Dear Editor,
Histone modifications play a vital role in the conformation and function of their associated chromatin templates 1. Histone H3K27 methylation mediated by the PRC2 complex is critical for transcriptional regulation, Polycomb silencing, Drosophila segmentation, mammalian X inactivation and cancer 1. Interestingly, H3K27me1 (H3 mono-methylated at residue K27) levels in vivo remain unaffected after PRC2 disruption 2, 3, which is an indication for the existence of other contributing histone methyltransferase(s) to H3K27me1. However, in animals, histone methyltransferases responsible for H3K27me1 levels in vivo remain undefined. On the other hand, G9a is a histone methyltransferase that controls H3K9me2 in vivo and predominantly represses genes at euchromatic regions 4. It has also been reported to act on H3K27 in vitro on H3 tail fused with a GST tag 5, but its role in H3K27 methylation has not been tested on the nucleosomes, the native substrates for histone methyltransferases. In addition, G9a's contribution to H3K27 methylation in vivo remains obscure.
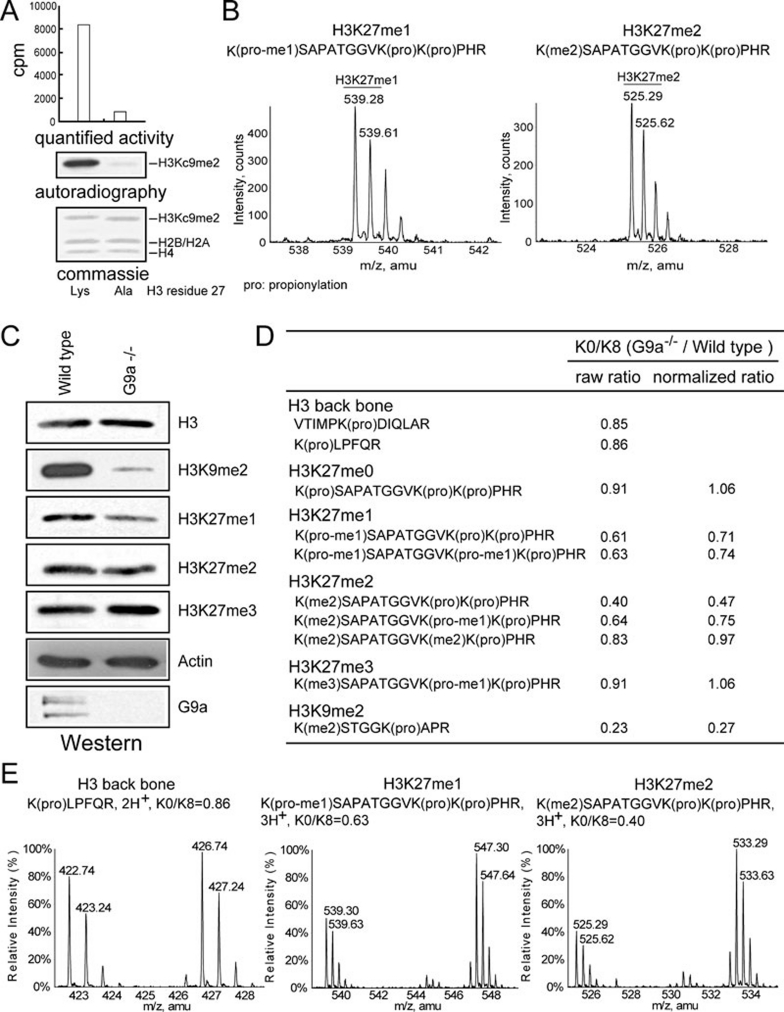
We previously reported that histones containing methyl-lysine analogues (MLA) 6 are compatible in biochemical reactions mediated by many histone methyltransferases, including Dot1L, HYPB, Suvar4-20 and Pr-Set7 7. We then extended this study to G9a. His-tagged H3 histones containing K9C mutation were converted into His-H3Kc9me2 with the MLA reaction to mimic homogenously di-methylated H3K9. To our surprise, recombinant G9a displayed robust activity on nucleosomes assembled with H3Kc9me2 (Figure 1A, left lane), which suggests that either G9a may be a trimethylase or may react with other H3 lysine residues on a nucleosomal substrate. Thus, we introduced a K27A mutation into the His-tagged pre-methylated H3, which nearly completely abolished G9a's activity (Figure 1A, right lane), indicating that G9a is mainly a dimethylase and also reacts with H3K27.
Figure 1.
G9a methylates H3K27 in vitro and contributes to H3K27 methylation in vivo. (A) G9a methylates nucleosomal H3K27 in vitro; (B) direct detection of H3K27me1 and H3K27me2 products by mass spectrometry on nucleosomal H3 reacted with G9a; (C) G9a−/− ES cells displayed reduced H3K27me1 levels in vivo; (D) stable isotope labeling-based quantitative mass spectrometry revealed reduced levels of H3K27me1 and a subset of H3K27me2 in G9a−/− ES cells.
To directly detect the reaction products, wild-type nucleosomes were reacted with G9a and S-adenosyl methionine. The reaction products were chemically propionylated 8 prior to mass spectrometry analysis to ensure the detection of H3K9 methylation marks in subsequent LC-MS (liquid chromatograph-coupled mass spectrometry) procedures. G9a-treated nucleosomal H3 samples contained H3K9me1, H3K9me2 signals and low amounts of H3K9me3 as expected (Supplementary information, Figure S1). Moreover, they also contained robust signals for H3K27me1 and H3K27me2 (Figure 1B). In addition, another histone methyltransferase GLP, a close homologue of G9a, can also methylate H3K27 in addition to H3K9 in vitro (Supplementary information, Figure S2).
The role of G9a as an H3K9 methyltransferase has been fully recognized 4, but its contribution to H3K27 methylation in vivo remains obscure. This is likely due to the overwhelming contribution of PRC2 in H3K27 methylation 2, 3, which may mask the contribution of G9a. Methylation status-specific antibodies were used to compare the levels of H3K27 methylation between wild-type and G9a−/− ES cells. Although no apparent changes of H3K27me2/3 levels were observed, H3K27me1 levels were clearly reduced in the G9a−/− ES cells (Figure 1C).
To quantitatively compare the histone modification levels between the wild-type and G9a−/− ES cells, stable isotope labeling-based quantitative mass spectrometry analysis 9, 10 was performed. Histone samples were prepared from wild-type ES cells fully labeled with lysine-8 ([13C6, 15N2] heavy isotope-labeled L-lysine, abbreviated as K8, Supplementary information, Figure S3) and then mixed with equal amounts of histones extracted from G9a−/− ES cells labeled with regular L-lysine (K0). H3K27me1 levels in the G9a−/− ES cells were reduced to about 70% of the wild-type levels (Figure 1D and 1E). Comparison of H3K27me2 levels between wild-type and G9a−/− ES cells revealed a more complicated and interesting pattern. G9a−/− ES cells contained similar levels (97%) of H3 carrying the combination of K27me2 and K36me2, reduced levels (75%) of H3 carrying the combination of K27me2 and K36me1, and further reduced levels (47%) of H3 carrying the combination of K27me2 and K36me0 (Figure 1D and 1E, Supplementary information, Figure S4). These results suggest a potential cross-talk between H3K27 and H3K36 methylations.
Taken together, our results collectively suggest G9a's contribution to H3K27 methylation in addition to its well-characterized role in H3K9 methylation in vivo.
PRC2 complexes containing Ezh2 and its closely related Ezh1 have been clearly shown to be the main contributors for H3K27me2/3 in vivo 3, 4. Identification of G9a and possibly GLP as novel contributors to H3K27me1 and H3K27me2 in vivo led to an intriguing question for further investigation: do G9a/GLP coordinate H3 K9 and K27 methylation at certain chromatin regions for a specific function?
Acknowledgments
We are grateful to Y Shinkai at Kyoto University for providing the G9a null ES cells and cDNA. This work was supported by the Chinese Ministry of Science and Technology 863 projects 2007AA02Z1A6 (to BZ) and 2007AA02Z1A3 (to SC).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
G9a's in vitro activity.
GLP's in vitro activity.
Full labeling of wild type ES cells with K8, determined by quantitative mass spectrometry.
Mass spectra for peptides listed in Figure 1D.
References
- Allis CD, Jenuwein T, Reinberg D.In: Allis CD, Jenuwein T, Reinberg D, eds. Epigenetics New York: Cold Spring Harbor Laboratory Press; 200623–56. [Google Scholar]
- Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell. 2004;15:57–67. doi: 10.1016/j.molcel.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Jensen MR, et al. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 2004;23:4061–4071. doi: 10.1038/sj.emboj.7600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Sugimoto K, Nozaki M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2004;16:1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem. 2001;276:25309–25317. doi: 10.1074/jbc.M101914200. [DOI] [PubMed] [Google Scholar]
- Simon MD, Chu F, Racki LR, et al. The site-specific installation of methyl-lysine analogs into recombinant histones. Cell. 2007;128:1003–1012. doi: 10.1016/j.cell.2006.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Wang W, Li H, et al. A systematic evaluation of the compatibility of histones containing methyl-lysine analogues with biochemical reactions. Cell Res. 2009;19:1217–1220. doi: 10.1038/cr.2009.110. [DOI] [PubMed] [Google Scholar]
- Garcia BA, Mollah S, Ueberheide BM, et al. Chemical derivatization of histones for facilitated analysis by mass spectrometry. Nat Protoc. 2007;2:933–938. doi: 10.1038/nprot.2007.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SE, Blagoev B, Kratchmarova I, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- Xu M, Long C, Chen X, Huang C, Chen S, Zhu B. Partitioning of histone H3-H4 tetramers during DNA replication-dependent chromatin assembly. Science. 2010;328:94–98. doi: 10.1126/science.1178994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
G9a's in vitro activity.
GLP's in vitro activity.
Full labeling of wild type ES cells with K8, determined by quantitative mass spectrometry.
Mass spectra for peptides listed in Figure 1D.