts unexpected function in mediating transcriptional co-activation. FEBS Lett. 2003;543:55–60. doi: 10.1016/s0014-5793(03)00401-0. [DOI] [PubMed] [Google Scholar]
- Ramirez-Carrozzi VR, Nazarian AA, Li CC, et al. Selective and antagonistic functions of SWI/SNF and Mi2β nucleosome remodeling complexes during an inflammatory response. Genes Dev. 2006;20:282–296. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-κB activation through Raf-1 and Syk. Nat Immunol. 2009;10:203–213. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- Rousseau S, Papoutsopoulou M, Symons A, et al. TPL2-mediated activation of ERK1 and ERK2 regulates the processing of pre-TNF alpha in LPS-stimulated macrophages. J Cell Sci. 2008;121:149–154. doi: 10.1242/jcs.018671. [DOI] [PubMed] [Google Scholar]
- Hirata K, Miyashiro M, Ogawa H, Taki H, Tobe K, Sugita T. Inhibition of tumor progression locus 2 protein kinase decreases lipopolysaccharide-induced tumor necrosis factor α production due to inhibition of the Tip-associated protein induction in RAW264.7 cells. Biol Pharm Bull. 2010;33:1233–1237. doi: 10.1248/bpb.33.1233. [DOI] [PubMed] [Google Scholar]
- Soond SM, Everson B, Riches DW, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFα-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci. 2005;118:2371–2380. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- Xiao N, Eidenschenk C, Krebs P, et al. The Tpl2 mutation sluggish impairs type I IFN production and increases susceptibility to group B Streptococcal disease. J Immunol. 2009;183:7975–7983. doi: 10.4049/jimmunol.0902718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomczak MF, Gadjeva M, Wang YY, et al. Defective activation of ERK in macrophages lacking the p50/p105 subunit of NF-κB is responsible for elevated expression of IL-12 p40 observed after challenge with Helicobacter hepaticus. J Immunol. 2006;176:1244–1251. doi: 10.4049/jimmunol.176.2.1244. [DOI] [PubMed] [Google Scholar]
- Windheim M, Stafford M, Peggie M, Cohen P. Interleukin-1 (IL-1) induces the Lys63-linked polyubiquitination of IL-1 receptor-associated kinase I to facilitate NEMO binding and the activation of IκBα kinase. Mol Cell Biol. 2008;28:1783–1791. doi: 10.1128/MCB.02380-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoyiannis D, Boulougouris G, Manoloukos M, et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn's-like inflammatory bowel disease. J Exp Med. 2002;196:1563–1574. doi: 10.1084/jem.20020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- Havell EA, Moldawer LI, Halfgott D, Kilian PL, Sehgal PB. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992;148:1486–1492. [PubMed] [Google Scholar]
- Rogers HW, Sheehan KC, Brunt LM, Dower SK, Unanue ER, Schreiber RD. Interleukin 1 participates in the development of anti-listerial responses in normal and SCID mice. Proc Natl Acad Sci USA. 1992;89:1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford WT, Wang CC, Tsatsanis C, et al. Ablation of tumor progression locus 2 promotes a type 2 Th cell response in ovalbumin-immunized mice. J Immunol. 2009;184:105–113. doi: 10.4049/jimmunol.0803730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap GS, Sher A. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiol. 1999;201:240–247. doi: 10.1016/S0171-2985(99)80064-3. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Ohata M, Miyoshi J, et al. A serine/threonine kinase, Cot/Tpl2, modulates bacterial DNA-induced IL-12 production and Th cell differentiation. J Clin Invest. 2004;114:857–866. doi: 10.1172/JCI20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto K, Musikacharoen T, Chiba N, Bandow K, Ohnishi T, Matsuguchi T. Cot/Tpl2 regulates IL-23 p19 expression in LPS-stimulated macrophages through ERK activation. J Physiol Biochem. 2010;66:47–53. doi: 10.1007/s13105-010-0007-9. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Gugasyan R, McMahon M, Gerondakis S. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci USA. 2006;103:3274–3279. doi: 10.1073/pnas.0511113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Grumont R, Gugasyan R, White C, Strasser A, Gerondakis S. NF-κB1 and c-Rel cooperate to promote the survival of TLR4-activated B cells by neutralizing Bim via distinct mechanisms. Blood. 2008;112:5063–5073. doi: 10.1182/blood-2007-10-120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated 'Bim(EL) kinases' that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J Biol Chem. 2004;279:8837–8847. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- van Acker GJ, Perides G, Weiss ER, Das S, Tsichlis PN, Steer ML. Tumor progression locus-2 is a critical regulator of pancreatic and lung inflammation during acute pancreatitis. J Biol Chem. 2007;282:22140–22149. doi: 10.1074/jbc.M702225200. [DOI] [PubMed] [Google Scholar]
- Jager J, Gremeaux T, Gonzalez T, et al. Tpl2 kinase is upregulated in adipose tissue in obesity and may mediate interleukin-1β and tumor necrosis factor-α effects on extracellular signal-regulated kinase activation and lipolysis. Diabetes. 2010;59:61–70. doi: 10.2337/db09-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M, Maini RN. Anti-TNFα therapy of rheumatoid arthritis: what have we learned. Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- Jacques C, Gosset M, Berenbaum F, Gabay C. The role of IL-1 and IL-1Ra in joint inflammation and cartilage degradation. Vitam Horm. 2006;74:371–403. doi: 10.1016/S0083-6729(06)74016-X. [DOI] [PubMed] [Google Scholar]
- Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009;21:1–8. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Wellbrock C, Karasarides M, Marais R. The Raf proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- George D, Salmeron A. Cot/TPL-2 protein kinase as a target for the treatment of inflammatory disease. Curr Topics Med Chem. 2009;9:611–622. doi: 10.2174/156802609789007345. [DOI] [PubMed] [Google Scholar]
- George D, Friedman M, Allen H, et al. Discovery of thieno [2,3-c]pyridines as potent COT inhibitors. Bioorg Med Chem Lett. 2008;18:4952–4955. doi: 10.1016/j.bmcl.2008.08.037. [DOI] [PubMed] [Google Scholar]
- Hall JP, Kurdi Y, Hsu S, et al. Pharmacologic inhibition of TPL-2 blocks inflammatory responses in primary human monocytes, synoviocytes, and blood. J Biol Chem. 2007;282:33295–33304. doi: 10.1074/jbc.M703694200. [DOI] [PubMed] [Google Scholar]
- Hu Y, Green N, Gavrin LK, et al. Inhibition of Tpl2 kinase and TNFα production with quinoline-3-carbonitriles for the treatment of rheumatoid arthritis. Bioorg Med Chem Lett. 2006;16:6067–6072. doi: 10.1016/j.bmcl.2006.08.102. [DOI] [PubMed] [Google Scholar]
- Inamdar GS, Madhunapantula SV, Robertson GP. Targeting the MAPK pathway in melanoma: why some approaches succeed and others fail. Biochem Pharm. 2010;80:624–637. doi: 10.1016/j.bcp.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratilas CA, Solit DB. Targeting the mitogen-activated protein kinase pathway: physiological feedback and drug response. Clin Cancer Res. 2010;16:3329–3334. doi: 10.1158/1078-0432.CCR-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt LM. Oncogenic activation of NF-κB. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane LP, Mollenauer MN, Xu Z, Turck CW, Weiss A. Akt-dependent phosphorylation specifically regulates Cot induction of NF-κB-dependent transcription. Mol Cell Biol. 2002;22:5962–5974. doi: 10.1128/MCB.22.16.5962-5974.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale ST. Selective transcription in response to an inflammatory stimulus. Cell. 2010;140:833–844. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyninck K, Kreike MM, Beyaert R. Structure-function analysis of the A20-binding inhibitor of NF-κB activation, ABIN-1. FEBS Lett. 2003;536:135–140. doi: 10.1016/s0014-5793(03)00041-3. [DOI] [PubMed] [Google Scholar]
- Skaug B, Jiang X, Chen ZJ. The role of ubiquitin in NF-κB regulatory pathways. Annu Rev Immunol. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Solan NJ, Miyoshi H, Bren GD, Paya CV. RelB cellular regulation and transcriptional activity are regulated by p100. J Biol Chem. 2001;277:1405–1418. doi: 10.1074/jbc.M109619200. [DOI] [PubMed] [Google Scholar]
- Soria Castro I, Krzyzanowska A, Pelaéz ML, et al. COT/TPL2 (MAP3K8) mediates myeloperoxidase activity and hypernociecption following peripheral inflammation. J Biol Chem. 2010;285:33805–33815. doi: 10.1074/jbc.M110.169409. [DOI] [PMC free article] [PubMed] [Google Scholar]