Abstract
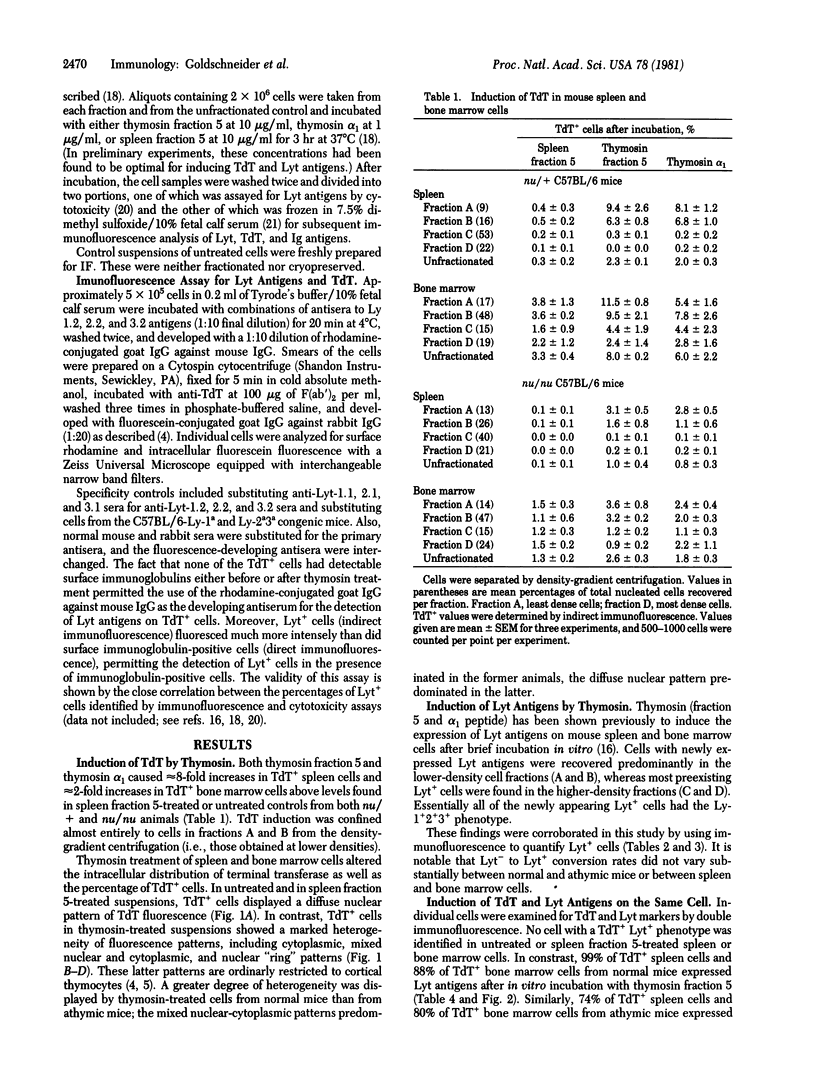
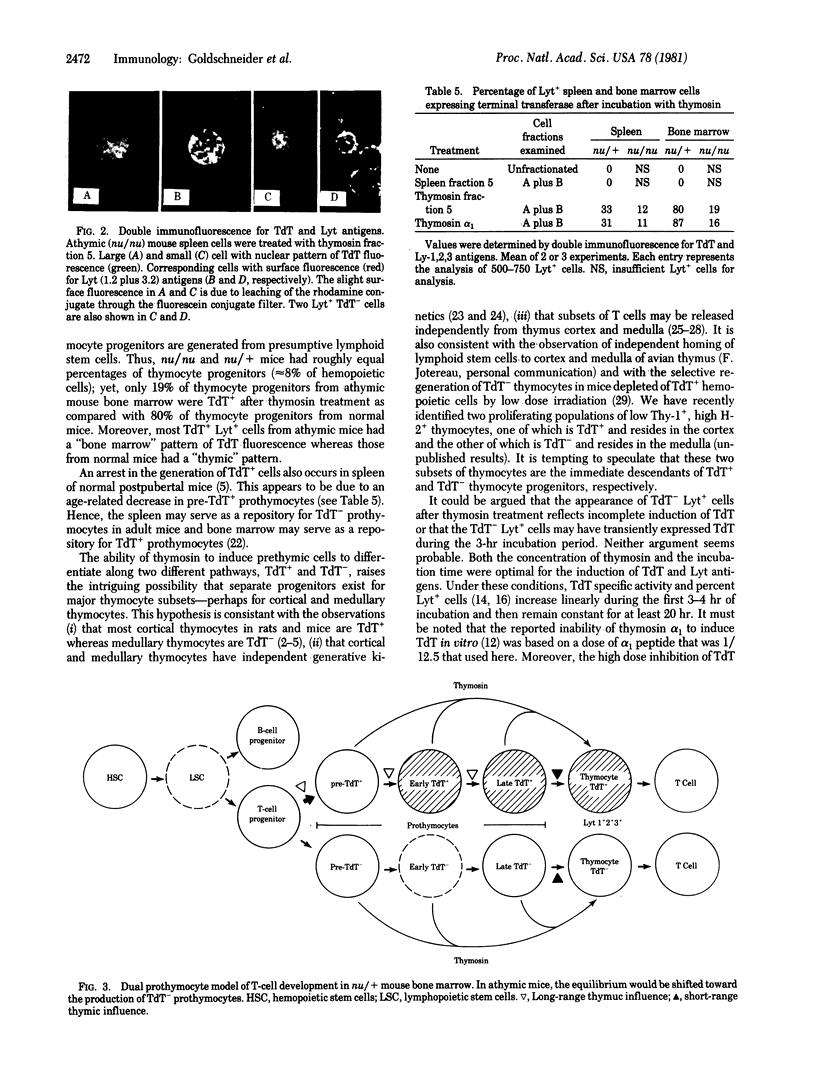
Thymosin (fraction 5 and synthetic alpha 1 peptide) induced prothymocytes in mouse bone marrow and spleen to express terminal deoxynucleotidyl transferase (TdT; DNA nucleotidylexotransferase; nucleosidetriphosphate:DNA deoxynucleotidylexotransferase, EC 2.7.7.31) or Lyt-1+, 2+, 3+ alloantigens (or both) after brief incubation in vitro. Three antigenic phenotypes were generated: (i) TdT+ Lyt+, (ii) TdT- Lyt+, and (iii) TdT+ Lyt-. The TdT+ Lyt+ phenotype was expressed by 80% of prothymocytes in bone marrow and 30% of prothymocytes in spleen from normal mice. The TdT- Lyt+ phenotype was expressed by 81% of prothymocytes in bone marrow from athymic mice. More than 80% of TdT+ bone marrow cells from normal and athymic mice expressed Lyt antigens after thymosin treatment. We interpret these observations as suggesting that (i) most TdT+ hemopoietic cells in normal and athymic mice are thymocyte progenitors; (ii) two independent lineages of prothymocytes exist, one that expresses TdT and another that does not, (iii) commitment of prothymocytes to the TdT+ cell pathway is partially regulated by a thymic feedback mechanism; and (iv) the bone marrow preferentially produces TdT+ prothymocytes, whereas the spleen may serve as a repository for TdT- prothymocytes. A model of T-cell development is presented in which the thymus functions as a compound organ to process TdT+ and TdT- thymocytes progenitors and to generate two lines of T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A., Scher I., Sharrow S. O., Smith A. H., Paul W. E., Sachs D. H., Sell K. W. B-lymphocyte heterogeneity: development and characterization of an alloantiserum which distinguishes B-lymphocyte differentiation alloantigens. J Exp Med. 1977 Jan 1;145(1):101–110. doi: 10.1084/jem.145.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A., Smith A. H., Wong D. M., Thurman G. B., Goldstein A. L., Sell K. W. In vitro induction of Lyt surface markers on precursor cells incubated with thymosin polypeptides. Cancer Treat Rep. 1978 Nov;62(11):1739–1747. [PubMed] [Google Scholar]
- Ahmed A., Wong D. M., Thurman G. B., Low T. L., Goldstein A. L., Sharkis S. J., Goldschneider I. T-lymphocyte maturation: cell surface markers and immune function induced by T-lymphocyte cell-free products and thymosin polypeptides. Ann N Y Acad Sci. 1979;332:81–94. doi: 10.1111/j.1749-6632.1979.tb47100.x. [DOI] [PubMed] [Google Scholar]
- Basch R. S., Kadish J. L., Goldstein G. Hematopoietic thymocyte precursors: IV. Enrichment of the precursors and evidence for heterogeneity. J Exp Med. 1978 Jun 1;147(6):1843–1848. doi: 10.1084/jem.147.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollum F. J. Antibody to terminal deoxynucleotidyl transferase. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4119–4122. doi: 10.1073/pnas.72.10.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollum F. J. Terminal deoxynucleotidyl transferase: biological studies. Adv Enzymol Relat Areas Mol Biol. 1978;47:347–374. doi: 10.1002/9780470122921.ch6. [DOI] [PubMed] [Google Scholar]
- Durkin H. G., Carboni J. M., Waksman B. H. Antigen-induced increase in migration of large cortical thymocytes (regulatory cells?) to the marginal zone and red pulp of the spleen. J Immunol. 1978 Sep;121(3):1075–1081. [PubMed] [Google Scholar]
- Fathman C. G., Small M., Herzenberg L. A., Weissman I. L. Thymus cell maturation. II. Differentiation of three "mature" subclasses in vivo. Cell Immunol. 1975 Jan;15(1):109–128. doi: 10.1016/0008-8749(75)90169-0. [DOI] [PubMed] [Google Scholar]
- Goldschneider I. Antigenic relationship between bone marrow lymphocytes cortical thymocytes and a subpopulation of peripheral T cells in the rat: description of a bone marrow lymphocyte antigen. Cell Immunol. 1976 Jun 15;24(2):289–307. doi: 10.1016/0008-8749(76)90213-6. [DOI] [PubMed] [Google Scholar]
- Goldschneider I. Antigenic relationship between medullary thymocytes and a subpopulation of peripheral T cells in the rat: description of a masked antigen. Cell Immunol. 1975 Apr;16(2):269–284. doi: 10.1016/0008-8749(75)90118-5. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gregoire K. E., Barton R. W., Bollum F. J. Demonstration of terminal deoxynucleotidyl transferase in thymocytes by immunofluorescence. Proc Natl Acad Sci U S A. 1977 Feb;74(2):734–738. doi: 10.1073/pnas.74.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Metcalf D., Mandel T., Bollum F. J. Analysis of rat hemopoietic cells on the fluorescence-activated cell sorter. II. Isolation of terminal deoxynucleotidyl transferase-positive cells. J Exp Med. 1980 Aug 1;152(2):438–446. doi: 10.1084/jem.152.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M., Verbi W., Vogler L., Cooper M., Ellis R., Ganeshaguru K., Hoffbrand V., Janossy G., Bollum F. J. Antigenic and enzymatic phenotypes of the pre-B subclass of acute lymphoblastic leukaemia. Leuk Res. 1979;3(6):353–362. doi: 10.1016/0145-2126(79)90032-8. [DOI] [PubMed] [Google Scholar]
- Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. Intracellular distribution of terminal deoxynucleotidyl transferase in rat bone marrow and thymus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3993–3996. doi: 10.1073/pnas.74.9.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues of rat and mouse. J Immunol. 1979 Sep;123(3):1347–1352. [PubMed] [Google Scholar]
- Hooper J. A., McDaniel M. C., Thurman G. B., Cohen G. H., Schulof R. S., Goldstein A. L. Purification and properties of bovine thymosin. Ann N Y Acad Sci. 1975 Feb 28;249:125–144. doi: 10.1111/j.1749-6632.1975.tb29063.x. [DOI] [PubMed] [Google Scholar]
- Kung P. C., Siverstone A. E., McCaffrey R. P., Baltimore D. Murine terminal deoxynucleotidyl transferase: cellular distribution and response to cortisone. J Exp Med. 1975 Apr 1;141(4):855–865. [PMC free article] [PubMed] [Google Scholar]
- Low T. L., Goldstein A. L. The chemistry and biology of thymosin. II. Amino acid sequence analysis of thymosin alpha1 and polypeptide beta1. J Biol Chem. 1979 Feb 10;254(3):987–995. [PubMed] [Google Scholar]
- Low T. L., Thurman G. B., McAdoo M., McClure J., Rossio J. L., Naylor P. H., Goldstein A. L. The chemistry and biology of thymosin. I. Isolation, characterization, and biological activities of thymosin alpha1 and polypeptide beta1 from calf thymus. J Biol Chem. 1979 Feb 10;254(3):981–986. [PubMed] [Google Scholar]
- Pazmiño N. H., Ihle J. N., Goldstein A. L. Induction in vivo and in vitro of terminal deoxynucleotidyl transferase by thymosin in bone marrow cells from athymic mice. J Exp Med. 1978 Mar 1;147(3):708–718. doi: 10.1084/jem.147.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazmiño N. H., Ihle J. N., McEwan R. N., Goldstein A. L. Control of differentiation of thymocyte precursors in the bone marrow by thymic hormones. Cancer Treat Rep. 1978 Nov;62(11):1749–1755. [PubMed] [Google Scholar]
- Pazmiño N. H., McEwan R., Ihle J. N. Radiation leukemia in C57BL/6 mice. III. Correlation of altered expression of terminal deoxynucleotidyl transferase to induction of leukemia. J Exp Med. 1978 Nov 1;148(5):1338–1350. doi: 10.1084/jem.148.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Jackson H. The differentiation of T lymphocytes. I. Proliferation kinetics and interrelationships of subpopulations of mouse thymus cells. Cell Immunol. 1974 May;12(2):230–246. doi: 10.1016/0008-8749(74)90075-6. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Cantor H., Goldstein G., Baltimore D. Terminal deoxynucleotidyl transferase is found in prothymocytes. J Exp Med. 1976 Aug 1;144(2):543–548. doi: 10.1084/jem.144.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong D. M., Ahmed A. A., Sell K. W., Greiff D. In vitro effects of cryoprotective agents on the response of murine T and B lymphoid subpopulations to mitogenic agents. Cryobiology. 1972 Oct;9(5):450–456. doi: 10.1016/0011-2240(72)90162-9. [DOI] [PubMed] [Google Scholar]
- Weissman I. L., Baird S., Gardner R. L., Papaioannou V. E., Raschke W. Normal and neoplastic maturation of T-lineage lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):9–21. doi: 10.1101/sqb.1977.041.01.005. [DOI] [PubMed] [Google Scholar]