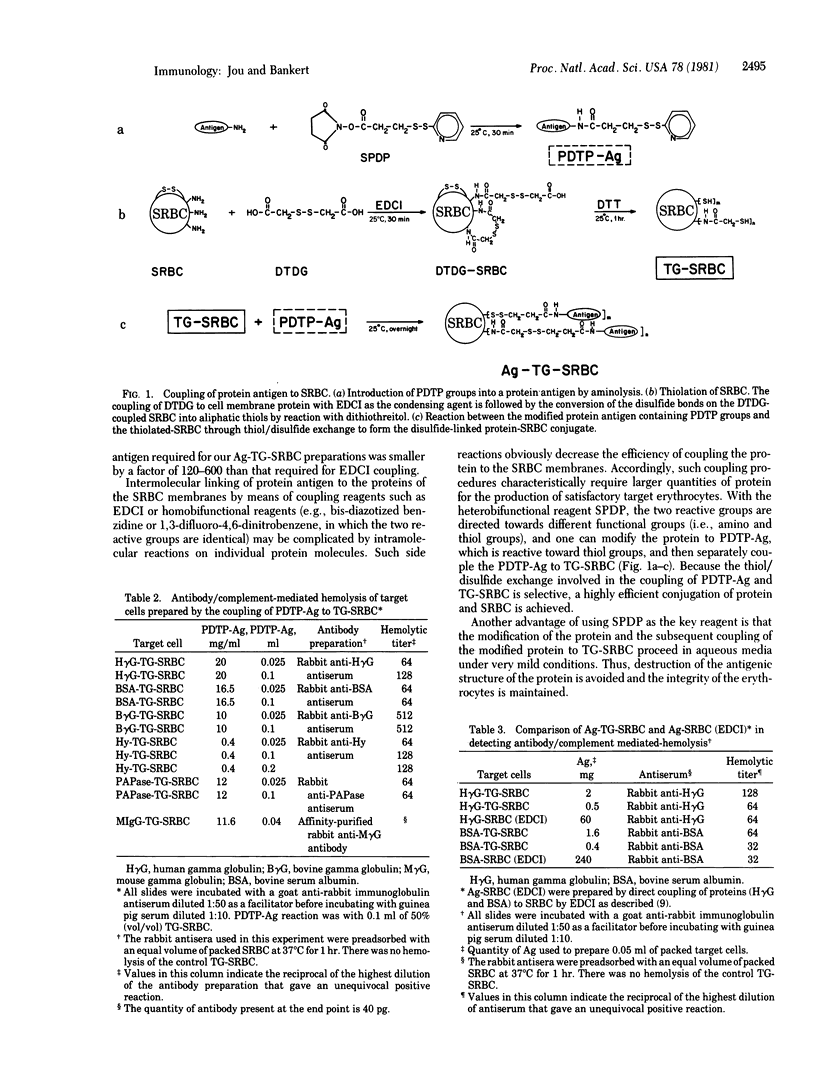
Abstract
An efficient technique has been developed for coupling protein antigens to erythrocyte membranes. The procedure involves three steps. First, 3-(2-pyridyldithio)propionyl residues are introduced into the protein by reaction with a heterobifunctional reagent, N-succinimidyl 3-(pyridyldithio) propionate. Second, the addition of disulfide groups to sheep erythrocytes (SRBC) is achieved by coupling dithiodiglycolic acid to SRBC with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide. The disulfide bonds of the dithiodiglycolyl-SRBC conjugate are then reduced with dithiothreitol. Finally, the 3-(2-pyridyldithio)propionyl-protein conjugate is covalently coupled to the thiolated SRBC through thiol/disulfide exchange to form the disulfide-linked antigen-SRBC conjugate. The procedure requires only 10-500 microgram of protein antigen for the preparation of 50 microliter of packed protein-coupled SRBC. Antibodies binding to antigen on the erythrocyte initiate a complement-dependent immune lysis of the target cells. Target cells prepared by this method are stable for at least 4 wk at 4 degrees C in phosphate buffer (pH 7.2) and are capable of detecting as little as 40 pg of antibody in a hemolytic assay without noticeable nonspecific lysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. V. The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. J Exp Med. 1951 Feb;93(2):107–120. doi: 10.1084/jem.93.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankert R. B., DesSoye D., Powers L. Screening and replica plating of anti-hapten hybridomas with a transfer template hemolytic spot assay. J Immunol Methods. 1980;35(1-2):23–32. doi: 10.1016/0022-1759(80)90147-7. [DOI] [PubMed] [Google Scholar]
- Bankert R. B., Mayers G. L., Pressman D. Mechanisms of B cell tolerance. I. Tolerance to dextran B1355 induced with the oxidized dextran. J Immunol. 1977 Apr;118(4):1265–1270. [PubMed] [Google Scholar]
- Bankert R. B., Mayers G. L., Pressman D. Synthesis of a lipopolysaccharide designed to conjugate haptens and proteins to erythrocyte for use as target cells in passive hemagglutination and hemolytic assays. J Immunol. 1979 Dec;123(6):2466–2474. [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citkowitz E. The hyaline layer: its isolation and role in echinoderm development. Dev Biol. 1971 Mar;24(3):348–362. doi: 10.1016/0012-1606(71)90085-6. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- GYENES L., SEHON A. H. THE USE OF TOLYLENE-2,4-DIISOCYANATE AS A COUPLING REAGENT IN THE PASSIVE HEMAGGLUTINATION REACTION. Immunochemistry. 1964 Apr;1:43–48. doi: 10.1016/0019-2791(64)90055-2. [DOI] [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Golub E. S., Mishell R. I., Weigle W. O., Dutton R. W. A modification of the hemolytic plaque assay for use with protein antigens. J Immunol. 1968 Jan;100(1):133–137. [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Brenner K., Hall H. E. The use of a wate-soluble carbodiimide as a coupling reagent in the passive hemagglutination test. J Immunol. 1966 Dec;97(6):791–796. [PubMed] [Google Scholar]
- LING N. R. The coupling of protein antigens to erythrocytes with difluorodinitrobenzene. Immunology. 1961 Jan;4:49–54. [PMC free article] [PubMed] [Google Scholar]
- Lee C. I., Wang M. C., Murphy G. P., Chu T. M. A solid-phase fluorescent immunoassay for human prostatic acid phosphatase. Cancer Res. 1978 Sep;38(9):2871–2878. [PubMed] [Google Scholar]
- Mayers G. L., Bankert R. B. Immunochemistry of monoclonal antibodies. Transplant Proc. 1980 Sep;12(3):413–416. [PubMed] [Google Scholar]
- ROWLEY D. A., FITCH F. W. HOMEOSTASIS OF ANTIBODY FORMATION IN THE ADULT RAT. J Exp Med. 1964 Dec 1;120:987–1005. doi: 10.1084/jem.120.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]


