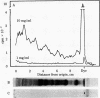
Abstract
Microvessels isolated from rat epididymal fat exhibit differential vesicular ingestion rates for unmodified and non-enzymatically glycosylated rat albumin. While unmodified rat albumin is excluded from ingestion by endothelial micropinocytic vesicles, glycosylated albumin is avidly taken up by endocytosis. Interaction of albumin and glycosylated albumin with endothelium was studied with a double-label fluorescence assay of micropinocytosis. When glycosylated albumin was present at a concentration of 6% with respect to total albumin (the level found in "non diabetic" serum), only glycosylated albumin was ingested. At higher concentrations of glycosylated albumin (those found in diabetic serum), both albumin and glycosylated albumin are ingested. Glycosylation of endothelial membrane components results in stimulated ingestion of glycosylated albumin, persistent exclusion of unmodified albumin, and unaltered micropinocytic ingestion of native ferritin. These results indicate that nonenzymatic glycosylation of serum albumin may result in rapid vesicle-mediated extravasation of albumin. Chronic microvascular leakage of glycosylated albumin could contribute to the pathogenesis of diabetic microangiopathy.
Full text
PDF

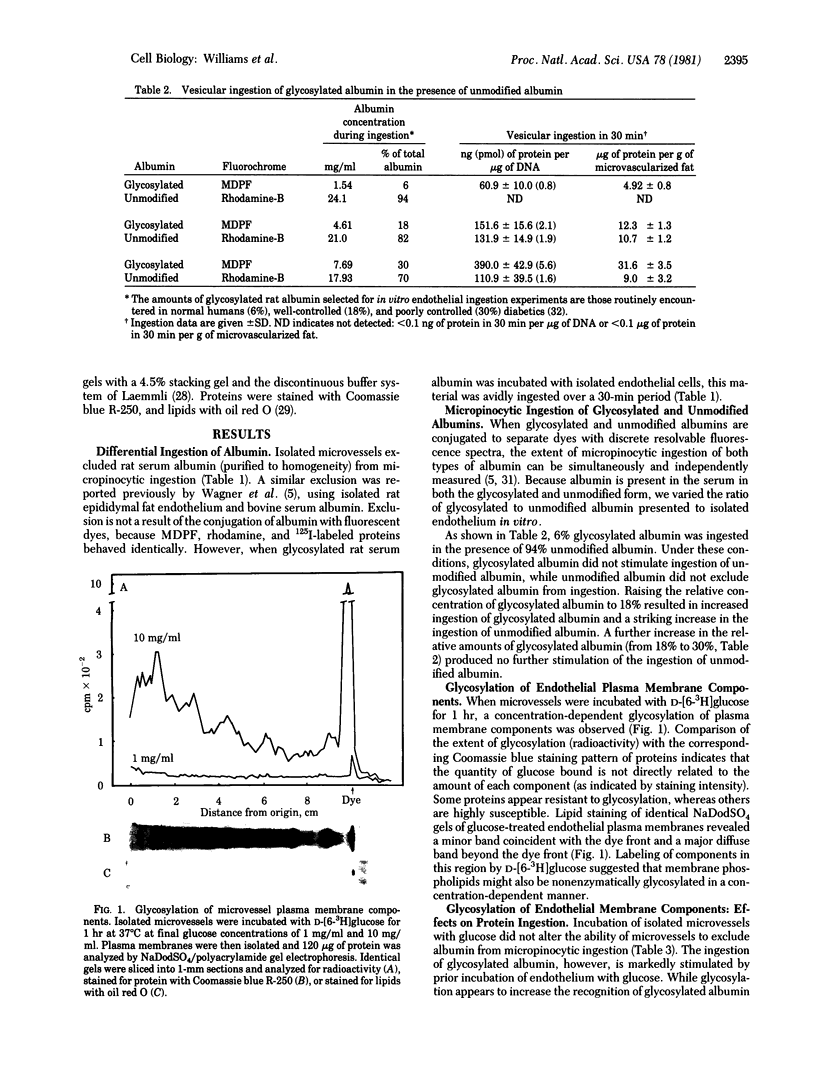

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Michiel H., Krans M. J., Rodbell M. The actions of hormones on the adenyl cyclase system. Adv Biochem Psychopharmacol. 1970;3:185–208. [PubMed] [Google Scholar]
- Bownds D., Gordon-Walker A., Gaide-Huguenin A. C., Robinson W. Characterization and analysis of frog photoreceptor membranes. J Gen Physiol. 1971 Sep;58(3):225–237. doi: 10.1085/jgp.58.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C. A fiber matrix model of capillary permeability. Microvasc Res. 1980 Jul;20(1):96–99. doi: 10.1016/0026-2862(80)90024-2. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J. F., Thorpe S. R., Baynes J. W. Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum. J Biol Chem. 1979 Feb 10;254(3):595–597. [PubMed] [Google Scholar]
- Dolhofer R., Wieland O. H. Increased glycosylation of serum albumin in diabetes mellitus. Diabetes. 1980 Jun;29(6):417–422. doi: 10.2337/diab.29.6.417. [DOI] [PubMed] [Google Scholar]
- Engerman R., Bloodworth J. M., Jr, Nelson S. Relationship of microvascular disease in diabetes to metabolic control. Diabetes. 1977 Aug;26(8):760–769. doi: 10.2337/diab.26.8.760. [DOI] [PubMed] [Google Scholar]
- Fox C. J., Darby S. C., Ireland J. T., Sönksen P. H. Blood glucose control and glomerular capillary basement membrane thickening in experimental diabetes. Br Med J. 1977 Sep 3;2(6087):605–607. doi: 10.1136/bmj.2.6087.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T., Tanaka K., Taniguchi Y. Disruption of blood-retinal barrier in experimental diabetic rats: an electron microscopic study. Exp Eye Res. 1980 Apr;30(4):401–410. doi: 10.1016/0014-4835(80)90055-x. [DOI] [PubMed] [Google Scholar]
- Johansson B. R. Movement of interstitially microinjected 125I-labeled albumin into blood capillaries of rat skeletal muscle demonstrated with electron microscopic autoradiography. Microvasc Res. 1978 Nov;16(3):354–361. doi: 10.1016/0026-2862(78)90068-7. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Neet K. E. The catalytic and regulatory properties of enzymes. Annu Rev Biochem. 1968;37:359–410. doi: 10.1146/annurev.bi.37.070168.002043. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landel A. M. Stability studies on fluorescein isothiocyanate--bovine serum albumin conjugate. Anal Biochem. 1976 Jun;73(2):280–289. doi: 10.1016/0003-2697(76)90173-1. [DOI] [PubMed] [Google Scholar]
- McFarland K. F., Catalano E. W., Day J. F., Thorpe S. R., Baynes J. W. Nonenzymatic glucosylation of serum proteins in diabetes mellitus. Diabetes. 1979 Nov;28(11):1011–1014. doi: 10.2337/diab.28.11.1011. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Gravallese E., Bunn H. F. Nonenzymatic glycosylation of erythrocyte membrane proteins. Relevance to diabetes. J Clin Invest. 1980 Apr;65(4):896–901. doi: 10.1172/JCI109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noer I., Lassen N. A. Evidence of active transport (filtration?) of plasma proteins across the capillary walls in muscle and subcutis. Acta Physiol Scand Suppl. 1979;463:105–110. [PubMed] [Google Scholar]
- Norton E. W., Gutman F. Diabetic retinopathy studied by fluorescein angiography. Ophthalmologica. 1965;150(1):5–17. doi: 10.1159/000304822. [DOI] [PubMed] [Google Scholar]
- PEDERSEN K. O. Exclusion chromatography. Arch Biochem Biophys. 1962 Sep;Suppl 1:157–168. [PubMed] [Google Scholar]
- Pardridge W. M. Carrier-mediated transport of thyroid hormones through the rat blood-brain barrier: primary role of albumin-bound hormone. Endocrinology. 1979 Sep;105(3):605–612. doi: 10.1210/endo-105-3-605. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J Clin Invest. 1979 Jul;64(1):145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbar S., Blumenfeld O., Ranney H. M. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun. 1969 Aug 22;36(5):838–843. doi: 10.1016/0006-291x(69)90685-8. [DOI] [PubMed] [Google Scholar]
- Renkin E. M. The microcirculatory society Eugene M. Landis award lecture. Transport pathways through capillary endothelium. Microvasc Res. 1978 Jan;15(1):123–135. doi: 10.1016/0026-2862(78)90013-4. [DOI] [PubMed] [Google Scholar]
- Richards F. F., Konigsberg W. H., Rosenstein R. W., Varga J. M. On the specificity of antibodies. Science. 1975 Jan 17;187(4172):130–137. doi: 10.1126/science.46122. [DOI] [PubMed] [Google Scholar]
- Stevens V. J., Vlassara H., Abati A., Cerami A. Nonenzymatic glycosylation of hemoglobin. J Biol Chem. 1977 May 10;252(9):2998–3002. [PubMed] [Google Scholar]
- Tchobroutsky G. Relation of diabetic control to development of microvascular complications. Diabetologia. 1978 Sep;15(3):143–152. doi: 10.1007/BF00421230. [DOI] [PubMed] [Google Scholar]
- Trivelli L. A., Ranney H. M., Lai H. T. Hemoglobin components in patients with diabetes mellitus. N Engl J Med. 1971 Feb 18;284(7):353–357. doi: 10.1056/NEJM197102182840703. [DOI] [PubMed] [Google Scholar]
- Trüeb B., Holenstein C. G., Fischer R. W., Winterhalter K. H. Nonenzymatic glycosylation of proteins. A warning. J Biol Chem. 1980 Jul 25;255(14):6717–6720. [PubMed] [Google Scholar]
- Wagner R. C., Andrews S. B., Matthews M. A. A fluorescense assay for micropinocytosis in isolated capillary endothelium. Microvasc Res. 1977 Jul;14(1):67–80. doi: 10.1016/0026-2862(77)90142-x. [DOI] [PubMed] [Google Scholar]
- Wagner R. C., Matthews M. A. The isolation and culture of capillary endothelium from epididymal fat. Microvasc Res. 1975 Nov;10(3):286–297. doi: 10.1016/0026-2862(75)90033-3. [DOI] [PubMed] [Google Scholar]
- Wagner R. C., Williams S. K., Matthews M. A., Andrews S. B. Exclusion of albumin from vesicular ingestion by isolated microvessels. Microvasc Res. 1980 Jan;19(1):127–130. doi: 10.1016/0026-2862(80)90088-6. [DOI] [PubMed] [Google Scholar]
- Weigele M., De Bernardo S., Leimgruber W. Fluorescent labeling of proteins. A new methodology. Biochem Biophys Res Commun. 1973 Oct 1;54(3):899–906. doi: 10.1016/0006-291x(73)90779-1. [DOI] [PubMed] [Google Scholar]
- Whayne T. F., Jr, Felts J. M. The determination of lipoprotein lipase activity: effect of different albumin preparations. Clin Biochem. 1972 Jun;5(2):109–114. doi: 10.1016/s0009-9120(72)80017-1. [DOI] [PubMed] [Google Scholar]
- Williams S. K., Gillis J. F., Matthews M. A., Wagner R. C., Bitensky M. W. Isolation and characterization of brain endothelial cells: morphology and enzyme activity. J Neurochem. 1980 Aug;35(2):374–381. doi: 10.1111/j.1471-4159.1980.tb06274.x. [DOI] [PubMed] [Google Scholar]
- Williams S. K., Matthews M. A., Wagner R. C. Metabolic studies on the micropinocytic process in endothelial cells. Microvasc Res. 1979 Sep;18(2):175–184. doi: 10.1016/0026-2862(79)90027-x. [DOI] [PubMed] [Google Scholar]
- Williams S. K., Sasaki A. W., Matthews M. A., Wagner R. C. Quantitative determination of deoxyribonucleic acid from cells collected on filters. Anal Biochem. 1980 Sep 1;107(1):17–20. doi: 10.1016/0003-2697(80)90485-6. [DOI] [PubMed] [Google Scholar]