Abstract
Hutchinson–Gilford progeria syndrome (HGPS) is a rare, progressive segmental premature aging disease that includes scleroderma-like skin, progressive joint contracture, and atherosclerosis. Affected individuals die prematurely of heart attacks or strokes. Extracellular matrix dysregulation is implicated as a factor in disease progression. We analyzed messenger RNA and protein levels for matrix metalloproteinases (MMPs)-2,-3, and -9 in HGPS primary human dermal fibroblasts using real-time polymerase chain reaction, enzyme-linked immunosorbent assay, and gelatin zymography. MMP-3 messenger RNA and protein levels decreased significantly with increasing donor age in HGPS fibroblasts but not in controls. MMP-2 messenger RNA also showed a donor age–dependent decrease in HGPS fibroblasts, but levels of secreted protein were unchanged. MMP-9 was similar in HGPS and control cultures. The decreased MMP-3 may represent a shift in the inherent extracellular matrix–degrading proteolytic balance in favor of matrix deposition in HGPS. This metalloproteinase has the potential to serve as a biomarker of therapeutic efficacy when assessing treatments for HGPS.
Keywords: Progeria, HGPS, MMP, Matrix metalloproteinase, Lamin A
HUTCHINSON–GILFORD progeria syndrome (HGPS) is a rare, progressive segmental premature aging disease in children. Manifestations of the disease suggest that disordered connective tissue contributes to morbidity and/or mortality as consistent disease phenotypes include short stature, progressive joint contractures, scleroderma-like skin (1), and atherosclerotic disease characterized by highly abnormal vessel wall extracellular matrix (ECM), including adventitial fibrosis (2). Ultimately, heart attacks and strokes claim the lives of these children at an average age of 13 years (3).
HGPS is caused by a silent mutation in LMNA, which creates increased use of a cryptic splice site and the production of progerin, a toxic, permanently farnesylated splicing variant of the nuclear envelope protein, lamin A (4). Lamin A is normally integral to chromatin organization, DNA replication, transcription, and repair (reviewed in (5)). Gene expression studies of primary human dermal fibroblasts have shown large differences in HGPS expression profiles compared with age-matched controls, confirming progerin’s broad downstream effects on gene transcription (6,7). How progerin causes the tissue-specific abnormalities associated with HGPS is unknown.
Matrix metalloproteinases (MMPs) are ECM-degrading enzymes, which, along with their endogenous tissue inhibitors (TIMPs), are essential for maintaining the proper balance between ECM synthesis and degradation. When the balance is disturbed, it results in various pathologic conditions, including arthritis (reviewed in (8)), coronary artery disease (reviewed in (9)), obesity (10), and cancer (11–13). MMP-3 (stromelysin-1) has the broadest substrate specificity of the MMP family and can degrade most components of the basement membrane, including proteoglycans, laminin, fibronectin, and collagens (14).
It is likely that altered ECM is a major downstream component driving disease. In gene expression studies using HGPS dermal fibroblasts, several genes involved in building and maintaining the ECM showed altered gene expression, including several collagens, proteoglycans, and MMPs (6,7). Additional reports describe altered expression of elastin, laminins, type IV collagen, and fibronectin as well as several proteoglycans (15–18). Of particular note, published gene array studies indicate that MMP-3 messenger RNA (mRNA) was downregulated in HGPS fibroblasts more than any other ECM gene when compared with donor age–matched controls (Ly et al. (7) [−29.5 fold] and Csoka et al. (6) [−6.7 fold]). However, in the study by Ly and colleagues (7), only two of three lines used were later shown to carry the classic HGPS mutation (ca. 1,824 C>T in LMNA), whereas Csoka and colleagues (6) specifically chose HGPS cell lines with validated classic HGPS mutations. Both studies pooled data from each line, so that only an average response was reported, precluding any analysis of more complex patterns of gene regulation, such as age dependence. No study has explored MMP-3 protein levels in HGPS.
In the current study, we asked whether regulation of both MMP-3 mRNA and MMP-3 protein is defective in HGPS. HGPS is progressive with major symptoms appearing in the first year of life and increasing in severity with age (3). It is of importance to understand the temporal nature of abnormalities in ECM homeostasis. We therefore analyzed fibroblast lines of various donor ages in order to determine if MMP-3 production in HGPS cells changes with donor age. Additionally, by analyzing MMP-2 and -9 expression, we could determine whether these potential changes are specific to MMP-3 or represent a more global disruption by MMPs.
MMP-3 can also act indirectly through its ability to activate the proforms of other MMPs, including the gelatinases, MMP-2, and MMP-9. These MMPs are expressed along with MMP-3 in tissues, such as skin and the vasculature, where they play an important role in remodeling collagenous ECM and specifically the basement membrane (19). As with MMP-3, perturbations in the activity of MMPs 2 and 9 are involved in the pathogenesis of a variety of diseases, including fibrosis (20), arthritis (8), cancer (13), and atherosclerosis (9).
Either elevation or depression of MMP-3 can contribute to disease. Although MMP-3 levels are elevated and destructive in diseases, such as arthritis and cancer, a reduction in MMP-3 activity can promote damaging ECM deposition in systemic sclerosis, hypertrophic scarring, liver fibrosis, and atherosclerosis (20–23). Mice deficient in both apolipoprotein E (ApoE) and MMP-3 develop larger atherosclerotic lesions, which are richer in fibrillar collagen compared with ApoE−/− and MMP-3+/+ mice (24).
MATERIALS AND METHODS
Compliance
This study was carried out with approval by the Institutional Review Boards of Tufts University, Medford, MA, and Brown University, Providence, RI.
Cell Lines
Primary skin fibroblasts were obtained from the Progeria Research Foundation Cell and Tissue Bank (http://www.progeriaresearch.org/cell_tissue_bank.html; HGPS lines and donor ages as follows: HGADFN001, 10 year old [yo]; HGADFN127, 3 yo; and HGADFN003, 2 yo) and from the Coriell Cell Repository (Camden, NJ; HGPS lines AG03513, 13 yo and AG10750, 9 yo and non-HGPS control lines GM02037, 13 yo; GM09503, 10 yo; GM00038, 9 yo; GM00498C, 3 yo; and GM00969, 2 yo). Cells were maintained in Eagle’s Minimum Essential Media with Earle’s salts (EMEM; Invitrogen, Carlsbad, CA) with 15% fetal bovine serum, 2 mM L-glutamine, 50 units/mL penicillin, 50 μg/mL streptomycin, and 10mM nonessential amino acids (Invitrogen) at 37°C in a humid incubator with 5% CO. All experiments were carried out with presenescent cultures. Control lines were used between Passages 5 and 24, whereas HGPS lines were between 6 and 16.
Cell Culture
On Day 0, cells were plated in maintenance medium at 80% confluence. On Day 1, maintenance medium was replaced with medium lacking serum and supplemented with 0.01% insulin-transferrin-selenium-X supplement (ITS-X; Invitrogen). On Day 2, when cultures were fully confluent, 24-hour conditioned media were collected and supplemented with protease inhibitors (10 mM benzamidine hydrochloride, 10 mM e-amino-H-caproic acid, 1 mM phenylmethylsulfonyl fluoride; Sigma, St. Louis, MO). The cell layer (cells plus ECM) was lysed with 45 mM Tris 2% sodium dodecyl sulfate pH 8 containing the same protease inhibitors. Protein content of the cell layer was determined by the bicinchoninic acid method (BCA assay; Pierce, Rockford, IL).
Western Blot
A total of 10 μg of cell lysate protein from each sample was run on a 12% sodium dodecyl sulfate/acrylamide gel and transferred to nitrocellulose (Amersham, Piscataway, NJ). Lamins A and C, prelamin A, and progerin were detected with an antihuman lamin A + C antibody (JOL2; ab40567; Abcam Cambridge, MA) diluted 1:50 and an horseradish peroxidase–conjugated goat antimouse secondary antibody (Amersham) diluted 1:3,000 and developed using the ECL technique (New England Nuclear Chemiluminescence Reagent Plus, Newton, MA).
Complementary DNA Synthesis and Real-Time PCR for MMP-3 Expression
Total RNA was isolated from cells by using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions and checked spectrophotometrically for quantity and purity. Equal amounts of mRNA were reverse transcribed using high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Gene expression was analyzed by one-step real-time polymerase chain reaction (PCR) using the Power SYBR Green PCR Master Mix (Applied Biosystems). A 126-bp product of human MMP-2 (NM_004530) was detected with specific primers (forward 5′-ATGACAGCTGCACCACTGAG-3′ and reverse 5′-AGTTCCCACCAACAGTGGAC-3′), a 129-bp product of human MMP-3 (NM_002422) was detected with specific primers (forward 5′-TGTTTTGGCCCATGCCTATGCCC-3′ and reverse 5′-TGGCCAATTTCATGAGCAGCAACG-3′), a 82-bp product of human MMP-9 (NM_004994) was detected with specific primers (forward 5′-ATAAGGACGACGTGAATGGC-3′ and reverse 5′-GGTGTGGTGGTGGTTGGA-3′), and a 80-bp product of the reference gene β-actin (hACTB; NT_007819) was detected with specific primers (forward 5′-AGAGCCTCGCCTTTGCCGATCC-3′ and reverse 5′-GACGAGCGCGGCGATATCATCA-3′). Reactions were carried out using a 384-well plate on an ABI HT 7900 (Applied Biosystems) using their standard methodology. Data acquisition and analysis were performed on ABI SDS Version 2.2 software.
MMP-3 Enzyme-Linked Immunosorbent Assay
MMP-3 concentrations in 24-hour conditioned media were determined using an enzyme-linked immunosorbent assay (ELISA; Human Biotrak ELISA, Amersham Biosciences, Piscataway NJ). Optical density of each 96-well plate was read at 405 nm on a microplate reader (Titertek Multiskan PLUS, ICN, Irvine, CA) monitored by computer using the Delta Soft II plate reader software from Biometallics (Princeton, NJ). The assay recognizes total MMP-3: proMMP-3, active MMP-3, and MMP-3/TIMP complexes. It does not cross-react with MMP-1, -2, and -9 and TIMP-1 and -2 nor do they interfere in the assay. Culture supernatant MMP-3 levels were normalized to total protein for comparative analysis. Results represent data from three separate experiments.
MMP-2 and MMP-9 Gel Zymography
Gelatin zymography for MMP-2 and MMP-9 was performed on the same conditioned media samples used in the MMP-3 ELISA, as previously described by us (25). Conditioned media sample volumes, normalized to equal cell lysate protein (20 μg), were loaded into wells of a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel containing 0.1% (wt/vol) gelatin (Bio-Rad Laboratories, Hercules, CA) on a mini gel apparatus. Gels were run at 200 V for 50 minutes, then soaked in 2.5% TritonX-100 with gentle shaking for 30 minutes at ambient temperature. After incubating overnight at 37°C in substrate buffer (50 mM Tris-HCL buffer pH 8, 5 mM CaCl2, and 0.02% NaN3), gels were stained for 30 minutes in 0.5% Coomassie Blue R-250 in acetic acid, ethanol, and water (1:3:6) and destained for 1 hour. MMP levels were quantified by scoring the band intensity of each type of MMP examined on the zymogram on a scale of zero to six, with zero indicating no detectable MMPs and six indicating strong intensity bands, as previously described (12,25,26). Enzyme levels were measured twice for each pair of the five pairs of fibroblast lines.
Statistical Methods
Reverse transcription-PCR estimates of MMP mRNA levels relative to β-actin using cycles to threshold were normally distributed and modeled as a function of age using a linear mixed model to account for the paired nature of the matched observations. The 2−ΔΔ technique was applied to transform differences in cycles to threshold to relative quantities. ELISA estimates of secreted MMP-3 concentrations were positively skewed and were modeled using a generalized linear mixed model for log-normal distributed data to account for the paired nature of the matched observations. Pearson’s correlation was used to analyze relationships between passage number and MMP expression. All other analyses were carried out using a paired, two-tailed standard Student’s t test.
RESULTS
Progerin Production in HGPS Dermal Fibroblasts Versus Donor Age–Matched Controls
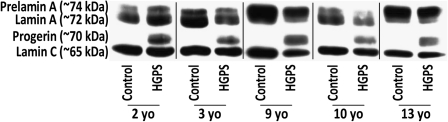
We assessed progerin and lamins A and C in five pairs of HGPS and donor age–matched control fibroblast lines by Western blot (Figure 1). Great effort was made to match cell lines as closely as possible in passage number to ensure minimal differences in growth rate and in vitro age as senescence can result in elevated levels of MMP-3 (27). As anticipated, all HGPS lines produced progerin, normal prelamin A, lamin A, and lamin C. In contrast, the donor age–matched counterparts produced prelamin A, lamin A, and lamin C but no detectable progerin. Densitometric measurements of the Western blots revealed that ratios of mature lamin A alone as well as total lamin A (prelamin A, lamin A, and progerin) to lamin C were similar between HGPS and controls and did not correlate with donor age (data not shown; p > .05). Progerin levels, normalized to lamin C, were also not significantly correlated with donor age or passage number (data not shown; p > .05).
Figure 1.
Western blotting demonstrates that progerin protein is produced by all Hutchinson–Gilford progeria syndrome (HGPS) cell lines but not by control cell lines. Prelamin A, lamin A, and lamin C proteins are produced by all cell lines.
Age-Dependent Reduction in MMP-3 and MMP-2 mRNA in HGPS Dermal Fibroblasts
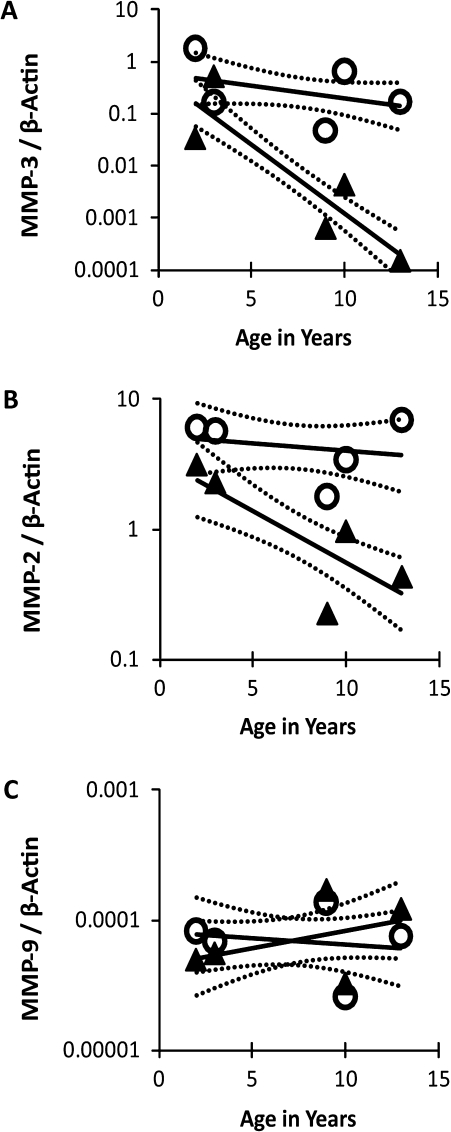
We used real-time Reverse transcription-PCR analysis to determine relative levels of MMP-3, -2, and -9 mRNA, normalized to β-actin, in the same five age-matched pairs of fibroblast lines shown in Figure 1 (Figure 2A–C). Mean MMP-3 mRNA levels in the five HGPS lines were 47-fold lower than in controls (range of ratios: 3.4–649.4; p = .0107), and mean MMP-2 levels were 4.8-fold lower than in controls (range of ratios: 1.3–18.0; p = .0275). In contrast, mean MMP-9 mRNA levels were not significantly different between HGPS and control cell lines (p = .8878). Notably, the levels of mRNA for both MMP-3 and -2 displayed a statistically significant donor age–dependent decline in the HGPS fibroblasts (Figure 2A; p < .001 and Figure 2B; p < .003, respectively) but not in controls (Figure 2A; p = .1665 and Figure 2B; p < .5574, respectively). There was no significant age-dependent change in MMP-9 mRNA expression for either HGPS fibroblasts (p = .2313) or controls (p = .8878; Figure 2C). Passage number had no significant relationship to mRNA levels for any of the MMP genes (data not shown; p > .05).
Figure 2.
Levels of messenger RNA for MMP genes were determined by real-time reverse-transcription polymerase chain reaction in five pairs of donor age–matched human dermal fibroblast lines. Symbols (○ = controls; ▲ = Hutchinson–Gilford progeria syndrome) indicate mean relative MMP-3 (A), -2 (B), and -9 (C) RNA levels standardized to β-actin for two separate experiments analyzed in duplicate; solid lines indicate the mixed linear model’s predicted mean with dashed lines representing upper and lower 95% confidence intervals.
Age-Dependent Reduction in MMP-3 Protein Levels in HGPS Dermal Fibroblasts
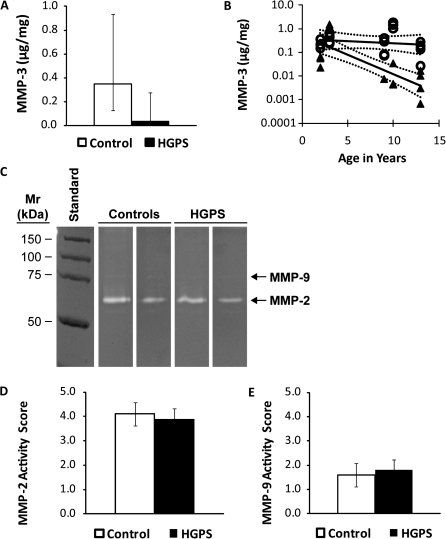
We next determined whether gene expression was reflected at the protein level for each MMP. Given that MMP-3 mRNA expression was greatly reduced in HGPS, we employed a sensitive ELISA method to measure total secreted MMP-3, given that its sensitivity is much greater than that of casein zymography for MMP-3 (400 ng/mL) (28). Similar to MMP-3 mRNA levels, there was a 10-fold mean reduction in MMP-3 protein secreted by HGPS fibroblast lines compared with donor age–matched controls (range of ratios: 0.3–76.7; p = .0382; Figure 3A). The decline in secreted MMP-3 protein became more significant with increasing donor age (Figure 3B, p < .001), whereas controls did not (p = .5292). There was no significant correlation between passage number and MMP-3 levels in either HGPS or control fibroblast lines (data not shown; p > .05).
Figure 3.
MMP-3 secretion (A and B) was determined in five donor age–matched pairs of human dermal fibroblast lines from 24-hour conditioned medium utilizing an enzyme-linked immunosorbent assay (n = 3 experiments per pair). (A) Bars (white = control; black = Hutchinson–Gilford progeria syndrome [HGPS]) indicate mean MMP-3 secretion of all five fibroblast lines per group, and error bars indicate upper and lower 95% confidence intervals. (B) Symbols (○ = controls; ▲= HGPS) indicate mean secreted protein levels from three replicate experiments for each individual cell line; solid lines indicate the mixed linear model’s predicted mean with dashed lines representing upper and lower 95% confidence intervals. Gelatin zymogram (C) of two representative samples of 24-hour conditioned media normalized to total cellular protein from control and HGPS cells. Enzyme levels of MMP-2 (D) and MMP-9 (E) were scored based on band intensity. Bars (white = control; black = HGPS) indicate means of all five fibroblast lines per group with standard errors (n = 2 experiments per pair).
MMP-2 and -9 levels in the conditioned media were assessed by gelatin zymography (Figure 3C). MMP-2 was present in its active form in both HGPS and control samples, with very little latent form detectable. However, only latent MMP-9 appeared in the HGPS and control samples. For MMP-2, the age-dependent reduction in mRNA levels was not reflected in differences in protein levels (p = .4713; Figure 3D). MMP-9 also failed to show differences in amount between HGPS and controls (Figure 3E) at any donor age (p = .7663).
DISCUSSION
The current study successfully characterized reduced MMP-3 in HGPS primary dermal fibroblast cultures at both the mRNA and the protein levels. Furthermore, by comparing separate donor age-matched pairs of HGPS and control fibroblasts, we demonstrated a decline to almost undetectable levels in both MMP-3 mRNA and MMP-3 protein with increasing donor age in HGPS fibroblasts but not in controls. These findings suggest that a decrease in MMP-3 correlates with in vivo disease severity.
Progerin has been reported to accumulate in the nucleus of cultured cells and effect increasing damage with successive passages (5). However, we did not find significant correlations between progerin levels and either donor age or in vitro age (passage number). We also did not find that in vitro age influenced MMP-3 levels. The observed influence of donor age in the absence of influence by passage number implies that there are in vivo regulatory elements responsible for the progressive, downstream effects of progerin that cannot be recapitulated simply by aging cells in vitro.
Premature senescence cannot account for differences in levels of MMP-3 in our study. In non-HGPS systems, MMP-3 has been found to increase with senescence (27) and with in vivo aging (29). Not only were our later passage HGPS cultures growing well, implying that they were not senescing, but we found almost undetectable MMP-3 in our later passage HGPS cultures. Control cultures were also not senescing at any of the passages used in this study; thus, MMP-3 would not be expected to increase with our later passage controls.
Dramatically low MMP-3 protein in HGPS skin fibroblast cultures suggests an altered balance in connective tissue remodeling in this disease. To determine whether reduced expression of MMP-3 was specific or indicative of a global reduction in ECM-degrading machinery, we also measured the activities of MMP -2 and -9. These MMPs are expressed in many of the same tissues as MMP-3 and are involved in many of the same pathologies (8,9,11,30,31). Although we found a modest age-dependent reduction in MMP-2 mRNA levels in our HGPS fibroblast lines, this change was not reflected in activity levels. Additionally, neither transcription nor translation of MMP-9 was altered in HGPS cultures. Our findings suggest that MMP-3 is specifically downregulated in HGPS.
Although we demonstrate reduced MMP-3 in progerin-expressing cells, our data do not demonstrate a specific causal relationship between progerin expression and MMP-3. One prior study does demonstrate a 25% increase in MMP-3 mRNA expression when aberrant LMNA splicing was partially corrected in a single HGPS fibroblast cell line (32). One possible mechanism by which progerin could inhibit MMP-3 is through a suppressive effect on the AP-1 transcription factor complex, which regulates many ECM genes, including MMP-3 (33,34). Recently, lamin A has been identified as a novel binding partner for the AP-1 subunit c-Fos (35). Overexpression of mature lamin A results in the accumulation of farnesylated prelamin A, a protein that closely resembles progerin in its farnesylation status and its ability to cause progeroid disease when overexpressed (36). Prelamin A accumulation indirectly causes reduced AP-1 DNA binding by sequestering c-Fos at the nuclear envelope, thus inhibiting its ability to regulate gene transcription (35). Suppression of AP-1 DNA-binding and transactivation capacities causes a loss of MMP-3 expression in normal dermal fibroblasts (33).
Several other nonprogerin-producing laminopathies manifest progeroid phenotypes (37), and it is of interest to determine whether altered ECM turnover due to changes in MMP-3 expression is a common disease mechanism. For example, patients with a related progeroid laminopathy, mandibuloacral dysplasia (MAD), have decreased serum MMP-3 levels as well as elevated levels of MMP-9 (38). MAD is caused by mutations in either LMNA or ZMPSTE24, which result in the production of a disease-causing amount of farnesylated prelamin A. We speculate that the retention of a farnesyl tail on lamin A may play a direct critical role in regulation of MMP-3 levels in both HGPS and MAD. Thus, in addition to exploring the specific relationship between progerin production and MMP abnormality, future studies should include other laminopathies to determine whether there is a more generalizable mechanism regulating ECM turnover in these closely related diseases.
The possible consequences of reduced MMP-3 in the pathobiology of HGPS are significant, given its wide range of substrates including fibronectins, collagens, gelatins, laminins, elastin, and various proteoglycans (14). For example, because tumor-derived fibroblasts exhibit greater invasive capacity through enhanced MMP-3 release (39), a lack of MMP-3 might contribute to the observed paucity of cancer in HGPS (40). Children with HGPS display skin changes that resemble systemic sclerosis, which has been shown to involve reduced ECM turnover due to reduced MMP-3 production in skin (41). Excisional wounds in MMP-3-deficient mice fail to contract, and healing is slower than in wild-type mice (42) due to inadequate organization of actin-rich stromal fibroblasts. Though children with HGPS do not exhibit obvious wound healing deficiencies, HGPS fibroblasts migrate more slowly in culture and are slower to heal an in vitro scratch wound than donor age–matched control fibroblasts (43).
In HGPS, atherosclerotic disease is the primary cause of mortality. Our findings, together with established studies correlating MMP-3 abnormalities in vascular pathology, suggest that MMP-3 could be involved in HGPS vascular disease. MMP-3 polymorphisms, which result in functional differences in MMP-3 activity, have been associated with a host of vascular pathologies in the normal aging population, including acute coronary events (44), aortic aneurysms (45), carotid intima-media thickening (46,47), and age-related aortic and large artery stiffening (reviewed in (48)). The processes by which these influences on vascular pathology occur are complex, with both an increased ECM accumulation and an increased ECM degradation being implicated. Although the pathology of HGPS strongly implicates alterations in ECM remodeling leading to abnormally functioning connective tissue, MMP-3 is only one component in a complex system that regulates ECM turnover. It remains to be determined whether a reduction in MMP-3 has functional consequences on vascular ECM turnover in HGPS.
In light of our findings, MMP-3 may be a potential in vitro biomarker to be used in monitoring therapeutic efficacy in children with HGPS. There are several compounds that inhibit protein farnesylation currently in clinical trials to treat HGPS (49), and the search continues for additional treatment regimens such as high throughput screening and genetic therapies (50). It is imperative that we identify as many pathobiological markers of this disease as possible to complement these therapeutics and to use them as part of preclinical treatment evaluations for children with HGPS.
FUNDING
This study was funded by the Progeria Research Foundation (PRF-1999-001), the American Heart Association (0030217N), and the National Institutes of Health (NIH P01 CA045548).
Acknowledgments
We gratefully acknowledge Susan Campbell, MS, Peter Geck, PhD, and Jeffrey Marchant, PhD, for their advice and technical assistance.
References
- 1.Merideth MA, Gordon LB, Clauss S, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358:592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olive M, Harten I, Mitchell R, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A. 2006;140:2603–2624. doi: 10.1002/ajmg.a.31346. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dechat T, Pfleghaar K, Sengupta K, et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csoka AB, English SB, Simkevich CP, et al. Genome-scale expression profiling of Hutchinson-Gilford progeria syndrome reveals widespread transcriptional misregulation leading to mesodermal/mesenchymal defects and accelerated atherosclerosis. Aging Cell. 2004;3:235–243. doi: 10.1111/j.1474-9728.2004.00105.x. [DOI] [PubMed] [Google Scholar]
- 7.Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287:2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- 8.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 9.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 10.Chavey C, Mari B, Monthouel MN, et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003;278:11888–11896. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal EL, Matrisian LM. Matrix metalloproteases in head and neck cancer. Head Neck. 2006;28:639–648. doi: 10.1002/hed.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy R, Louis G, Loughlin KR, et al. Tumor-specific urinary matrix metalloproteinase fingerprinting: identification of high molecular weight urinary matrix metalloproteinase species. Clin Cancer Res. 2008;14:6610–6617. doi: 10.1158/1078-0432.CCR-08-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]
- 14.Okada Y, Nagase H, Harris ED., Jr. A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986;261:14245–14255. [PubMed] [Google Scholar]
- 15.Beavan LA, Quentin-Hoffmann E, Schonherr E, Snigula F, Leroy JG, Kresse H. Deficient expression of decorin in infantile progeroid patients. J Biol Chem. 1993;268:9856–9862. [PubMed] [Google Scholar]
- 16.Colige A, Roujeau JC, De la Rocque F, Nusgens B, Lapiere CM. Abnormal gene expression in skin fibroblasts from a Hutchinson-Gilford patient. Lab Invest. 1991;64:799–806. [PubMed] [Google Scholar]
- 17.Giro M, Davidson JM. Familial co-segregation of the elastin phenotype in skin fibroblasts from Hutchinson-Gilford progeria. Mech Ageing Dev. 1993;70:163–136. doi: 10.1016/0047-6374(93)90046-t. [DOI] [PubMed] [Google Scholar]
- 18.Lemire JM, Patis C, Gordon LB, Sandy JD, Toole BP, Weiss AS. Aggrecan expression is substantially and abnormally upregulated in Hutchinson-Gilford Progeria Syndrome dermal fibroblasts. Mech Ageing Dev. 2006;127:660–669. doi: 10.1016/j.mad.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJ. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987;248:265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquez-Aguirre A, Sandoval-Rodriguez A, Gonzalez-Cuevas J, et al. Adenoviral delivery of dominant-negative transforming growth factor beta type II receptor up-regulates transcriptional repressor SKI-like oncogene, decreases matrix metalloproteinase 2 in hepatic stellate cell and prevents liver fibrosis in rats. J Gene Med. 2009;11:207–219. doi: 10.1002/jgm.1303. [DOI] [PubMed] [Google Scholar]
- 21.Dasu MR, Hawkins HK, Barrow RE, Xue H, Herndon DN. Gene expression profiles from hypertrophic scar fibroblasts before and after IL-6 stimulation. J Pathol. 2004;202:476–485. doi: 10.1002/path.1539. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005;102:15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishijima C, Hayakawa I, Matsushita T, et al. Autoantibody against matrix metalloproteinase-3 in patients with systemic sclerosis. Clin Exp Immunol. 2004;138:357–363. doi: 10.1111/j.1365-2249.2004.02615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silence J, Lupu F, Collen D, Lijnen HR. Persistence of atherosclerotic plaque but reduced aneurysm formation in mice with stromelysin-1 (MMP-3) gene inactivation. Arterioscler Thromb Vasc Biol. 2001;21:1440–1445. doi: 10.1161/hq0901.097004. [DOI] [PubMed] [Google Scholar]
- 25.Novak KB, Le HD, Christison-Lagay ER, et al. Effects of metalloproteinase inhibition in a murine model of renal ischemia-reperfusion injury. Pediatr Res. 2010;67:257–262. doi: 10.1203/PDR.0b013e3181ca0aa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan LW, Moses MA, Goley E, et al. Urinary VEGF and MMP levels as predictive markers of 1-year progression-free survival in cancer patients treated with radiation therapy: a longitudinal study of protein kinetics throughout tumor progression and therapy. J Clin Oncol. 2004;22:499–506. doi: 10.1200/JCO.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Millis AJ, Hoyle M, McCue HM, Martini H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp Cell Res. 1992;201:373–379. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- 28.Manicourt DH, Lefebvre V. An assay for matrix metalloproteinases and other proteases acting on proteoglycans, casein, or gelatin. Anal Biochem. 1993;215:171–179. doi: 10.1006/abio.1993.1572. [DOI] [PubMed] [Google Scholar]
- 29.Cartwright MJ, Schlauch K, Lenburg ME, et al. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci. 2010;65:242–251. doi: 10.1093/gerona/glp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eltarhouny SA, Elsawy WH, Radpour R, Hahn S, Holzgreve W, Zhong XY. Genes controlling spread of breast cancer to lung “gang of 4”. Exp Oncol. 2008;30:91–95. [PubMed] [Google Scholar]
- 31.Kahari VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 32.Scaffidi P, Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med. 2005;11:440–445. doi: 10.1038/nm1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kajanne R, Miettinen P, Mehlem A, et al. EGF-R regulates MMP function in fibroblasts through MAPK and AP-1 pathways. J Cell Physiol. 2007;212:489–497. doi: 10.1002/jcp.21041. [DOI] [PubMed] [Google Scholar]
- 34.Yee J, Kuncio GS, Bhandari B, Shihab FS, Neilson EG. Identification of promoter activity and differential expression of transcripts encoding the murine stromelysin-1 gene in renal cells. Kidney Int. 1997;52:120–129. doi: 10.1038/ki.1997.311. [DOI] [PubMed] [Google Scholar]
- 35.Ivorra C, Kubicek M, Gonzalez JM, et al. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev. 2006;20:307–320. doi: 10.1101/gad.349506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Candelario J, Sudhakar S, Navarro S, Reddy S, Comai L. Perturbation of wild-type lamin A metabolism results in a progeroid phenotype. Aging Cell. 2008;7:355–367. doi: 10.1111/j.1474-9726.2008.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg A, Subramanyam L, Agarwal AK, et al. Atypical progeroid syndrome due to heterozygous missense LMNA mutations. J Clin Endocrinol Metab. 2009;94:4971–4983. doi: 10.1210/jc.2009-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lombardi F, Fasciglione GF, D’Apice MR, et al. Increased release and activity of matrix metalloproteinase-9 in patients with mandibuloacral dysplasia type A, a rare premature ageing syndrome. Clin Genet. 2008;74:374–383. doi: 10.1111/j.1399-0004.2008.01034.x. [DOI] [PubMed] [Google Scholar]
- 39.Holliday DL, Hughes S, Shaw JA, Walker RA, Jones JL. Intrinsic genetic characteristics determine tumor-modifying capacity of fibroblasts: matrix metalloproteinase-3 5A/5A genotype enhances breast cancer cell invasion. Breast Cancer Res. 2007;9:R67. doi: 10.1186/bcr1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King CR, Lemmer J, Campbell JR, Atkins AR. Osteosarcoma in a patient with Hutchinson-Gilford progeria. J Med Genet. 1978;15:481–484. doi: 10.1136/jmg.15.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bou-Gharios G, Osman J, Black C, Olsen I. Excess matrix accumulation in scleroderma is caused partly by differential regulation of stromelysin and TIMP-1 synthesis. Clin Chim Acta. 1994;231:69–78. doi: 10.1016/0009-8981(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 42.Bullard KM, Lund L, Mudgett JS, et al. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–265. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verstraeten VL, Ji JY, Cummings KS, Lee RT, Lammerding J. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell. 2008;7:383–393. doi: 10.1111/j.1474-9726.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terashima M, Akita H, Kanazawa K, et al. Stromelysin promoter 5A/6A polymorphism is associated with acute myocardial infarction. Circulation. 1999;99:2717–2719. doi: 10.1161/01.cir.99.21.2717. [DOI] [PubMed] [Google Scholar]
- 45.Yoon S, Tromp G, Vongpunsawad S, Ronkainen A, Juvonen T, Kuivaniemi H. Genetic analysis of MMP3, MMP9, and PAI-1 in Finnish patients with abdominal aortic or intracranial aneurysms. Biochem Biophys Res Commun. 1999;265:563–568. doi: 10.1006/bbrc.1999.1721. [DOI] [PubMed] [Google Scholar]
- 46.Gnasso A, Motti C, Irace C, et al. Genetic variation in human stromelysin gene promoter and common carotid geometry in healthy male subjects. Arterioscler Thromb Vasc Biol. 2000;20:1600–1605. doi: 10.1161/01.atv.20.6.1600. [DOI] [PubMed] [Google Scholar]
- 47.Rauramaa R, Vaisanen SB, Luong LA, et al. Stromelysin-1 and interleukin-6 gene promoter polymorphisms are determinants of asymptomatic carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2657–2662. doi: 10.1161/01.atv.20.12.2657. [DOI] [PubMed] [Google Scholar]
- 48.Agrotis A. The genetic basis for altered blood vessel function in disease: large artery stiffening. Vasc Health Risk Manag. 2005;1:333–344. doi: 10.2147/vhrm.2005.1.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kieran MW, Gordon L, Kleinman M. New approaches to progeria. Pediatrics. 2007;120:834–841. doi: 10.1542/peds.2007-1356. [DOI] [PubMed] [Google Scholar]
- 50.Gordon LB, Harling-Berg CJ, Rothman FG. Highlights of the 2007 Progeria Research Foundation scientific workshop: progress in translational science. J Gerontol A Biol Sci Med Sci. 2008;63:777–787. doi: 10.1093/gerona/63.8.777. [DOI] [PubMed] [Google Scholar]