Abstract
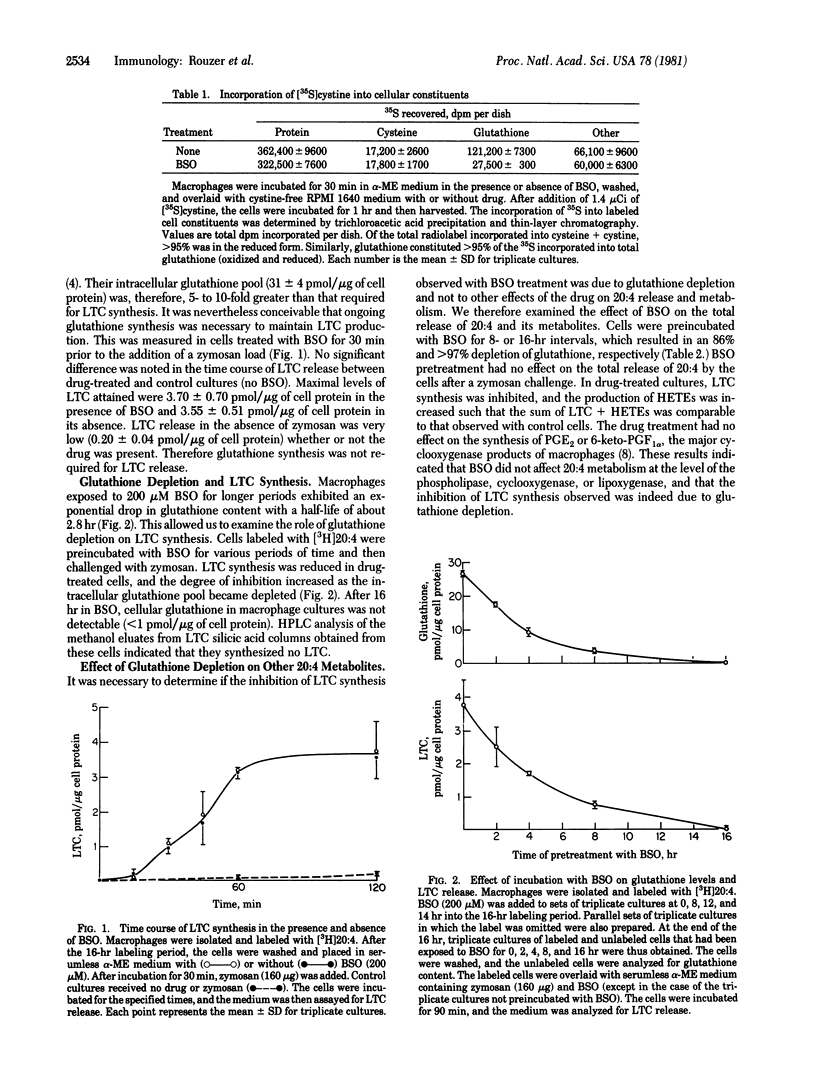
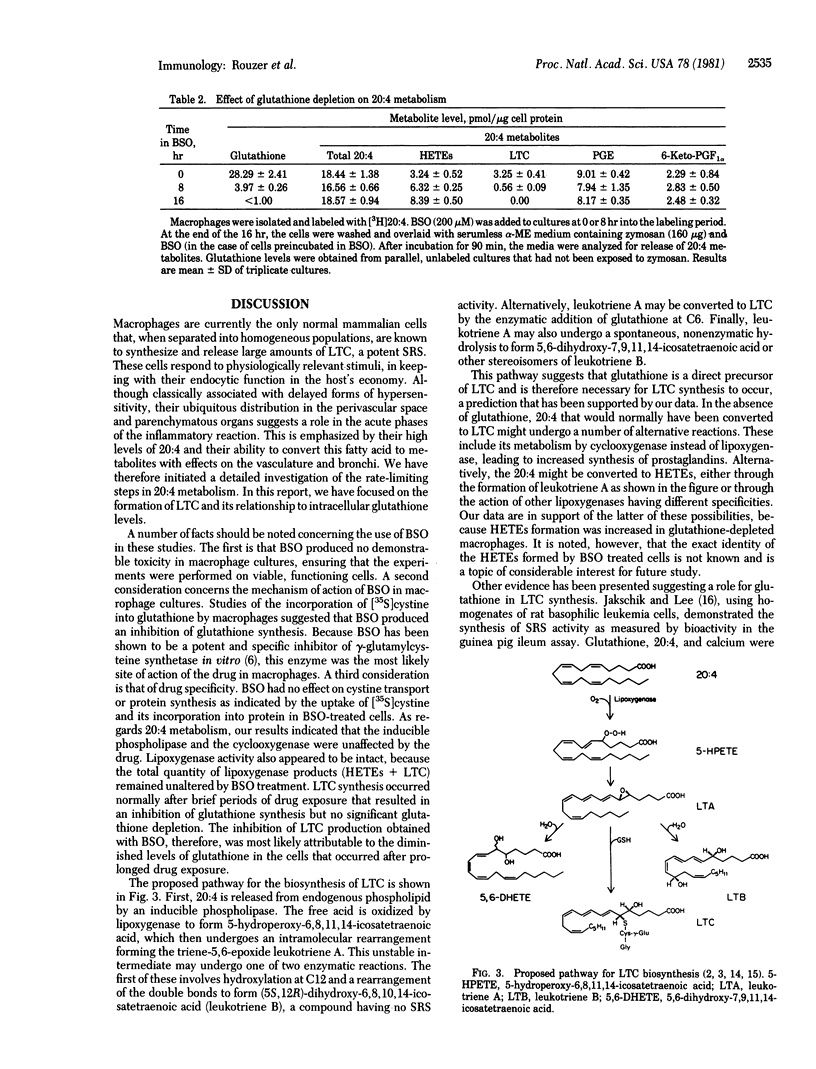
We have examined the role of glutathione synthesis and intracellular glutathione content in the formation of leukotriene C (LTC) by mouse peritoneal macrophages. For this purpose, we utilized the drug buthionine sulfoximine (BSO), a specific inhibitor of glutathione synthesis. Thirty minutes after the addition of BSO (200 microM) to macrophage cultures, when glutathione synthesis was inhibited approximately 80%, the cells responded to a zymosan challenge with a normal release of LTC. During this period, intracellular glutathione stores were not significantly depleted. Cells exposed to BSO for 2 hr or more exhibited marked decreases in glutathione levels and a progressive inhibition of LTC synthesis. After exposure to BSO for 16 hr, intracellular glutathione was undetectable, and no LTC was synthesized by the cells. Treatment of macrophages with BSO for 16 hr had no effect on cell viability, phagocytosis, total release of arachidonic acid, or prostaglandin synthesis. However, an increased synthesis of hydroxyicosatetraenoic acids in BSO-treated cells compensated for the diminished production of LTC. We conclude that BSO produces a specific, time-dependent inhibition of LTC synthesis as a result of intracellular glutathione depletion. This is consistent with a biosynthetic pathway for LTC in which glutathione is a direct precursor of this arachidonic acid metabolite.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannai S., Tsukeda H. The export of glutathione from human diploid cells in culture. J Biol Chem. 1979 May 10;254(9):3444–3450. [PubMed] [Google Scholar]
- Bonney R. J., Wightman P. D., Davies P., Sadowski S. J., Kuehl F. A., Jr, Humes J. L. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978 Nov 15;176(2):433–442. doi: 10.1042/bj1760433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3213–3217. doi: 10.1073/pnas.76.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979 Aug 25;254(16):7865–7869. [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Murphy R. C., Samuelsson B., Clark D. A., Mioskowski C., Corey E. J. Structure of leukotriene C. Identification of the amino acid part. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1266–1272. doi: 10.1016/0006-291x(79)91203-8. [DOI] [PubMed] [Google Scholar]
- Jakschik B. A., Lee L. H. Enzymatic assembly of slow reacting substance. Nature. 1980 Sep 4;287(5777):51–52. doi: 10.1038/287051a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murphy R. C., Hammarström S., Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4275–4279. doi: 10.1073/pnas.76.9.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning L., Hammarström S. Inhibition of leukotriene C and leukotriene D biosynthesis. J Biol Chem. 1980 Sep 10;255(17):8023–8026. [PubMed] [Google Scholar]
- Orning L., Hammarström S., Samuelsson B. Leukotriene D: a slow reacting substance from rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2014–2017. doi: 10.1073/pnas.77.4.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. W., Fischman C. M., Wedner H. J. Relationship of biosynthesis of slow reacting substance to intracellular glutathione concentrations. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6870–6873. doi: 10.1073/pnas.77.11.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Cohn Z. A., Blackburn P., Manning J. M. Mouse peritoneal macrophages release leukotriene C in response to a phagocytic stimulus. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4928–4932. doi: 10.1073/pnas.77.8.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Hamill A. L., Cohn Z. A. Dynamics of leukotriene C production by macrophages. J Exp Med. 1980 Nov 1;152(5):1236–1247. doi: 10.1084/jem.152.5.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Zrike J. M., Hamill A. L., Kempe J., Cohn Z. A. Regulation of arachidonic acid metabolites in macrophages. J Exp Med. 1980 Aug 1;152(2):324–335. doi: 10.1084/jem.152.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]


