Abstract
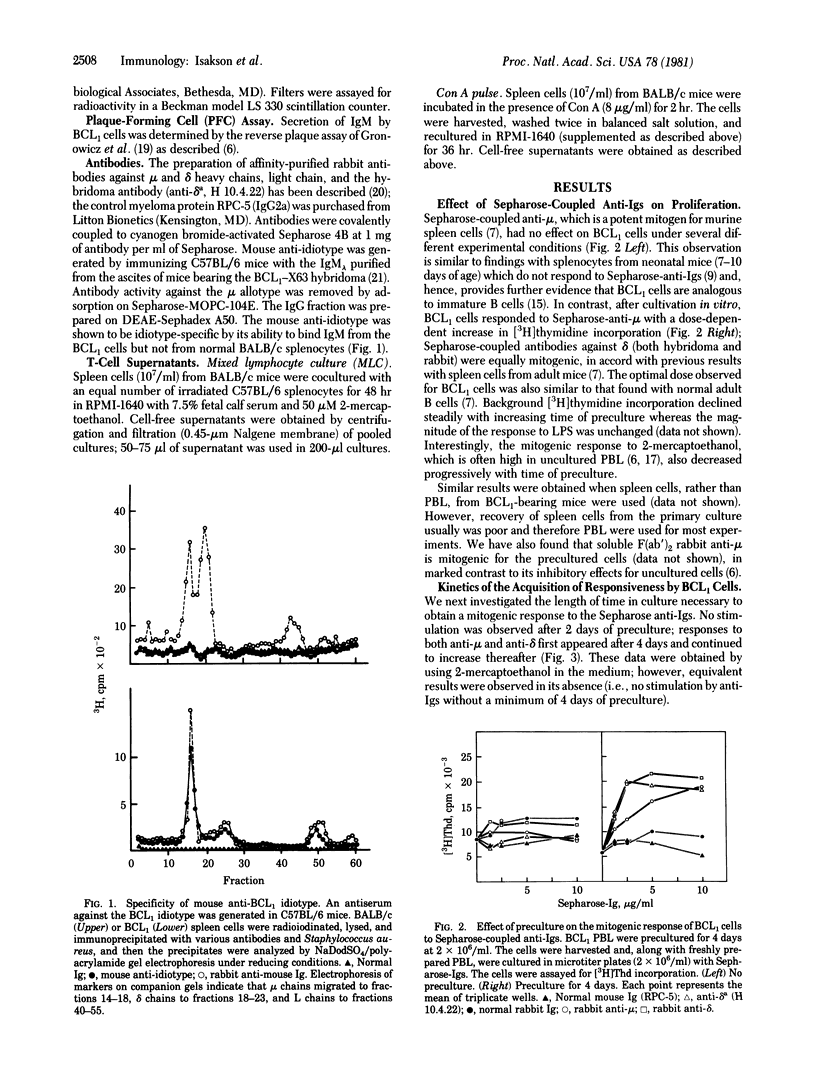
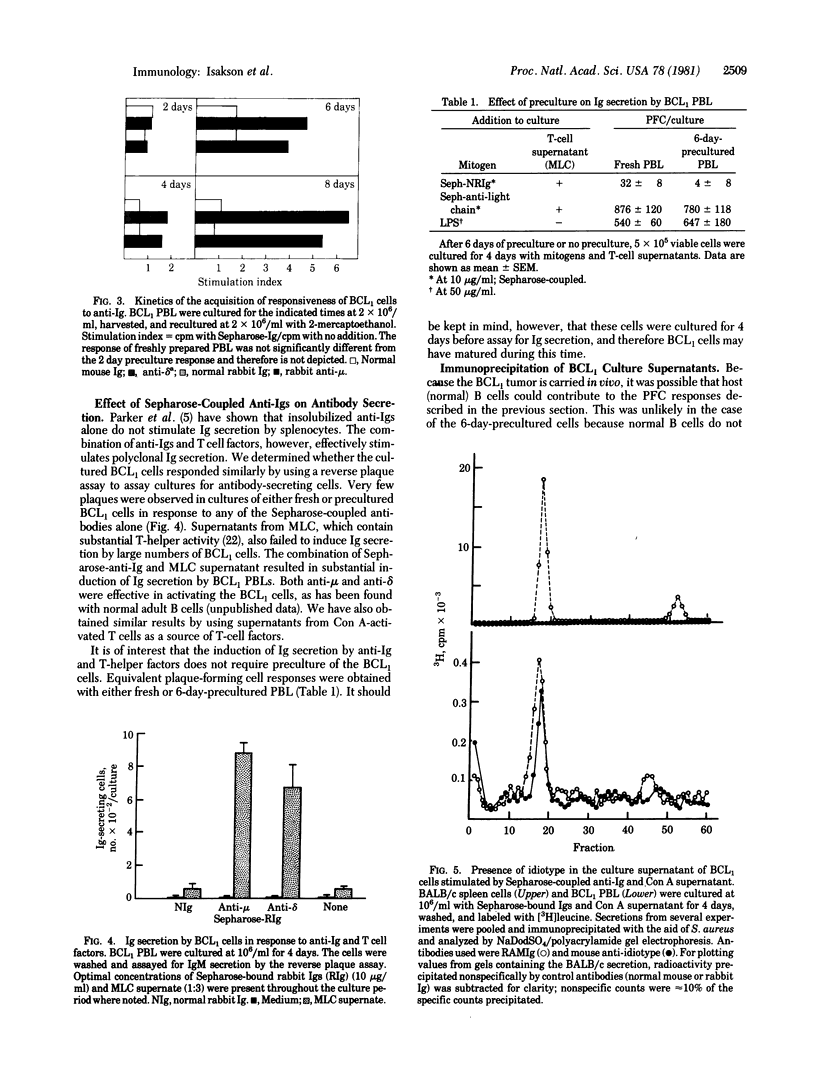
Sepharose-bound anti-immunoglobulins, which are potent mitogens for normal adult B cells, are not mitogenic for tumor cells freshly isolated from mice carrying the B-cell leukemia BCL1. However, after 4 or more days of in vitro cultivation, BCL1 cells can be stimulated to divide by either anti-mu or anti-delta antibodies. These results suggest that in vitro cultivation of BCL1 cells results in their differentiation into more mature cells which can be triggered to proliferate by their interaction with anti-Ig antibodies. Addition of T-cell helper factors to anti-Ia treated BCL1 cells results in their differentiation into Ig-secreting cells. These results indicate that surface Ig molecules on BCL1 cells are capable of delivering an activation signal to the cells but that the cells require a second signal from T cells for induction of Ig secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Cohen H. J. Human lymphocyte surface immunoglobulin capping. Normal characteristics and anomalous behavior of chronic lymphocytic leukemic lymphocytes. J Clin Invest. 1975 Jan;55(1):84–93. doi: 10.1172/JCI107921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger M. W., Hart D. A., Wells J. V., Nisonoff A. Requirement for cross-linkage in the stimulation of transformation of rabbit peripheral lymphocytes by antiglobulin reagents. J Immunol. 1970 Dec;105(6):1484–1492. [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Kunkel H. G. Differentiation capacity and other properties of the leukemic cells of chronic lymphocytic leukemia. Immunol Rev. 1979;48:23–44. doi: 10.1111/j.1600-065x.1979.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Kunkel H. G., Halper J. P., Harris S. R. Induction of in vitro differentiation and immunoglobulin synthesis of human leukemic B lymphocytes. J Exp Med. 1978 Dec 1;148(6):1570–1578. doi: 10.1084/jem.148.6.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S. M., Winchester R. J., Feizi T., Walzer P. D., Kunkel H. G. Idiotypic specificity of surface immunoglobulin and the maturation of leukemic bone-marrow-derived lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4487–4490. doi: 10.1073/pnas.71.11.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Harwell L., Kappler J. W., Marrack P. Antigen-specific and nonspecific mediators of T cell/B cell cooperation. III. Characterization of the nonspecific mediator(s) from different sources. J Immunol. 1976 May;116(5):1379–1384. [PubMed] [Google Scholar]
- Harwell L., Skidmore B., Marrack P., Kappler J. Concanavalin A-inducible, interleukin-2-producing T cell hybridoma. J Exp Med. 1980 Oct 1;152(4):893–904. doi: 10.1084/jem.152.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson P. C., Krolick K. A., Uhr J. W., Vitetta E. S. The effect of anti-immunoglobulin antibodies on the in vitro proliferation and differentiation of normal and neoplastic murineB cells. J Immunol. 1980 Aug;125(2):886–892. [PubMed] [Google Scholar]
- Isakson P. C., Uhr J. W., Krolick K. A., Finkelman F., Vitetta E. S. Acquisition of cell surface IgD after in vitro culture of neoplastic B cells from the murine tumor BCL1. J Exp Med. 1980 Mar 1;151(3):749–754. doi: 10.1084/jem.151.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Lawton A. R. B lymphocyte differentiation induced by lipopolysaccharide. I. Generation of cells synthesizing four major immunoglobulin classes. J Immunol. 1975 Sep;115(3):671–676. [PubMed] [Google Scholar]
- Knapp M. R., Jones P. P., Black S. J., Vitetta E. S., Slavin S., Strober S. Characterization of a spontaneous murine B cell leukemia (BCL1). I. Cell surface expression of IgM, IgD, Ia, and FcR. J Immunol. 1979 Sep;123(3):992–999. [PubMed] [Google Scholar]
- Knapp M. R., Severinson-Gronowicz E., Schröder J., Strober S. Characterization of a spontaneous murine B cell leukemia (BCL1). II. Tumor cell proliferation and IgM secretion after stimulation by LPS. J Immunol. 1979 Sep;123(3):1000–1006. [PubMed] [Google Scholar]
- Krolick K. A., Isakson P. C., Uhr J. W., Vitetta E. S. BCL1, a murine model for chronic lymphocytic leukemia: use of the surface immunoglobulin idiotype for the detection and treatment of tumor. Immunol Rev. 1979;48:81–106. doi: 10.1111/j.1600-065x.1979.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Krolick K. A., Villemez C., Isakson P., Uhr J. W., Vitetta E. S. Selective killing of normal or neoplastic B cells by antibodies coupled to the A chain of ricin. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5419–5423. doi: 10.1073/pnas.77.9.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. C., Fothergill J. J., Wadsworth D. C. B lymphocyte activation by insoluble anti-immunoglobulin: induction of immunoglobulin secretion by a T cell-dependent soluble factor. J Immunol. 1979 Aug;123(2):931–941. [PubMed] [Google Scholar]
- Parker D. C. Induction and suppression of polyclonal antibody responses by anti-Ig reagents and antigen-nonspecific helper factors: a comparison of the effects of anti-Fab, anti-IgM, and anti IgD on murine B cells. Immunol Rev. 1980;52:115–139. doi: 10.1111/j.1600-065x.1980.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Parker D. C. Stimulation of mouse lymphocytes by insoluble anti-mouse immunoglobulin. Nature. 1975 Nov 27;258(5533):361–363. doi: 10.1038/258361a0. [DOI] [PubMed] [Google Scholar]
- Puré E., Vitetta E. S. The murine B cell response to TNP-polyacrylamide beads: the relationship between the epitope density of the antigen and the requirements for T cell help and surface IgD. J Immunol. 1980 Jul;125(1):420–427. [PubMed] [Google Scholar]
- Puré E., Vitetta E. Induction of murine B cell proliferation by insolubilized anti-immunoglobulins. J Immunol. 1980 Sep;125(3):1240–1242. [PubMed] [Google Scholar]
- Saiki O., Kishimoto T., Kuritani T., Muraguchi A., Yamamura Y. In vitro induction of IgM secretion and switching to IgG production in human B leukemic cells with the help of T cells. J Immunol. 1980 Jun;124(6):2609–2614. [PubMed] [Google Scholar]
- Scribner D. J., Weiner H. L., Moorhead J. W. Anti-immunoglobulin stimulation of murine lymphocytes. V. Age-related decline in Fc receptor-mediated immunoregulation. J Immunol. 1978 Jul;121(1):377–382. [PubMed] [Google Scholar]
- Sidman C. L., Unanue E. R. Control of proliferation and differentiation in B lymphocytes by anti-Ig antibodies and a serum-derived cofactor. Proc Natl Acad Sci U S A. 1978 May;75(5):2401–2405. doi: 10.1073/pnas.75.5.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieckmann D. G., Asofsky R., Mosier D. E., Zitron I. M., Paul W. E. Activation of mouse lymphocytes by anti-immunoglobulin. I. Parameters of the proliferative response. J Exp Med. 1978 Mar 1;147(3):814–829. doi: 10.1084/jem.147.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieckmann D. G., Scher I., Asofsky R., Mosier D. E., Paul W. E. Activation of mouse lymphocytes by anti-immunoglobulin. II. A thymus-independent response by a mature subset of B lymphocytes. J Exp Med. 1978 Dec 1;148(6):1628–1643. doi: 10.1084/jem.148.6.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K., Tanaka K., Tominaga A., Kumahara Y., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). III. Establishment of T cell hybrid clone continuously producing TRF and functional analysis of released TRF. J Immunol. 1980 Dec;125(6):2646–2653. [PubMed] [Google Scholar]
- Vitetta E., Puré E., Isakson P., Buck L., Uhr J. The activation of murine B cells: the role of surface immunoglobulins. Immunol Rev. 1980;52:211–231. doi: 10.1111/j.1600-065x.1980.tb00336.x. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Liebes L. F., Silber R. Differences in the behavior of the membrane and membrane-associated filamentous structures in normal and chronic lymphocytic leukemia (CLL) lymphocytes. J Immunol. 1979 Jan;122(1):97–107. [PubMed] [Google Scholar]



