Abstract
A reduction in calorie intake (Caloric restriction), appears to consistently decrease the biological rate of aging in a variety of organisms as well as protect against age-associated diseases including chronic inflammatory disorders such as cardiovascular disease and diabetes. Although the mechanisms behind this observation are not fully understood, identification of the main metabolic pathways affected by caloric restriction has generated interest in finding molecular targets that could be modulated by caloric restriction mimetics. This review describes the general concepts of caloric restriction and caloric restriction mimetics as well as discusses evidence related to their effects on inflammation and chronic inflammatory disorders. Additionally, emerging evidence related to the effects of caloric restriction on periodontal disease in non-human primates is presented. While the implementation of this type of dietary intervention appears to be challenging in our modern society where obesity is a major public health problem, caloric restriction mimetics could offer a promising alternative to control and perhaps prevent several chronic inflammatory disorders including periodontal disease.
Keywords: Caloric restriction, mimetics, chronic inflammation, periodontal disease, aging
Introduction
The quest for the proverbial fountain of youth has been pursued for many years. Although our understanding of aging has advanced, the mechanisms behind increased longevity are still uncertain. Understanding the causes of aging and extending the lifespan of multiple species, including humans, has captured the interest of scientists for decades (McCay et al., 1956). An interesting finding from research in aging, was that many mutations capable of extending life span, also decreased the activity of nutrient-signaling pathways suggesting that aging and nutrition could be linked (Fontana et al., 2010, Harrison et al., 2009, Jia et al., 2004, Wu et al., 2009). A growing body of evidence has demonstrated that the balance between energy (calorie) consumption and energy expenditure is critical not only for lifespan but also for quality of life across the lifespan (Minor et al., 2010). Thus, potential variations in this energy equation would be expected to impact not only longevity but also health/disease states as has been demonstrated in several organisms from yeast to humans (Fontana et al., 2010). A clear example of this fundamental observation is how the chronic accumulation of energy in obesity (declared as one of the major public health problems worldwide by the World Health Organization), increases the risk for chronic inflammatory disorders such as hypertension, type 2 diabetes, coronary heart disease, stroke, osteoarthritis and periodontitis among others (Palacios et al., 2009, Pischon et al., 2007, Saito et al., 1998, Waxman, 2003). On the other hand, in marked contrast to obesity, a reduction in energy/calorie consumption (caloric restriction-CR), appears to consistently decrease the biological rate of aging as well as protect against age-associated diseases including chronic inflammatory disorders (Fontana, 2009, Morgan et al., 2007, Omodei & Fontana, 2011). In spite of the promising anti-inflammatory properties exhibited by this nutritional approach to control the increasing prevalence of chronic inflammatory diseases, reducing caloric intake in a modern western society, which is conditioned to ad libitum feeding, has brought new challenges to the field. Thus, a better understanding of the cellular and molecular pathways modified by CR, that may impact the control of chronic inflammatory disorders, seems to be critical for the identification of new molecular targets that could be modulated through the use of caloric restriction mimetics (CRMs) as a potential therapeutic alternative. The purpose of this review is to convey general concepts of CR and CRMs as well as the evidence related to their therapeutic potential to control chronic inflammatory disorders including periodontal disease.
Caloric restriction
In general, CR is set at around a 40% reduction in energy consumption that does not result in malnutrition (Martin-Montalvo et al., 2011, Opalach et al., 2010). In addition to increased lifespan, mammalian studies found CR to benefit other processes that contribute to overall health, such as cardiovascular function and blood glucose regulation (Colman et al., 2009). As this phenomenon has been scrutinized further, it appears that prevention of free radical formation and the inflammatory response contributes to the benefits of CR (Anderson & Weindruch, 2010). One of the most common theories of aging is the ‘free radical theory’ which proposes that reactive oxygen species (ROS), the daily byproducts of cellular metabolism, can accumulate and damage other molecules, thereby driving senescence. Although the inflammatory response is a natural and essential reaction to stress experienced by an organism, the accumulation and improper disposal of ROS can cause pathology, primarily inflammation (Anderson & Weindruch, 2010, Figueiredo et al., 2009). Concentrations of ROS, such as 8-hydroxydeoxyguanosine (8-OHdG), a product of DNA oxidation, have been found to have a direct relationship with aging in various mammals (Sohal et al., 1994). In addition, it has been shown that species with long life expectancies have lower rates of ROS generation (Pamplona et al., 1998). CR may also prevent an energy excess, which is capable of causing systemic inflammation as adipose tissues expand to accommodate the extra storage of these nutrients (Ye & Keller, 2010). Therefore, regulation of ROS and their site of production, the mitochondria, have been targeted in understanding the mechanisms of CR and disease prevention. The National Institute on Aging and the University of Wisconsin-Madison have been studying the effects of CR on aging for several years and the preliminary results suggest that CR will delay the onset of cardiovascular disease, diabetes, and perhaps cancer supporting the concept that the beneficial effects of CR on aging in rodents will also apply to nonhuman primates and perhaps ultimately to humans (Colman et al., 2009, Lane et al., 2001, Mattison et al., 2003). Evidence related to the beneficial effects of CR to control chronic inflammatory disorders will be discussed in the next sections.
Effect of caloric restriction on chronic inflammatory disorders
Caloric restriction and inflammation
Chronic inflammation is defined as an unresolved low-grade immunoinflammatory response associated with persistent tissue and organ damage that is commonly observed with aging (Schottenfeld & Beebe-Dimmer, 2006), and is considered a major risk factor underlying age-related diseases such as atherosclerosis, arthritis, cancer, diabetes, osteoporosis, dementia, cardiovascular diseases, obesity and metabolic syndrome (Chung et al., 2009). A growing body of evidence suggests that the dysregulation of the immune system with age, and the impaired redox balance during aging are important causes of chronic inflammation (Chung et al., 2009, Chung et al., 2006) (Figure 1). The altered redox balance is mainly generated by the net effect of weakened anti-oxidative defense systems (i.e., superoxide dismutase-SOD, glutathione-GSH, and thioredoxin-Trx), along with the continuous production of reactive species (RS) including reactive oxygen species (ROS: superoxide- O2−, hydroxyl radical- •OH, hydrogen peroxide- H2O2), and reactive nitrogen species (RNS: reactive nitric oxide- NO, peroxynitrite- ONOO−). In fact, higher levels of the pro-inflammatory cytokines IL-1aβ, TNFα, IL-6 and inflammatory-associated enzymes such as cyclooxygenase (COX) and inducible nitric oxide synthase (iNOS), as well as persistence of the inflammatory infiltrate of macrophages, lymphocytes and plasma cells within the tissues observed in aging and chronic inflammation, have been related with the redox imbalance (i.e., oxidative stress) induced by RS accumulation (Kim et al., 2002). Evidence suggests that the mechanisms by which RS induce chronic inflammation relies on the RS ability to activate cell signaling cascades that include IκB kinase and MAPKs which further turn on the NFκB transcription factor downstream that is considered a master regulator for the expression of several pro-inflammatory genes (Kim et al., 2000). Since a natural metabolic consequence of converting nutrients to usable energy (ATP) is the production of RS, it would be expected as mentioned earlier in this review, that an imbalance between intake and expenditure of energy/calories could impact the levels of RS and subsequently the inflammatory response (Figure 1). Accordingly, evidence supporting the anti-oxidant and anti-inflammatory properties as well as the beneficial effects on insulin sensitivity via CR, using mainly animal models, has shown a rapid growth during the last decade and has been recently reviewed (Fontana, 2009). Of note, the anti-inflammatory effects of CR, rather than being simply a passive mechanism linked to the reduction of inflammatory stimuli such as RS, may also exert active and positive actions on metabolic, hormonal and gene expression products that repress pathways of inflammation in several types of tissues including liver, hearth, muscle, white adipose tissue, neural tissue, kidney, lung and colon among others (Clement et al., 2004, Higami et al., 2004, Lamas et al., 2003, Matsuzaki et al., 2001, Swindell, 2009). Thus, while the expression of genes encoding proteins with anti-inflammatory properties such as NFκB inhibitor alpha (Nfkbia), tissue inhibitor of metalloproteinases-3 (Timp3), and peroxisome proliferator-activated receptors (PPARs), are enhanced by CR (Sung et al., 2004, Swindell, 2009), pro-inflammatory genes such as TNFα, IL-6, COX-2, iNOS, VCAM-1 and ICAM-1 appear to be inhibited (Higami et al., 2006, Jung et al., 2009). In support of these observations, in vivo studies using murine models have shown that CR appears to reduce the expression and binding activity of specific transcription factors such as C/EBPalpha, STAT5b, nuclear factor kappa B (NFκB), forkhead transcription factor-1 (FOXO1), and activator protein-1 (AP-1) to the promoters of genes encoding acute phase response proteins (e.g. haptoglobin), pro-inflammatory cytokines as well as oxidative stress-related genes (Jung et al., 2009, Kim et al., 2008, Kim et al., 2002, Uskokovic et al., 2009).
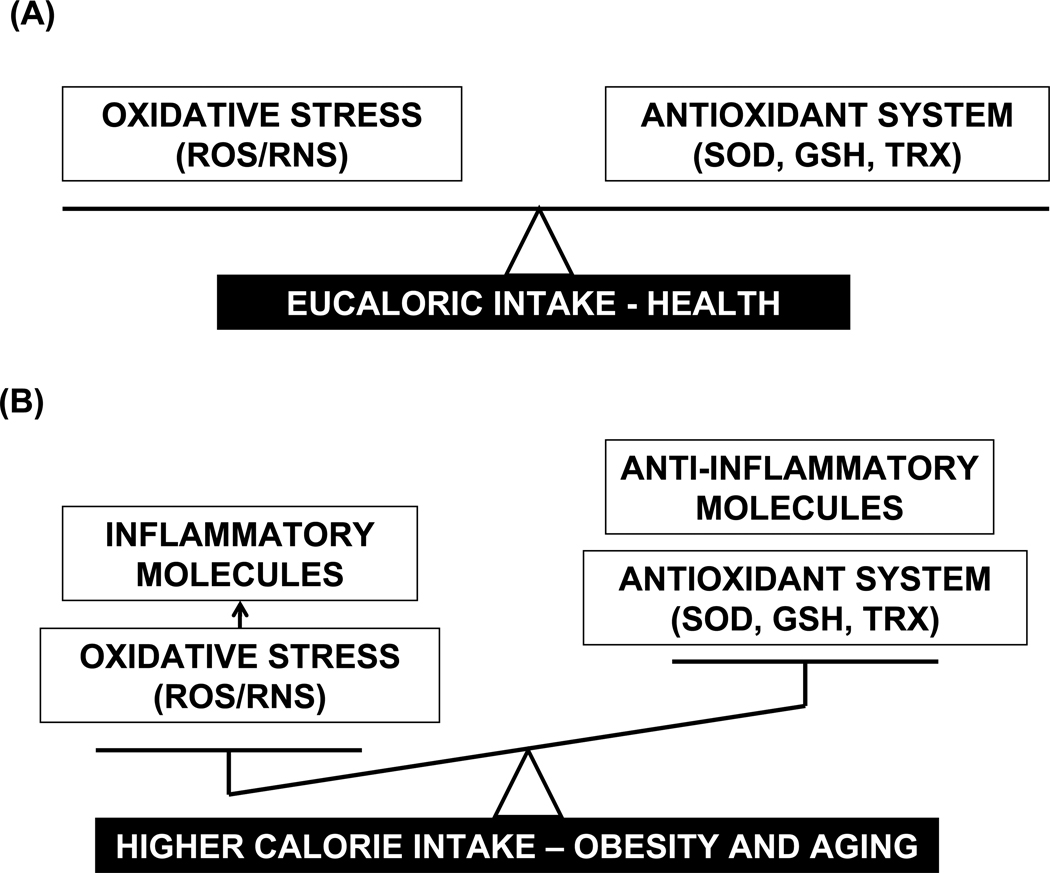
Figure 1. Calorie intake levels and inflammation.
The normal balance between the production of oxidative stress (Reactive oxygen -ROS, and Nitrogen-RNS species) and the antioxidant enzymatic system (superoxide dismutase-SOD, glutathione-GSH, and thioredoxin-TRX) observed during a normal/healthy caloric intake (A), is lost with either higher levels of calorie intake commonly observed in obesity or during aging, which leads to the accumulation of oxidative stress and production of inflammatory molecules and mediators (B). Caloric restriction would compensate the balance by reducing production of oxidative stress and inflammation as well as enhancing the production and activity of anti-oxidant enzymes and anti-inflammatory mediators.
Evidence for the potential anti-inflammatory mechanisms of CR in humans is more limited, and most of the studies addressing this have been developed in obese patients, since CR is perhaps the most efficient way to promote weight loss in this population. Circulating levels of serum amyloid A protein, IL-6, CRP, TNFα, and IFNγ were reduced in obese patients after CR, improving their general inflammatory profile (Lee et al., 2010, Salas-Salvado et al., 2006, Viguerie et al., 2005). Nevertheless, CR does not seem to have the same anti-inflammatory effect on severe obesity (Sola et al., 2009). It is still controversial if the reduction in the systemic levels of pro-inflammatory molecules is due to the reduction in adipose tissue mass as well as adipocyte-secreted cytokines (i.e. adipokines), or it could also involve a direct effect on immune cells (i.e. macrophages, lymphocytes) after CR (Lee et al., 2010, Salas-Salvado et al., 2006, Viguerie et al., 2005). Recent observations suggest that a low calorie diet has an impact on the expression of inflammatory genes in peripheral mononuclear blood cells of obese patients (Crujeiras et al., 2008). Since a tight coupling of metabolic and immune systems is mediated by neuro-endocrine peptides as well as several cytokines and chemokines (Dixit, 2008, Ouchi et al., 2011), both immune and non-immune cells could be impacted either directly or indirectly by energy balance changes experienced during obesity and CR. Nevertheless, the results of these studies with the obese population provide information more relevant to the effects of weight loss than of chronic CR. Thus, the main challenge now is developing future studies involving humans with a normal body mass index (below 25 kg/m2) that will be subject to CR. The potential benefits of CR in a non-obese population are supported by the preliminary and promising results of pilot studies such as the Comprehensive Assessment of Long-Term Effects of Reducing Calorie intake (CALERIE phase I), as well as studies involving members of the caloric restriction society (Holloszy & Fontana, 2007).
Caloric restriction and cardiovascular disease (CVD)
Cardiovascular disease is considered one of the leading causes of morbidity and mortality in Western countries especially in older populations (Callow, 2006). Among the risk factors for CVD, smoking, hypertension, dyslipidemia, abdominal obesity, impaired insulin sensitivity, and sedentary lifestyle have been identified (Dahlof, 2010). Additionally, accumulating evidence suggests that biomarkers such as redox state, elevated levels of pro-inflammatory cytokines and enzymes, as well as adhesion molecules, increase the risk for CVD (Chung et al., 2009). Therefore, the anti-oxidant and anti-inflammatory properties of CR make this nutritional intervention approach an interesting and promising alternative to prevent and control CVD. Evidence for mechanisms of cardioprotection by CR has been recently reviewed (Han & Ren, 2010, Marzetti et al., 2009). Briefly, it has been demonstrated in animal studies (including rats, mice and nonhuman primates) that the attenuation of oxidative stress, mitochondrial dysfunction and inflammation, and a favorable modulation of cardiomyocyte apoptosis and autophagy (biological process that allows eukaryotic cells to degrade and recycle long-lived proteins and organelles) are critical mechanisms modulated by CR which could be associated with reduced risk of CVD (Marzetti et al., 2009).
Evidence suggests that brief periods of ischemia paradoxically protect the heart and limit necrosis caused by a subsequent more prolonged period of ischemia (a phenomenon known as preconditioning); nevertheless, this protective mechanism is attenuated with aging. An exposure to CR for 6 months was effective in maintaining the preconditioning response in aged Sprague-Dawley rats, as compared to young adults (Long et al., 2002a). The mechanisms, by which CR improves recovery of heart function following ischemia, are not completely understood. However it was shown that rats under CR diets (55% reduced calorie intake for 8 months), exhibited better energy production and oxidative phosphorylation compared with control animals, suggesting that changes in mitochondrial respiration could be related to such an effect (Broderick et al., 2002). Similar results were reported by Shinmura et al, whereby improved myocardial ischemic tolerance was observed in rats under short- (2–4 weeks) and prolonged-term (6 months) CR via a mechanism that appears to involve an oxide-dependent increase in nuclear silent information regulator (Sirt1) (Shinmura et al., 2005, Shinmura et al., 2008). Most recently, it has been suggested that increased production of adiponectin and activation of the PI3kinase/Akt and vascular endothelial growth factor (VEGF) pathways could also be involved in the cardioprotective effects of CR on cardiac preconditioning (Katare et al., 2009, Shinmura et al., 2007). Interestingly, beyond the anti-oxidant and anti-inflammatory properties of CR, it has been shown that a 40% CR for 1 month also improved the cardiac autonomic activity in rats (Mager et al., 2006). Whether or not gender could play a role in the beneficial effects of CR in reducing CVD remains unclear. Nonetheless a recent study demonstrated that cardiac muscle mitochondrial metabolism (i.e. H2O2 production and oxidative damage), was most efficiently reduced by CR (40% reduced calorie intake for 3 months) in female rather than in male rats (Colom et al., 2007). Endothelial dysfunction commonly found in obesity can be reversed by CR in mice and results suggest that CR can do so in a mechanism that appears to involve the enzyme endothelial nitric oxide synthase (eNOS) (Ketonen et al., 2010). Consistent with these results, Dolinsky et al demonstrated that CR prevents hypertension in rats via stimulation of an adiponectin/AMPK/eNOS signaling axis (Dolinsky et al., 2010). Notably, CR showed an ability to correct pre-existing mitochondrial dysfunction and apoptotic activation commonly observed in senescent myocardium, which prevented deterioration of left ventricular function in rats (Niemann et al., 2010).
The effects of CR on atherosclerosis, using different models, have shown controversial results. Specifically, CR (30% reduction of calorie intake) improved visceral fat accumulation and insulin sensitivity in adult cynomolgus monkeys but did not have an effect on plasma lipid levels or atherosclerotic lesion extent in the abdominal aorta (Cefalu et al., 1999, Cefalu et al., 2004). Nevertheless, it was shown that CR reduced circulating lipoprotein a (lpa) levels in Rhesus monkeys compared with the animals receiving a control diet. Plasma levels of lpa are considered an independent risk factor for age-associated development of atherosclerosis (Edwards et al., 2001). Most recently, Fontana et al demonstrated that individuals on CR for 6 years had markedly lower levels of serum total cholesterol, low-density lipoprotein cholesterol, triglycerides, fasting glucose, fasting insulin, CRP, platelet-derived growth factor AB, and systolic and diastolic blood pressure compared with age-matched healthy individuals on a typical American diet. Of note, carotid artery intima-media thickness was about 40% less in the CR group compared to the control group, which suggested that CR has a critical effect on the risk for atherosclerosis (Fontana et al., 2004).
Evidence of the cardio-protective effects of CR in humans continues to expand. A prospective study evaluating the effects of a low-calorie diet (800kcal/d for 2 weeks) on endothelial function in obese hypertensive Japanese patients vs. healthy control found that CR improved endothelial-dependent vasodilatation through an increased release of nitric oxide in obese hypertensive patients (Sasaki et al., 2002). Furthermore, a group of 12 obese men, after 12 weeks of CR, showed a significant decrease of endothelin-1 and increase of nitric oxide, which would suggest an improvement in endothelial function (Miyaki et al., 2009). Serum advanced glycation end products (AGEs), have been linked to increased atherogenicity and inflammation in diabetes and renal failure. Significant reduction of serum AGE levels were reported in a clinical study of 37 Japanese overweight and obese individuals under CR (5,023 kJ/day) for 2 months (Gugliucci et al., 2009). Similarly, 34 obese individuals, after consuming a low calorie diet for 6 weeks, exhibited significant reduction in myocardial fatty acid uptake, which suggests that the increased fatty acid uptake found in hearts of obese patients can be reversed by weight loss-induced CR (Viljanen et al., 2009). Of note, levels of IL-18 and IL-20 have been related to atherosclerosis and coronary heart disease (Jefferis et al., 2011, Maiorino et al., 2010), and CR significantly reduced the circulating levels of these two cytokines in obese women compared with their matched normal-weight controls (Esposito et al., 2002). CR also appears to improve insulin sensitivity, glucose, and triglyceride levels when combined with exercise and dietary approaches to treat hypertension (Blumenthal et al., 2010). Nevertheless, the results of a recent randomized clinical trial showed that CR did not improve the effects of exercise on metabolic and cardiovascular risk factors of aged and obese individuals diagnosed with metabolic syndrome, which suggests that exercise alone could be an effective non-pharmacological treatment strategy for insulin resistance, metabolic syndrome and CVD (Yassine et al., 2009). In general, emerging clinical evidence indicates that dietary restriction provides significant improvements in traditional cardiovascular risk factors (i.e., blood pressure, blood glucose, lipids, and body composition) in overweight and obese subjects as well as in lean individuals. Nevertheless, the effect of CR on biomarkers of oxidative stress and inflammation in humans has not yet been thoroughly explored and will require further analysis (Marzetti et al., 2009).
Caloric restriction and Diabetes
Diabetes is a group of diseases characterized by high blood glucose levels that result from defects in the body’s ability to produce and/or use insulin. Diabetes has been diagnosed in about 26 million people in the United States, although the prevalence is increasing since about 79 million people have pre-diabetes. Among the diabetic population approximately 5% have type 1 diabetes (insulin-dependent diabetes mellitus) which involves a failure to produce insulin by the beta cells of the pancreas, and 95% have type 2 diabetes (non-insulin-dependent diabetes mellitus) that can be developed at any age and is generally associated with insulin resistance, i.e., fat, muscle, and liver cells do not use insulin properly which leads to the loss of the ability to secrete enough insulin by the pancreas in response to glucose load. Importantly, aging, obesity, family history of diabetes, history of gestational diabetes, impaired glucose tolerance, physical inactivity, and race/ethnicity are considered risk factors for type 2 diabetes (http://www.cdc.gov/diabetes). Growing evidence shows that obese individuals gradually progress to type 2 diabetes (Whitmore, 2010); however, the biological mechanisms behind this observation remain unclear. It could involve an increase in oxidative stress, induced by hyperglycemia and free fatty acids which would worsen both insulin secretion and action in type 2 diabetes (Davi et al., 2005). CR has been suggested as the cornerstone of therapy with exercise and oral hypoglycemic agents to control type 2 diabetes; especially in obese patients. In fact, growing evidence suggests that CR appears to be beneficial in improving pancreatic beta-cell function, glycemic control, insulin action in the liver and peripheral tissues (i.e. decrease insulin resistance), as well as insulin secretion (Davidson, 1980, Henry & Gumbiner, 1991, Ichihara et al., 1975, Wheeler et al., 1987, Wing et al., 1994). Specifically, CR increases insulin responsiveness in obese type 2 diabetic rats (Kalant et al., 1988), and prevents the clinical expression of diabetes mellitus in rats that spontaneously develop the disease compared to their ad libitum fed controls, through a mechanism that appears to involve increased insulin sensitivity (Okauchi et al., 1995). In addition, prevention of beta-cell depletion has also been observed after CR in rodents (Ohneda et al., 1995). The effects of long term CR (about 4–10 years) have shown similar results in non-human primates; improving glycogen metabolism and insulin sensitivity (Ortmeyer et al., 1994, Roth et al., 2001, Wang et al., 2009). The findings in animal models have been replicated in several human studies and have shown that either short or long term CR regimens in obese patients with type 2 diabetes, improve glycemic control and insulin resistance (Escalante-Pulido et al., 2003, Harder et al., 2004, Hughes et al., 1984). Of note, CR appears to have sustained beneficial metabolic effects (up to 18 months) in obese, insulin-treated patients with type 2 diabetes, although to a lesser extent in patients who regained body weight (Jazet et al., 2007). Even though CR seems to improve the markers of type 2 diabetes in obese patients, there is no supportive clinical evidence about its potential to prevent the onset of the disease (Baker et al., 2009), as has been reported in non-human primates (Colman et al., 2009).
The biological mechanisms by which CR decreases insulin resistance and improves glucose metabolism are not fully understood. Nevertheless, it has been proposed that CR may have a direct impact on the reduction of plasma levels of pro-inflammatory cytokines (i.e. adipokines), peptides, and complement factors that are normally produced in adipose tissue (Barzilai & Gabriely, 2001). Recent studies have demonstrated that the sir2 homologue (SIRT1; an NAD+- dependent deacetylase) controls the gluconeogenic/glycolytic pathways in the liver in response to the CR-activated transcriptional cofactor PGC-1alpha (Rodgers et al., 2005), and also appears to reduce the apoptosis ratio of islet beta cells in diabetic rats (Deng et al., 2010). Additionally, CR enhanced the expression of the glucose transporter type 4 (GLUT4) in rat adipocytes, which correlated with improved glucose translocation into fat cells and insulin resistance (Park et al., 2005). Most recently, microarray analyses showed that CR enhanced differential expression of genes involved in glucose and lipid metabolism in skeletal muscle, liver, and pancreatic islets in diabetic fatty rodents (Colombo et al., 2006, Selman et al., 2006).
Pre-clinical and clinical studies provide controversial results about the impact of short term CR on oxidative stress. Despite a decrease in lipid peroxidation, improvement in antioxidant activity, and restoration of the overall redox status observed in mice following CR (Ugochukwu & Figgers, 2007), only a marginal anti-oxidant effect could be associated with CR in type 2 diabetic patients compared with non-diabetic obese individuals (Skrha et al., 2005, Wycherley et al., 2008). Interestingly, recent evidence shows that bariatric surgery can abolish type 2 diabetes within days of surgery, even before weight loss has occurred, which suggests that CR alone may not entirely account for this effect (Andreelli et al., 2009). A clear explanation of this postsurgical outcome is a topic of an ongoing debate (Gumbs et al., 2005). Significantly, animal and human studies have shown that genotype contributes to insulin resistance and obesity, and therefore CR may not have the same beneficial effect in all individuals (Corella et al., 2005, Metzger, 2003).
Caloric restriction and immunity
It has been broadly established that a healthy and adequate nutrition is critical to maintain normal immune function, as malnutrition can increase the incidence of infectious diseases due to immunosuppression (Fernandes, 2008, Good et al., 1976, Latshaw, 1991). Even though CR is defined as a reduction in caloric intake without malnutrition, it has been shown that both short or long term CR may have an impact on immunity (Jolly, 2004). There is limited evidence of the effects of CR on the immune response; however, animal studies (i.e. rodents) suggest that while CR (40–60% intake reduction) could have beneficial effects on the adaptive immune response, particularly T-cells, it may have a detrimental effect on the innate immune response with impaired ability to control infections by monocytes/macrophages (Jolly, 2004, Nayak et al., 2009). Specifically, it has been shown that CR improves IL-2 production and T cell proliferation normally reduced in aging (Goonewardene & Murasko, 1995, Pahlavani et al., 1997), and reduces the incidence, duration and severity of experimental autoimmune uveoretinits in rats correlating with lower levels of IFNγ and auto-antibody production (Abe et al., 2001). Furthermore, using a validated mouse model for studying systemic lupus erythematosis, it has been shown that CR delayed autoimmune renal disease in a process that seems to involve specific immune compartments and different T cell subsets (Fernandes et al., 1978, Jolly et al., 1999, Jolly et al., 2001). CR also has been shown to improve mouse survival after Salmonella typhimurium infection (Peck et al., 1992), ameliorate experimental colitis in mice (Shibolet et al., 2002), and enhance the production of IFNγ (a cytokine that plays a critical role against intracellular infections and cancer) by LPS-stimulated peripheral mononuclear cells from aged non-human primates (Mascarucci et al., 2002). In marked contrast, a significant reduction in H2O2, TNFα, IL-6, NO and some signal transduction pathways in macrophages, as well as impaired natural killer (NK) cell function have been reported in mice/rats after CR. These observations could correlate with impaired control of certain viral infections, such as influenza (Dong et al., 1998, Gardner, 2005, Spaulding et al., 1997, Stapleton et al., 2001, Vega et al., 2004). Similarly, CR reduces IgA levels in mouse small intestine, probably due to a reduction of IgA+ cells in the lamina propria (Lara-Padilla et al., 2010). These controversial results could be associated with the variability of time and degree of CR used in different models. In fact, it was recently suggested that there could be an optimal window during adulthood where CR can delay immune senescence and improve immunity (Messaoudi et al., 2008). Although human studies addressing this aspect are limited, similar effects on the adaptive and innate components of the immune response have been reported in obese patients under CR for 3 and 6 months, including reduction in pro-inflammatory cytokines (e.g. TNFα and IL-6) and increase of anti-inflammatory cytokines (i.e. IL-10) (Ahmed et al., 2009, Jung et al., 2008, McMurray et al., 1990, Santos et al., 2003).
As suggested by Jolly, all the animal studies have used very aggressive CR regimens which could not be practically translated to the clinical settings in humans (Jolly, 2004). It is becoming clear that CR has important anti-inflammatory properties, prolongs life and health span in several organisms including humans; nevertheless, it could have chronic detrimental effects on some components of immunity (Jolly, 2007). A better understanding of the cellular and molecular pathways affected by CR has created an opportunity to identify specific molecular targets that could be modulated by similar molecules referred to as CRMs, which will be discussed in the following section of this review. Perhaps this growing group of molecules will help to overcome the potential adverse effects on the immune response and improve the specificity of the beneficial effects observed with CR.
Caloric restriction mimetics
Given the wide range of benefits mediated by CR in preventing chronic inflammation and associated diseases in both invertebrate and mammalian studies, prescription of such a regimen seems promising for application to humans to incur these same benefits. Unfortunately, a reduction in the dietary consumption of calories of around 30–40% would be undesirable for most individuals, given the current global eating habits. A decrease in time spent preparing food at home and an increase in time spent eating per day among Americans has been reported over the last few decades (Zick & Stevens, 2010), and certainly suggests that people would have a hard time accepting the rigors of a CR diet. As a result, there has been emerging interest in identifying compounds that modulate the same signaling pathways as CR so that similar anti-inflammatory and disease prevention benefits may be provided without dietary sacrifice. Currently CRMs that affect SIRT, Target of rapamycin (TOR), and Insulin growth factor (IGF) signaling have all been investigated since modulating these pathways in prior studies has resulted in an increased lifespan (Bjedov et al., 2010, Boily et al., 2008, Harrison et al., 2009, Howitz et al., 2003, Kaeberlein et al., 2005, Onken & Driscoll, Rogina & Helfand, 2004) (Figure 2). Further research into these molecules will be necessary to provide clinically significant health benefits of CR in the absence of dietary limitations.
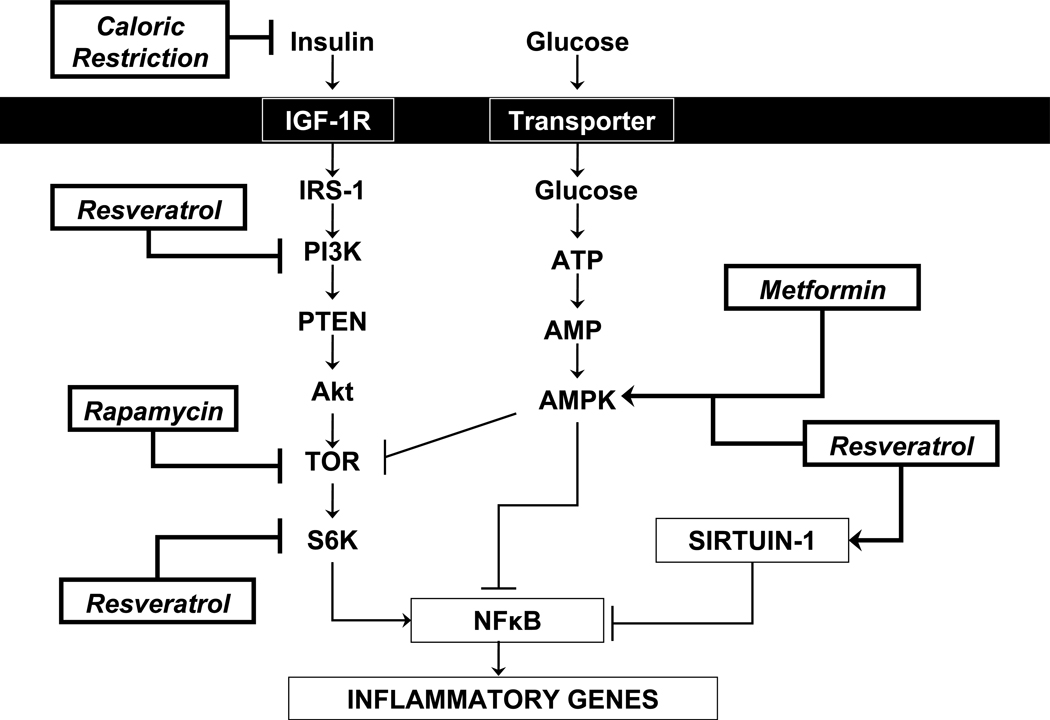
Figure 2. Molecular targets for caloric restriction mimetics.
Normally, the target of rapamycin (TOR) pathway is activated by nutrients (Glucose and amino acids), growth factors and insulin, which enhances the activation downstream of the transcription factor nuclear factor kappa B (NFκB) that is critical regulator of the pro-inflammatory gene expression. TOR is directly inhibited by rapamycin, and indirectly by the oral anti-diabetic drug metformin and resveratrol, which activate AMPK, an important inhibitor of TOR. The histone deacetylase, Sirtuin-1, considered a master metabolic regulator (involved in Cholesterol and fat metabolism, and glucose homeostasis), exhibits ability to modulate and control numerous transcription factors including de-acetylating (inactivation) of NFκB with the consequent reduction in the transcription of pro-inflammatory genes. Resveratrol also enhances activation of Sirtuin-1 (negative regulator of NFκB) and inhibits PI3K and S6K (positive regulators of NFκB). The levels of nutrients and insulin are reduced during caloric restriction which decreases activation of TOR pathway. IGF-1R: Insulin growth factor type 1 receptor; IRS-1: Insulin receptor substrate-1; PI3K: Phosphoinositide 3-kinase; PTEN: Phosphatase and tensin homolog; Akt: Serine-threonine protein kinase; S6K: 70KDa protein kinase; ATP: Adenosine triphosphate; AMP: Adenosine monophosphate; AMPK: 5’ adenosine monophosphate-activated protein kinase.
Resveratrol
The observation made during the early 90s that the rate of mortality from coronary heart disease was relatively low in France despite the relatively high levels of dietary saturated fat and cigarette smoking, led to the idea that regular consumption of red wine might provide additional protection from CVD; phenomenon named “French paradox” (Criqui & Ringel, 1994). Since then, several studies investigating the biological and clinical associations of red wine consumption with CVD and mortality led to the identification of specific red wine chemical components related with these beneficial effects including resveratrol (Lippi et al., 2010). Resveratrol is a polyphenolic compound found in grapes, red wine, purple grape juice, peanuts, and some berries. It is a member of the stilbene class of polyphenols (i.e. 3, 4’, 5-trihydroxystilbene), and has been widely cited as a CR mimetic because of its ability to activate SIRT and extend lifespan in cell cultures and animal models (Baur & Sinclair, 2006, Marques et al., 2009), as well as its potential to inhibit the development of cancer (Jang et al., 1997). In mammals, there are seven members of the sirtuin family, classified as SIRT1-7 (Borra et al., 2005, Frye, 2000, Howitz et al., 2003). SIRT1 is a target of resveratrol and is commonly studied because of its interaction with other pro-inflammatory molecules such as NF-κB, IL-6, TNF-α and PPARγ (Shakibaei et al., 2011). SIRT1 acts as a NAD-dependent deacetylase and has been found to regulate cellular processes such as metabolism and apoptosis by modifying proteins (Frye, 1999, Sinclair & Guarente, 2006). Therefore, SIRT1 activity may be related to disease prevention and longevity by regulating ROS and maintaining cellular integrity. In addition, SIRT1 also seems to play a role in glucose metabolism in the liver, which is essential for glucose homeostasis (Rodgers et al., 2005, Sun et al., 2007). Several studies have shown that resveratrol supplementation directly suppress the release of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-10, MCP-1, IFNα, and IFNβ in a wide range of tissues, including the brain in rodents (Knobloch et al., 2011, Liu et al., 2011, Lu et al., 2010, Mbimba et al., 2011, Yen et al., 2011). Resveratrol also displays antioxidant activity in cell culture, contributing to a reduction in inflammation. In laboratory conditions, resveratrol neutralizes free radicals and other oxidizing molecules (Stojanovic et al., 2001). Studies have also found a powerful effect of resveratrol in preventing lipid peroxidation, specifically low density lipoprotein (LDL) (Brito et al., 2002). Improved endothelial function and enhanced cardioprotective effects have been observed with resveratrol through its anti-inflammatory and anti-oxidant properties on various animal models of myocardial injury, hypertension and type 2 diabetes (Csiszar, 2011).
Initial clinical trials, mainly focused on determining the safety and tolerability, as well as the pharmacokinetic and metabolism profiles of resveratrol, have suggested that doses of <1.0 g/day seem to be safe and reasonably well tolerated (Patel et al., 2011). Nevertheless, the therapeutic or protective effects of resveratrol have not yet been demonstrated although several clinical trials are currently in process to test its effects on Alzheimer’s disease, colorectal cancer, type 2 diabetes and metabolic syndrome (http://clinicaltrials.gov). A recent study showed significant reductions in the generation of ROS and the expression of proinflammatory cytokines, TNF-α and IL-6 and C - reactive protein, in mononuclear cells from healthy adults with normal weight receiving 40 mg/day of a plant extract containing resveratrol for 6 weeks (Ghanim et al., 2010). In addition to systemic administration, topical application of resveratrol has been effective in reducing inflammation in response to dermatological irritants (Fabbrocini et al., 2011). Interestingly, resveratrol treatment in human adipose tissue explants was able to decrease gene expression of the inflammatory adipokines IL-6, IL-8, MCP-1, IL-1β (Olholm et al., 2010). This is particularly exciting given the fact that chronic inflammation in adipose tissue is implicated in metabolic syndrome and obesity (Despres & Lemieux, 2006).
Rapamycin
TOR is another gene commonly studied as a target for CRMs that is present in all eukaryotes, and its conservation seems to imply an essential role in homeostasis and mediating longevity (Wullschleger et al., 2006). Mammals have one copy of TOR (mTOR) with two distinct complexes, TOR complex 1 (TORC1), which responds to rapamycin, and TOR complex 2 (TORC2), which does not (Wullschleger et al., 2006). Interestingly, yeast have 2 TOR genes (TOR1&2) (Barbet et al., 1996). As a serine/threonine kinase protein, TORs appear to be involved with cellular growth via regulation of protein synthesis, especially during development due to nutrient sensing (Long et al., 2002b). Conversely, TOR is also involved with cellular catabolism and macro-autophagy in yeast and higher eukaryotes (Lum et al., 2005). This is particularly important since dysregulation of autophagy is often associated with pathology and an altered inflammatory response. Metabolic processes, specifically fat deposition, are also related to TOR signaling (Kim & Chen, 2004); therefore, benefits mediated through TOR may be the result of multiple areas of regulation.
Rapamycin, a macrolide drug, works by binding the cyclophilin protein FKBP12, which then binds and inhibits the function of mTOR (Gregory et al., 2006). In yeast, this rapamycin-FKBP complex inhibits both TOR1 and TOR2, arresting cellular growth (Barbet et al., 1996). In mice, it has been found that over-activity of TOR results in elevated levels of ROS and that inflammation is mediated by cytokine release through TOR activation (Chen et al., 2008, Chen et al., 2010). Consistently, rapamycin has been shown to prevent ROS accumulation and subsequent inflammation and infection after transplantation surgery in animals and humans (Ozdemir et al., 2011, Stepkowski et al., 1991). A recent study by Song et al has shown that mTOR overexpression mitigates the consequences of pressure overload in cardiomyocytes and that mTOR activation could reduce cytokine IL-6 expression, providing a reduction in inflammation and a cardioprotective effect (Song et al., 2010). Moreover, rapamycin also acts as an immunosuppressant by suppressing T cell activation and proliferation via mTOR (McMahon et al., 2011). Thus it appears that rapamycin treatment may not be appropriate in all cases, and that more research is necessary to understand the possible role for rapamycin as a CR mimetic.
Metformin
Insulin growth factor (IGF) genes are also considered a target of CRMs because of their well established role in cellular proliferation differentiation and development, particularly during the fetal stage but also in anabolic processes in adults (Lighten et al., 1998). Although there are several members of the IGF family (IGF-I, IGF-II, somatomedin A, and somatomedin C), IGF-1 and 2 are most commonly studied as the target of CR mimetics because of their structural similarity to insulin (Kasuga et al., 1981). CR studies have used anti-diabetic biguanides to target the insulin/IGF signaling pathway and have observed improved health outcomes (Anisimov et al., 2008); one commonly used drug from this family is metformin because of its specificity for IGF-1 (Scheiwiller et al., 1986). As a member of the biguanide family, characterized by a structure of two linked guanidine rings, metformin appears to work by inhibiting complex 1 of the respiratory chain and thereby preventing mitochondrial oxidation (Owen et al., 2000, Witters, 2001). In addition to increased longevity, anti-inflammatory benefits may result from this inhibition of ROS production. Furthermore, metformin helps regulate blood glucose levels by decreasing hepatic glucose output and enhancing peripheral glucose uptake (DeFronzo et al., 1991), possibly through a mechanism that involves increased intestinal use and disposal of glucose, as well as reduced ROS production and inflammation (Bailey & Turner, 1996).
Studies with metformin have shown that markers of inflammation, such as TNF-α, IL-6, IFN-γ and NF-κβ decrease with its supplementation (Bergheim et al., 2006, Chen et al., 2005, Hattori et al., 2006), and metformin effectively reduces high neutrophil levels in women with polycystic ovarian syndrome (Ibanez et al., 2005). In addition to IGF-1, 5’ adenosine monophosphate-activated protein kinase (AMPK), which regulates energy homeostasis and metabolic stress, is also activated by metformin (Hattori et al., 2006, Zhou et al., 2001). Therefore, the anti-inflammatory effects of metformin may be due to activation of multiple signaling pathways.
Although there are gaps in the research surrounding proposed CR mimetics, most of the effective candidates seem to play a role in regulating metabolism and generation of ROS, two processes that are undoubtedly intertwined. Thus far, regulation of SIRT, TOR and IGF by CRMs, seems to be a promising target to investigate for the prevention of inflammatory processes and promoting longevity. Continued investigation into these mechanisms will better serve our understanding of how they may reduce inflammation and thereby reduce the incidence of various chronic diseases in the future.
Caloric restriction and periodontal disease
Periodontal disease is considered to be an inflammatory condition of the supporting structures of the teeth that may result in destruction of connective tissue and bone leading to tooth loss if left untreated (Tatakis & Kumar, 2005). A complex microbial biofilm, known commonly as dental plaque, is thought to be the initiating agent for the development of periodontal inflammation and the removal of the biofilm is important in controlling the disease process (Haffajee et al., 2008). The oral cavity has proven to be an excellent environment to study both the microbial colonization and formation of the plaque biofilm and the inflammatory periodontal response that occurs in response to the plaque challenge. Due to its ease of access, the oral cavity is frequently used for clinical, microbiologic, and immunologic studies of the etiology and pathogenesis of periodontal disease. In addition, animal studies have been used to explore the kinetics of the interaction between plaque microorganisms and the periodontal tissues and its modification by topical and/or systemic modulators of plaque formation and periodontal inflammation. Previous cross sectional studies have noted that maintenance of normal body weight by a healthy diet and exercise significantly reduces the prevalence and severity of periodontitis (Pischon et al., 2007). However, there have been no studies of the effects of CR on periodontal infection, inflammation, and disease in humans, although a recent series of studies, performed by our group in non-human primates, have laid the foundation for future studies in humans (Branch-Mays et al., 2008, Ebersole et al., 2008, Reynolds et al., 2009).
As part of a longitudinal investigation of the effects of CR on aging being conducted through the National Institute on Aging (Ingram et al., 1990), we were invited to examine a cohort of rhesus monkeys (Macaca mulatta) for the effects of CR on the clinical, microbiologic, and immunologic characteristics of periodontal disease using a cross-sectional experimental design (Reynolds et al., 2009). This study was then followed by a prospective study, measuring the same parameters, using an experimental ligature-induced periodontitis model to evaluate the kinetics of the development of periodontitis in CR and control animals (Branch-Mays et al., 2008). The CR monkeys had been subjected to a 30% reduction in dietary caloric intake relative to control animals for periods of 13–17 years (Ingram et al., 1990). The CR diet was supplemented with minerals and vitamins up to 100% daily allowance for CR animals. The diet was supplemented once weekly with fresh fruit for all monkeys.
Clinical, microbiologic and immunologic effects on the periodontium of long term exposure to CR (Reynolds et al., 2009)
In this cross sectional study, we demonstrated that a long-term exposure to CR had a significant effect on decreasing the severity of naturally occurring chronic periodontal disease. CR males had significantly less periodontal pocketing compared to control males with no differences between same sex CR and control monkeys for mean scores of plaque index, calculus index, and bleeding on probing. In the absence of CR, males showed significantly greater periodontal breakdown, as reflected by higher mean clinical attachment level and periodontal probing depth scores, than females. Subgingival samples of the dental plaque biofilms were evaluated by “checkerboard hybridization” using digoxigenin-labeled whole genomic DNA probes to a panel 40 different representative oral bacteria (Socransky et al., 1994), and by quantitative real-time polymerase chain reaction (qPCR) (Kirakodu et al., 2008) for the following target periodontal pathogens: Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Prevotella intermedia, Fusobacterium nucleatum, and Campylobacter rectus. Using both methods, we were able to demonstrate that the CR diet had no demonstrable effects on the subgingival periodontal microbiota in male or female monkeys.
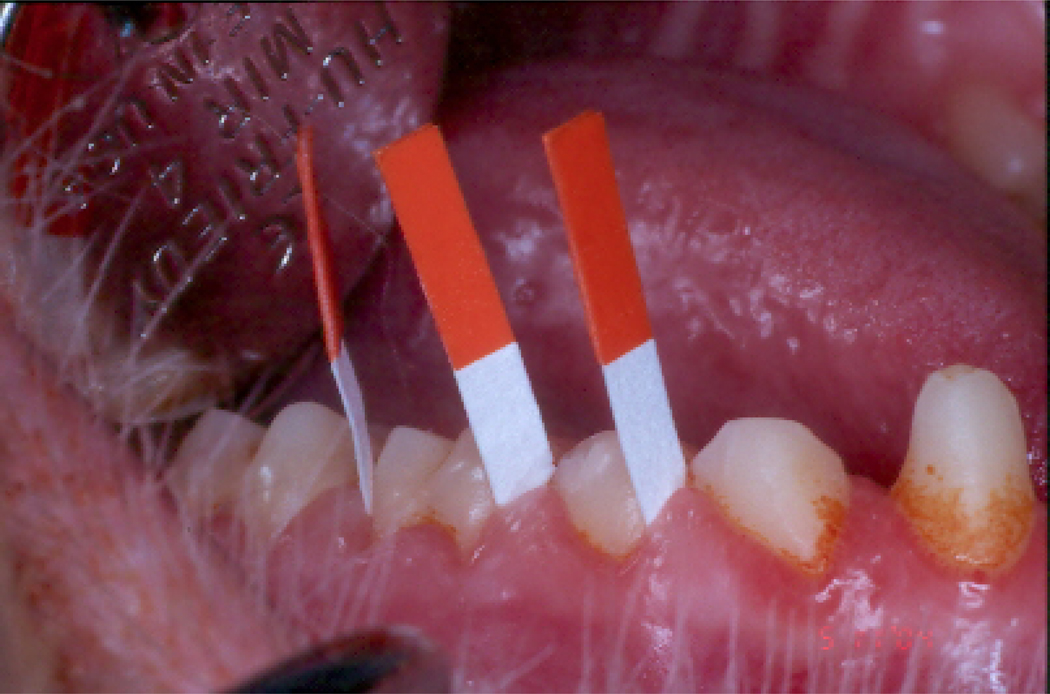
The potential for CR to demonstrate an immuno-modulatory effect has been clearly demonstrated and described in detail in previous sections of this review. In the cross-sectional study of the effects of CR on the inflammatory response associated with periodontal status, we assessed biomarkers of inflammation in gingival crevicular fluid (GCF), which is a serum like transudate that can be collected from the periodontal pockets around teeth with inflamed periodontal tissues, and contains molecules released as part of the local and systemic inflammatory responses. The GCF is collected on paper strips inserted into the periodontal pocket (Figure 3), and the molecules contained in the collected fluid are eluted off the strips and evaluated by normal laboratory methods. CR males, who had demonstrated a significant reduction in clinical probing pocket depth compared to their controls, also demonstrated a significantly lower IgG antibody response and lower levels of IL-8 and β-glucuronidase in GCF compared to control males. A similar, but non-significant, reduction was found for IL-1β. In contrast, CR females, who had exhibited clinical measures comparable to control females, also demonstrated a lower IgG antibody response, but with comparable levels of inflammatory markers in GCF compared to control females. It was therefore concluded from the cross-sectional study that a long term exposure to CR, although having no effect on the periodontal microbiota, appears to have a modulatory effect on the local immune responses and that this is associated with a reduction in the clinical manifestations of periodontal disease in males, but not females. The gender effects on inflammation and the immune response were also seen in serum analyzed from the same animals (Ebersole et al., 2008). The results demonstrated that haptoglobin and α1-acid glycoprotein, were elevated in serum of male monkeys versus females. Serum IgG antibody responses to Campylobacter rectus, Aggregatibacter actinomycetemcomitans, and Pophyromonas gingivalis were significantly elevated in female monkeys. While only the antibody to Fusobacterium nucleatum was significantly affected by the CR diet in females, antibody levels to Prevotella intermedia, C. rectus and Treponema denticola demonstrated a similar trend.
Figure 3.
Collection of gingival crevicular fluid (GCF) from the periodontal pocket of Rhesus monkeys using Periopaper absorbent strips.
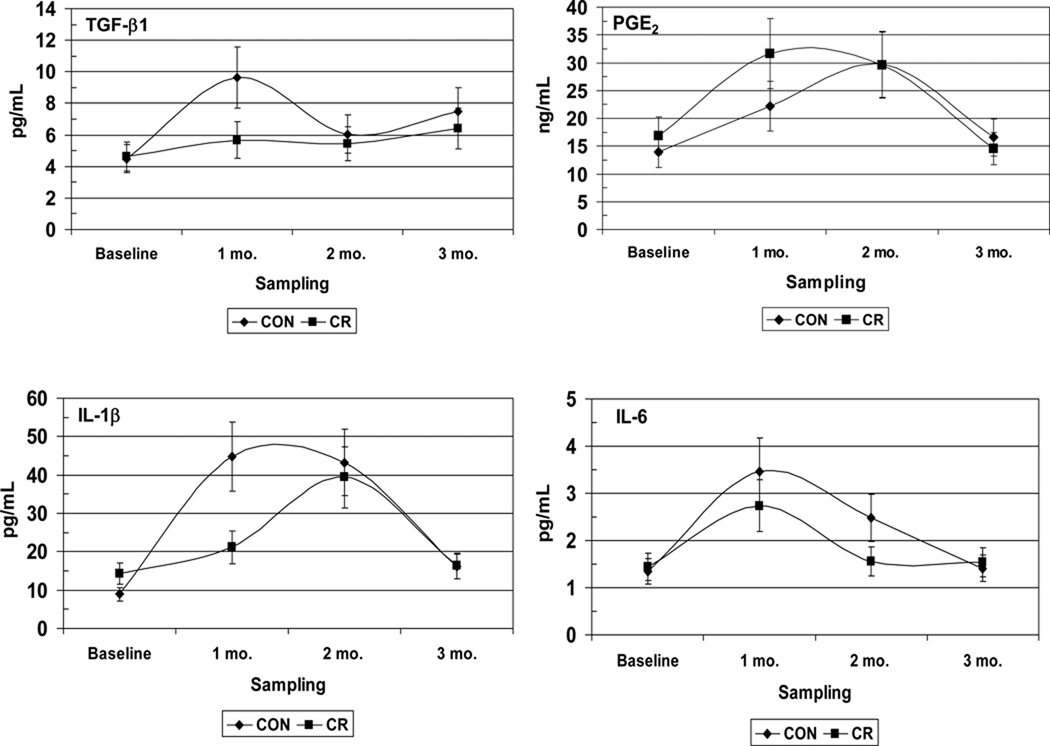
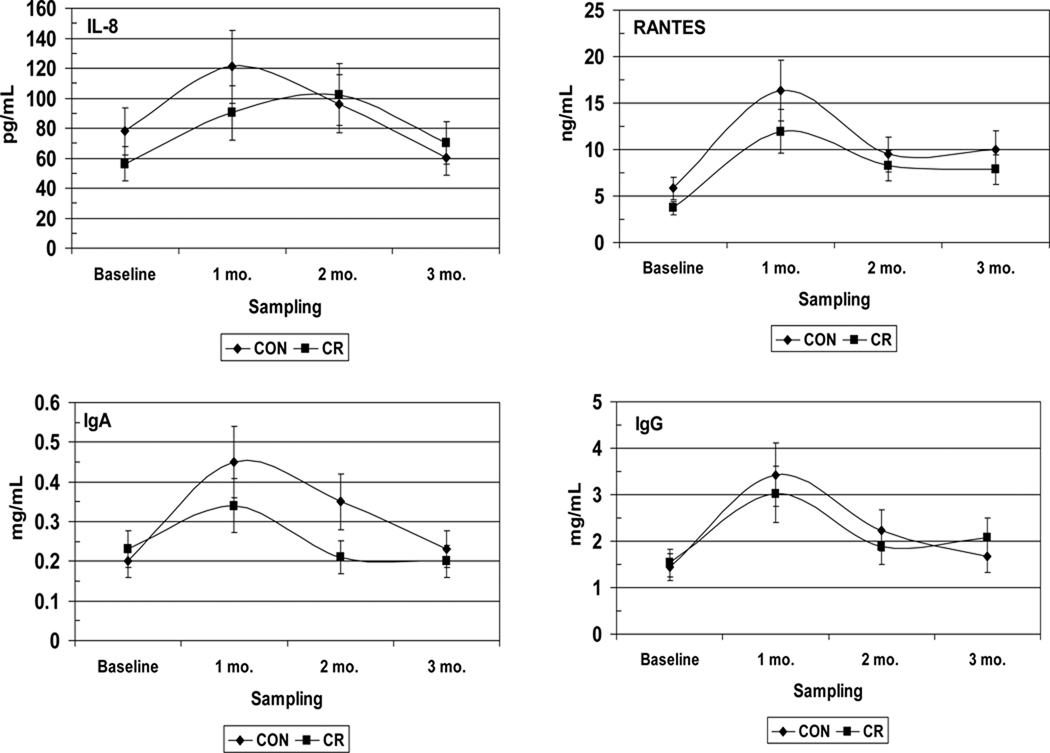
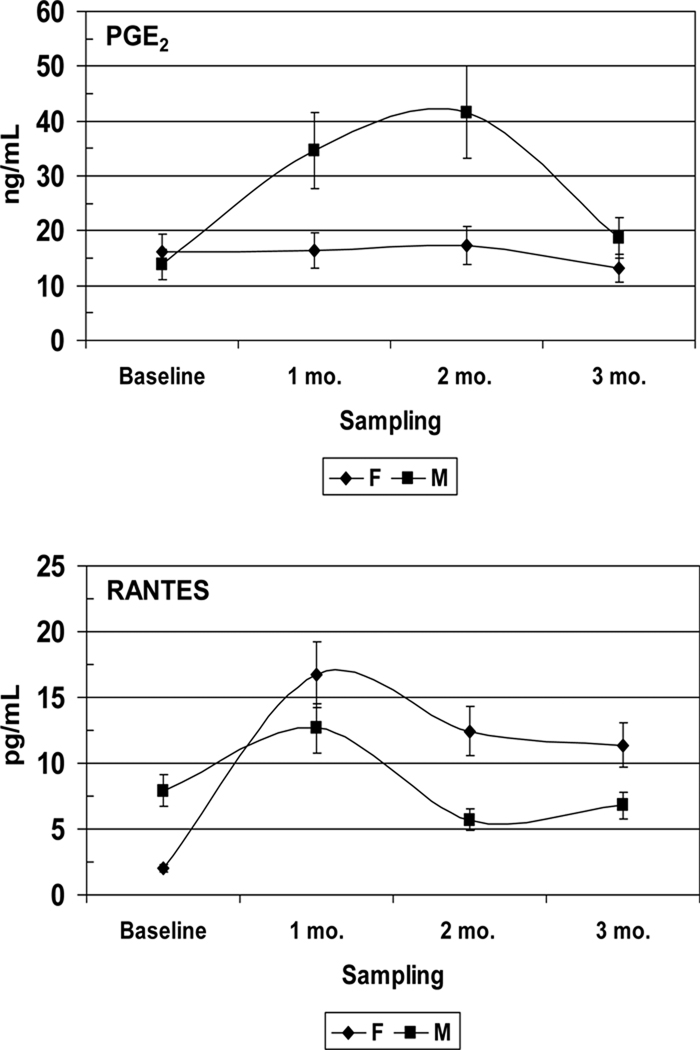
A cross-sectional analysis of the effects of long term CR exposure provides us with the potential impact of CR on disease progression. However, since the prevalence of periodontal disease in this type of study is a single measure of what may be a series of disease events that have taken place over an extended interval of time; these data give us very little insight into how CR affects the kinetics of disease activity. An additional problem is that naturally occurring periodontal disease in healthy non-human primates is a relatively rare occurrence in young adults and is mainly observed in aged animals; the reason by which it has been necessary to develop models that can induce periodontitis in healthy non-human primates. The ligature-induced periodontitis model has been used extensively in New and Old World non-human primates to facilitate the accumulation of microbial plaque on silk ligatures that are placed at the cemento-enamel junctions of premolar and molar teeth (Kennedy & Polson, 1973, Madden & Caton, 1994, Page, 1982). In a follow up study to the one previously described and in collaboration with the National Institute on Aging at the National Institutes for Health, experimental periodontitis was induced in 55 young, healthy, adult rhesus monkeys (Macaca mulatta) by tying 2.0 silk ligatures at the gingival margins of maxillary premolar/molar teeth (Branch-Mays et al., 2008). The experimental animals were maintained on a CR diet (30% CR; n=23) and were compared to ad libitum diet controls (n=32). Clinical periodontal measures, including plaque (PLI), probing pocket depth (PD), clinical attachment level (CAL), modified Gingival Index (GI) and bleeding on probing (BOP), were taken at baseline and 1, 2, and 3 months after ligature placement. Significant effects of CR were observed on the development of inflammation and the progression of periodontal destruction in this model. When compared to controls, CR resulted in a significant reduction in ligature induced GI (p<0.0001), BOP (p<0.0015), PD (p<0.0016), and CAL (p<0.0038). When viewed over time, periodontal destruction, as measured by CAL, progressed significantly more slowly in the CR animals than in the controls (p<0.001). An assessment of the effects of CR on inflammatory mediators in GCF (currently unpublished data), demonstrated that CR had a significant effect on decreasing the magnitude and kinetics of the inflammatory and humoral response molecules such as TGF-β1, IL-1β, PGE2, IL-8, IL-6, RANTES, IgA, and IgG (Figure 4). In addition, there were significant differences between male and female animals for PGE2, and RANTES in GCF (Figure 5), confirming our gender differences from the cross-sectional study.
Figure 4.
Effect of caloric restriction on inflammatory mediators and IgA/IgG levels in gingival crevicular fluid (GCF) during ligature-induced periodontitis in nonhuman primates. CON: Control, CR: Caloric restriction.
Figure 5.
Effect of gender on inflammatory mediators levels in gingival crevicular fluid (GCF) during ligature-induced periodontitis in nonhuman primates under caloric restriction. F: Female, M: Male.
Calorie restricted diet programs have become extremely popular and successful and the potential benefits for improving systemic conditions are becoming increasingly evident, as described in previous sections of this review. However, little is known of the effects of a CR diet on the initiation and progression of periodontal disease. The results of these preliminary animal studies suggested that CR may have a significant effect on the progression of periodontal disease and that this effect may be associated with selective local (periodontal tissues) and systemic (serum) alterations in the magnitude and kinetics of the immune response.
To implement diets with substantial food intake restriction appears to be a great challenge, especially with a growing epidemic of obesity. More animal and clinical studies focused on CRM are needed as the regulation of CRM-modulated cellular pathways appears to provide promising results in the prevention and control of chronic inflammatory disorders including periodontal disease.
ACKNOWLEDGMENTS
This work was supported by United States Health Service grant U01 AG-021406 from the National Institute on Aging to MJN.
REFERENCES
- Abe T, Nakajima A, Satoh N, Ohkoshi M, Sakuragi S, Koizumi A. Suppression of experimental autoimmune uveoretinitis by dietary calorie restriction. Jpn J Ophthalmol. 2001;45:46–52. doi: 10.1016/s0021-5155(00)00303-8. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Das SK, Golden JK, Saltzman E, Roberts SB, Meydani SN. Calorie restriction enhances T-cell-mediated immune response in adult overweight men and women. J Gerontol A Biol Sci Med Sci. 2009;64:1107–1113. doi: 10.1093/gerona/glp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21:134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreelli F, Amouyal C, Magnan C, Mithieux G. What can bariatric surgery teach us about the pathophysiology of type 2 diabetes? Diabetes Metab. 2009;35:499–507. doi: 10.1016/S1262-3636(09)73456-1. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- Baker S, Jerums G, Proietto J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract. 2009;85:235–242. doi: 10.1016/j.diabres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Barbet NC, Schneider U, Helliwell SB, Stansfield I, Tuite MF, Hall MN. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell. 1996;7:25–42. doi: 10.1091/mbc.7.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Gabriely I. The role of fat depletion in the biological benefits of caloric restriction. J Nutr. 2001;131:903S–906S. doi: 10.1093/jn/131.3.903S. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bergheim I, Luyendyk JP, Steele C, Russell GK, Guo L, Roth RA, Arteel GE. Metformin prevents endotoxin-induced liver injury after partial hepatectomy. J Pharmacol Exp Ther. 2006;316:1053–1061. doi: 10.1124/jpet.105.092122. [DOI] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Sherwood A, Craighead L, Lin PH, Johnson J, Watkins LL, Wang JT, Kuhn C, Feinglos M, Hinderliter A. Effects of the dietary approaches to stop hypertension diet alone and in combination with exercise and caloric restriction on insulin sensitivity and lipids. Hypertension. 2010;55:1199–1205. doi: 10.1161/HYPERTENSIONAHA.109.149153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, Evans M, Harper ME, McBurney MW. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Branch-Mays GL, Dawson DR, Gunsolley JC, Reynolds MA, Ebersole JL, Novak KF, Mattison JA, Ingram DK, Novak MJ. The effects of a calorie-reduced diet on periodontal inflammation and disease in a non-human primate model. J Periodontol. 2008;79:1184–1191. doi: 10.1902/jop.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito P, Almeida LM, Dinis TC. The interaction of resveratrol with ferrylmyoglobin and peroxynitrite; protection against LDL oxidation. Free Radic Res. 2002;36:621–631. doi: 10.1080/10715760290029083. [DOI] [PubMed] [Google Scholar]
- Broderick TL, Belke T, Driedzic WR. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol Cell Biochem. 2002;233:119–125. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- Callow AD. Cardiovascular disease 2005--the global picture. Vascul Pharmacol. 2006;45:302–307. doi: 10.1016/j.vph.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Cefalu WT, Wagner JD, Bell-Farrow AD, Edwards IJ, Terry JG, Weindruch R, Kemnitz JW. Influence of caloric restriction on the development of atherosclerosis in nonhuman primates: progress to date. Toxicol Sci. 1999;52:49–55. doi: 10.1093/toxsci/52.2.49. [DOI] [PubMed] [Google Scholar]
- Cefalu WT, Wang ZQ, Bell-Farrow AD, Collins J, Morgan T, Wagner JD. Caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): metabolic, physiologic, and atherosclerotic measures from a 4-year intervention trial. J Gerontol A Biol Sci Med Sci. 2004;59:1007–1014. doi: 10.1093/gerona/59.10.b1007. [DOI] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Zheng P. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. J Clin Invest. 2010;120:4091–4101. doi: 10.1172/JCI43873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SQ, Liu Q, Sun H, Tang L, Deng JC. Effects of metformin on fatty liver in insulin-resistant rats. Zhonghua Gan Zang Bing Za Zhi. 2005;13:915–918. [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R, Langin D. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 2007;74:456–465. doi: 10.1016/j.cardiores.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Colombo M, Kruhoeffer M, Gregersen S, Agger A, Jeppesen P, Oerntoft T, Hermansen K. Energy restriction prevents the development of type 2 diabetes in Zucker diabetic fatty rats: coordinated patterns of gene expression for energy metabolism in insulin-sensitive tissues and pancreatic islets determined by oligonucleotide microarray analysis. Metabolism. 2006;55:43–52. doi: 10.1016/j.metabol.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Corella D, Qi L, Sorli JV, Godoy D, Portoles O, Coltell O, Greenberg AS, Ordovas JM. Obese subjects carrying the 11482G>A polymorphism at the perilipin locus are resistant to weight loss after dietary energy restriction. J Clin Endocrinol Metab. 2005;90:5121–5126. doi: 10.1210/jc.2005-0576. [DOI] [PubMed] [Google Scholar]
- Criqui MH, Ringel BL. Does diet or alcohol explain the French paradox? Lancet. 1994;344:1719–1723. doi: 10.1016/s0140-6736(94)92883-5. [DOI] [PubMed] [Google Scholar]
- Crujeiras AB, Parra D, Milagro FI, Goyenechea E, Larrarte E, Margareto J, Martinez JA. Differential expression of oxidative stress and inflammation related genes in peripheral blood mononuclear cells in response to a low-calorie diet: a nutrigenomics study. OMICS. 2008;12:251–261. doi: 10.1089/omi.2008.0001. [DOI] [PubMed] [Google Scholar]
- Csiszar A. Anti-inflammatory effects of resveratrol: possible role in prevention of age-related cardiovascular disease. Ann N Y Acad Sci. 2011;1215:117–122. doi: 10.1111/j.1749-6632.2010.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlof B. Cardiovascular disease risk factors: epidemiology and risk assessment. Am J Cardiol. 2010;105:3A–9A. doi: 10.1016/j.amjcard.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Davi G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7:256–268. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- Davidson JK. Newer approaches to diet management of diabetes: calorie control. Med Times. 1980;108:35–40. [PubMed] [Google Scholar]
- DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1991;73:1294–1301. doi: 10.1210/jcem-73-6-1294. [DOI] [PubMed] [Google Scholar]
- Deng X, Cheng J, Zhang Y, Li N, Chen L. Effects of caloric restriction on SIRT1 expression and apoptosis of islet beta cells in type 2 diabetic rats. Acta Diabetol. 2010;47:177–185. doi: 10.1007/s00592-009-0159-7. [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukoc Biol. 2008;84:882–892. doi: 10.1189/jlb.0108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, Des Rosiers C, Walsh K, Davidge ST, Dyck JR. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56:412–421. doi: 10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- Dong W, Selgrade MK, Gilmour IM, Lange RW, Park P, Luster MI, Kari FW. Altered alveolar macrophage function in calorie-restricted rats. Am J Respir Cell Mol Biol. 1998;19:462–469. doi: 10.1165/ajrcmb.19.3.3114. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Steffen MJ, Reynolds MA, Branch-Mays GL, Dawson DR, Novak KF, Gunsolley JC, Mattison JA, Ingram DK, Novak MJ. Differential gender effects of a reduced-calorie diet on systemic inflammatory and immune parameters in nonhuman primates. J Periodontal Res. 2008;43:500–507. doi: 10.1111/j.1600-0765.2008.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards IJ, Rudel LL, Terry JG, Kemnitz JW, Weindruch R, Zaccaro DJ, Cefalu WT. Caloric restriction lowers plasma lipoprotein (a) in male but not female rhesus monkeys. Exp Gerontol. 2001;36:1413–1418. doi: 10.1016/s0531-5565(01)00107-3. [DOI] [PubMed] [Google Scholar]
- Escalante-Pulido M, Escalante-Herrera A, Milke-Najar ME, Alpizar-Salazar M. Effects of weight loss on insulin secretion and in vivo insulin sensitivity in obese diabetic and non-diabetic subjects. Diabetes Nutr Metab. 2003;16:277–283. [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Ciotola M, Di Palo C, Grella E, Nicoletti G, Giugliano D. Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab. 2002;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- Fabbrocini G, Staibano S, De Rosa G, Battimiello V, Fardella N, Ilardi G, La Rotonda MI, Longobardi A, Mazzella M, Siano M, Pastore F, De Vita V, Vecchione ML, Ayala F. Resveratrol-containing gel for the treatment of acne vulgaris: a single-blind, vehicle-controlled, pilot study. Am J Clin Dermatol. 2011;12:133–141. doi: 10.2165/11530630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Fernandes G. Progress in nutritional immunology. Immunol Res. 2008;40:244–261. doi: 10.1007/s12026-007-0021-3. [DOI] [PubMed] [Google Scholar]
- Fernandes G, Friend P, Yunis EJ, Good RA. Influence of dietary restriction on immunologic function and renal disease in (NZB x NZW) F1 mice. Proc Natl Acad Sci U S A. 1978;75:1500–1504. doi: 10.1073/pnas.75.3.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo PA, Powers SK, Ferreira RM, Appell HJ, Duarte JA. Aging impairs skeletal muscle mitochondrial bioenergetic function. J Gerontol A Biol Sci Med Sci. 2009;64:21–33. doi: 10.1093/gerona/gln048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L. Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Exp Gerontol. 2009;44:41–45. doi: 10.1016/j.exger.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci. 2005;60:688–694. doi: 10.1093/gerona/60.6.688. [DOI] [PubMed] [Google Scholar]
- Ghanim H, Sia CL, Abuaysheh S, Korzeniewski K, Patnaik P, Marumganti A, Chaudhuri A, Dandona P. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab. 2010;95:E1–E8. doi: 10.1210/jc.2010-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good RA, Fernandes G, Yunis EJ, Cooper WC, Jose DC, Kramer TR, Hansen MA. Nutritional deficiency, immunologic function, and disease. Am J Pathol. 1976;84:599–614. [PMC free article] [PubMed] [Google Scholar]
- Goonewardene IM, Murasko DM. Age-associated changes in mitogen-induced lymphoproliferation and lymphokine production in the long-lived brown-Norway rat: effect of caloric restriction. Mech Ageing Dev. 1995;83:103–116. doi: 10.1016/0047-6374(95)01609-4. [DOI] [PubMed] [Google Scholar]
- Gregory MA, Hong H, Lill RE, Gaisser S, Petkovic H, Low L, Sheehan LS, Carletti I, Ready SJ, Ward MJ, Kaja AL, Weston AJ, Challis IR, Leadlay PF, Martin CJ, Wilkinson B, Sheridan RM. Rapamycin biosynthesis: Elucidation of gene product function. Org Biomol Chem. 2006;4:3565–3568. doi: 10.1039/b608813a. [DOI] [PubMed] [Google Scholar]
- Gugliucci A, Kotani K, Taing J, Matsuoka Y, Sano Y, Yoshimura M, Egawa K, Horikawa C, Kitagawa Y, Kiso Y, Kimura S, Sakane N. Short-term low calorie diet intervention reduces serum advanced glycation end products in healthy overweight or obese adults. Ann Nutr Metab. 2009;54:197–201. doi: 10.1159/000217817. [DOI] [PubMed] [Google Scholar]
- Gumbs AA, Modlin IM, Ballantyne GH. Changes in insulin resistance following bariatric surgery: role of caloric restriction and weight loss. Obes Surg. 2005;15:462–473. doi: 10.1381/0960892053723367. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Socransky SS, Patel MR, Song X. Microbial complexes in supragingival plaque. Oral Microbiol Immunol. 2008;23:196–205. doi: 10.1111/j.1399-302X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- Han X, Ren J. Caloric restriction and heart function: is there a sensible link? Acta Pharmacol Sin. 2010;31:1111–1117. doi: 10.1038/aps.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder H, Dinesen B, Astrup A. The effect of a rapid weight loss on lipid profile and glycemic control in obese type 2 diabetic patients. Int J Obes Relat Metab Disord. 2004;28:180–182. doi: 10.1038/sj.ijo.0802529. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care. 1991;14:802–823. doi: 10.2337/diacare.14.9.802. [DOI] [PubMed] [Google Scholar]
- Higami Y, Barger JL, Page GP, Allison DB, Smith SR, Prolla TA, Weindruch R. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr. 2006;136:343–352. doi: 10.1093/jn/136.2.343. [DOI] [PubMed] [Google Scholar]
- Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA, Weindruch R. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. FASEB J. 2004;18:415–417. doi: 10.1096/fj.03-0678fje. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med. 1984;77:7–17. doi: 10.1016/0002-9343(84)90429-7. [DOI] [PubMed] [Google Scholar]
- Ibanez L, Jaramillo AM, Ferrer A, de Zegher F. High neutrophil count in girls and women with hyperinsulinaemic hyperandrogenism: normalization with metformin and flutamide overcomes the aggravation by oral contraception. Hum Reprod. 2005;20:2457–2462. doi: 10.1093/humrep/dei072. [DOI] [PubMed] [Google Scholar]
- Ichihara K, Shima K, Nonaka K, Tarui S. Dietary therapy and insulin secretory response to glucose in adult-onset non-obese diabetic subjects. Endocrinol Jpn. 1975;22:399–408. doi: 10.1507/endocrj1954.22.399. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, et al. Dietary restriction and aging: the initiation of a primate study. J Gerontol. 1990;45:B148–B163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jazet IM, de Craen AJ, van Schie EM, Meinders AE. Sustained beneficial metabolic effects 18 months after a 30-day very low calorie diet in severely obese, insulin-treated patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;77:70–76. doi: 10.1016/j.diabres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Jefferis BJ, Papacosta O, Owen CG, Wannamethee SG, Humphries SE, Woodward M, Lennon LT, Thomson A, Welsh P, Rumley A, Lowe GD, Whincup PH. Interleukin 18 and coronary heart disease: Prospective study and systematic review. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Jolly CA. Dietary restriction and immune function. J Nutr. 2004;134:1853–1856. doi: 10.1093/jn/134.8.1853. [DOI] [PubMed] [Google Scholar]
- Jolly CA. Is dietary restriction beneficial for human health, such as for immune function? Curr Opin Lipidol. 2007;18:53–57. doi: 10.1097/MOL.0b013e3280115416. [DOI] [PubMed] [Google Scholar]
- Jolly CA, Fernandez R, Muthukumar AR, Fernandes G. Calorie restriction modulates Th-1 and Th-2 cytokine-induced immunoglobulin secretion in young and old C57BL/6 cultured submandibular glands. Aging (Milano) 1999;11:383–389. doi: 10.1007/BF03339817. [DOI] [PubMed] [Google Scholar]
- Jolly CA, Muthukumar A, Reddy Avula CP, Fernandes G. Maintenance of NF-kappaB activation in T-lymphocytes and a naive T-cell population in autoimmune-prone (NZB/NZW)F(1) mice by feeding a food-restricted diet enriched with n-3 fatty acids. Cell Immunol. 2001;213:122–133. doi: 10.1006/cimm.2001.1866. [DOI] [PubMed] [Google Scholar]
- Jung KJ, Lee EK, Kim JY, Zou Y, Sung B, Heo HS, Kim MK, Lee J, Kim ND, Yu BP, Chung HY. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res. 2009;58:143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, Kim SM, Lee SY, Ahn SM, Kim YK, Kim HJ, Kim DJ, Lee KW. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem. 2008;19:371–375. doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kalant N, Stewart J, Kaplan R. Effect of diet restriction on glucose metabolism and insulin responsiveness in aging rats. Mech Ageing Dev. 1988;46:89–104. doi: 10.1016/0047-6374(88)90117-0. [DOI] [PubMed] [Google Scholar]
- Kasuga M, Van Obberghen E, Nissley SP, Rechler MM. Demonstration of two subtypes of insulin-like growth factor receptors by affinity cross-linking. J Biol Chem. 1981;256:5305–5308. [PubMed] [Google Scholar]
- Katare RG, Kakinuma Y, Arikawa M, Yamasaki F, Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J Mol Cell Cardiol. 2009;46:405–412. doi: 10.1016/j.yjmcc.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Kennedy JE, Polson AM. Experimental marginal periodontitis in squirrel monkeys. J Periodontol. 1973;44:140–144. doi: 10.1902/jop.1973.44.3.140. [DOI] [PubMed] [Google Scholar]
- Ketonen J, Pilvi T, Mervaala E. Caloric restriction reverses high-fat diet-induced endothelial dysfunction and vascular superoxide production in C57Bl/6 mice. Heart Vessels. 2010;25:254–262. doi: 10.1007/s00380-009-1182-x. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim JY, Yu BP, Chung HY. The activation of NF-kappaB through Akt-induced FOXO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology. 2008;9:33–47. doi: 10.1007/s10522-007-9114-6. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY. Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev. 2002;123:1589–1595. doi: 10.1016/s0047-6374(02)00094-5. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim KW, Yu BP, Chung HY. The effect of age on cyclooxygenase-2 gene expression: NF-kappaB activation and IkappaBalpha degradation. Free Radic Biol Med. 2000;28:683–692. doi: 10.1016/s0891-5849(99)00274-9. [DOI] [PubMed] [Google Scholar]
- Kim JE, Chen J. regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes. 2004;53:2748–2756. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- Kirakodu SS, Govindaswami M, Novak MJ, Ebersole JL, Novak KF. Optimizing qPCR for the Quantification of Periodontal Pathogens in a Complex Plaque Biofilm. Open Dent J. 2008;2:49–55. doi: 10.2174/1874210600802010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch J, Hag H, Jungck D, Urban K, Koch A. Resveratrol Impairs the Release of Steroid-resistant Cytokines from Bacterial Endotoxin-Exposed Alveolar Macrophages in COPD. Basic Clin Pharmacol Toxicol. 2011 doi: 10.1111/j.1742-7843.2011.00707.x. [DOI] [PubMed] [Google Scholar]
- Lamas O, Moreno-Aliaga MJ, Martinez JA, Marti A. NF-kappa B-binding activity in an animal diet-induced overweightness model and the impact of subsequent energy restriction. Biochem Biophys Res Commun. 2003;311:533–539. doi: 10.1016/j.bbrc.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Lane MA, Black A, Handy A, Tilmont EM, Ingram DK, Roth GS. Caloric restriction in primates. Ann N Y Acad Sci. 2001;928:287–295. doi: 10.1111/j.1749-6632.2001.tb05658.x. [DOI] [PubMed] [Google Scholar]
- Lara-Padilla E, Campos-Rodriguez R, Jarillo-Luna A, Reyna-Garfias H, Rivera-Aguilar V, Miliar A, Berral de la Rosa FJ, Navas P, Lopez-Lluch G. Caloric restriction reduces IgA levels and modifies cytokine mRNA expression in mouse small intestine. J Nutr Biochem. 2010 doi: 10.1016/j.jnutbio.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Latshaw JD. Nutrition--mechanisms of immunosuppression. Vet Immunol Immunopathol. 1991;30:111–120. doi: 10.1016/0165-2427(91)90012-2. [DOI] [PubMed] [Google Scholar]
- Lee IS, Shin G, Choue R. A 12-week regimen of caloric restriction improves levels of adipokines and pro-inflammatory cytokines in Korean women with BMIs greater than 23 kg/m2. Inflamm Res. 2010;59:399–405. doi: 10.1007/s00011-009-0113-8. [DOI] [PubMed] [Google Scholar]
- Lighten AD, Moore GE, Winston RM, Hardy K. Routine addition of human insulin-like growth factor-I ligand could benefit clinical in-vitro fertilization culture. Hum Reprod. 1998;13:3144–3150. doi: 10.1093/humrep/13.11.3144. [DOI] [PubMed] [Google Scholar]
- Lippi G, Franchini M, Favaloro EJ, Targher G. Moderate red wine consumption and cardiovascular disease risk: beyond the "French paradox". Semin Thromb Hemost. 2010;36:59–70. doi: 10.1055/s-0030-1248725. [DOI] [PubMed] [Google Scholar]
- Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 2011;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- Long P, Nguyen Q, Thurow C, Broderick TL. Caloric restriction restores the cardioprotective effect of preconditioning in the rat heart. Mech Ageing Dev. 2002a;123:1411–1413. doi: 10.1016/s0047-6374(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Long X, Spycher C, Han ZS, Rose AM, Muller F, Avruch J. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr Biol. 2002b;12:1448–1461. doi: 10.1016/s0960-9822(02)01091-6. [DOI] [PubMed] [Google Scholar]
- Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang Z, Wang Z, Wang JM, Le Y. Resveratrol differentially modulates inflammatory responses of microglia and astrocytes. J Neuroinflammation. 2010;7:46. doi: 10.1186/1742-2094-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- Madden TE, Caton JG. Animal models for periodontal disease. Methods Enzymol. 1994;235:106–119. doi: 10.1016/0076-6879(94)35135-x. [DOI] [PubMed] [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Maiorino MI, Schisano B, Di Palo C, Vietri MT, Cioffi M, Giugliano G, Giugliano D, Esposito K. Interleukin-20 circulating levels in obese women: effect of weight loss. Nutr Metab Cardiovasc Dis. 2010;20:180–185. doi: 10.1016/j.numecd.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Marques FZ, Markus MA, Morris BJ. Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int J Biochem Cell Biol. 2009;41:2125–2128. doi: 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Martin-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30:505–520. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Wohlgemuth SE, Anton SD, Bernabei R, Carter CS, Leeuwenburgh C. Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin Geriatr Med. 2009;25:715–732. doi: 10.1016/j.cger.2009.07.002. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarucci P, Taub D, Saccani S, Paloma MA, Dawson H, Roth GS, Lane MA, Ingram DK. Cytokine responses in young and old rhesus monkeys: effect of caloric restriction. J Interferon Cytokine Res. 2002;22:565–571. doi: 10.1089/10799900252982043. [DOI] [PubMed] [Google Scholar]
- Matsuzaki J, Kuwamura M, Yamaji R, Inui H, Nakano Y. Inflammatory responses to lipopolysaccharide are suppressed in 40% energy-restricted mice. J Nutr. 2001;131:2139–2144. doi: 10.1093/jn/131.8.2139. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- Mbimba T, Awale P, Bhatia D, Geldenhuys WJ, Darvesh AS, Carroll RT, Bishayee A. Alteration of Hepatic Proinflammatory Cytokines is Involved in the Resveratrol-Mediated Chemoprevention of Chemically-Induced Hepatocarcinogenesis. Curr Pharm Biotechnol. 2011 doi: 10.2174/138920112798868575. [DOI] [PubMed] [Google Scholar]
- McCay CM, Pope F, Lunsford W. Experimental prolongation of the life span. Bull N Y Acad Med. 1956;32:91–101. [PMC free article] [PubMed] [Google Scholar]
- McMahon G, Weir MR, Li XC, Mandelbrot DA. The evolving role of mTOR inhibition in transplantation tolerance. J Am Soc Nephrol. 2011;22:408–415. doi: 10.1681/ASN.2010040351. [DOI] [PubMed] [Google Scholar]
- McMurray RW, Bradsher RW, Steele RW, Pilkington NS. Effect of prolonged modified fasting in obese persons on in vitro markers of immunity: lymphocyte function and serum effects on normal neutrophils. Am J Med Sci. 1990;299:379–385. doi: 10.1097/00000441-199006000-00005. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Fischer M, Warner J, Park B, Mattison J, Ingram DK, Totonchy T, Mori M, Nikolich-Zugich J. Optimal window of caloric restriction onset limits its beneficial impact on T-cell senescence in primates. Aging Cell. 2008;7:908–919. doi: 10.1111/j.1474-9726.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger BL. The effect of a genetic variant for obesity and Type 2 Diabetes on the therapeutic potential of exercise and calorie restrictive diets in Zucker rats. Res Theory Nurs Pract. 2003;17:321–333. doi: 10.1891/rtnp.17.4.321.53194. discussion 335-8. [DOI] [PubMed] [Google Scholar]
- Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci. 2010;65:695–703. doi: 10.1093/gerona/glq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki A, Maeda S, Yoshizawa M, Misono M, Saito Y, Sasai H, Endo T, Nakata Y, Tanaka K, Ajisaka R. Effect of weight reduction with dietary intervention on arterial distensibility and endothelial function in obese men. Angiology. 2009;60:351–357. doi: 10.1177/0003319708325449. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Wong AM, Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip Top Gerontol. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- Nayak BN, Friel JK, Rempel CB, Jones PJ. Energy-restricted diets result in higher numbers of CD4+, CD8+, immunoglobulins (A, M, and G), and CD45RA cells in spleen and CD4+, immunoglobulin A, and CD45RA cells in colonic lamina propria of rats. Nutr Res. 2009;29:487–493. doi: 10.1016/j.nutres.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Niemann B, Chen Y, Issa H, Silber RE, Rohrbach S. Caloric restriction delays cardiac ageing in rats: role of mitochondria. Cardiovasc Res. 2010;88:267–276. doi: 10.1093/cvr/cvq273. [DOI] [PubMed] [Google Scholar]
- Ohneda M, Inman LR, Unger RH. Caloric restriction in obese pre-diabetic rats prevents beta-cell depletion, loss of beta-cell GLUT 2 and glucose incompetence. Diabetologia. 1995;38:173–179. doi: 10.1007/BF00400091. [DOI] [PubMed] [Google Scholar]
- Okauchi N, Mizuno A, Yoshimoto S, Zhu M, Sano T, Shima K. Is caloric restriction effective in preventing diabetes mellitus in the Otsuka Long Evans Tokushima fatty rat, a model of spontaneous non-insulin-dependent diabetes mellitus? Diabetes Res Clin Pract. 1995;27:97–106. doi: 10.1016/0168-8227(95)01029-d. [DOI] [PubMed] [Google Scholar]
- Olholm J, Paulsen SK, Cullberg KB, Richelsen B, Pedersen SB. Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int J Obes (Lond) 2010;34:1546–1553. doi: 10.1038/ijo.2010.98. [DOI] [PubMed] [Google Scholar]
- Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011 doi: 10.1016/j.febslet.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opalach K, Rangaraju S, Madorsky I, Leeuwenburgh C, Notterpek L. Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation Res. 2010;13:65–74. doi: 10.1089/rej.2009.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmeyer HK, Bodkin NL, Hansen BC. Chronic calorie restriction alters glycogen metabolism in rhesus monkeys. Obes Res. 1994;2:549–555. doi: 10.1002/j.1550-8528.1994.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- Ozdemir BH, Ozdemir AA, Erdal R, Ozdemir FN, Haberal M. Rapamycin Prevents Interstitial Fibrosis in Renal Allografts through Decreasing Angiogenesis and Inflammation. Transplant Proc. 2011;43:524–526. doi: 10.1016/j.transproceed.2011.01.080. [DOI] [PubMed] [Google Scholar]
- Page SH. A comparative review. New York: Karger; 1982. Periodontitis in man and other animals; pp. 208–212. [Google Scholar]
- Pahlavani MA, Harris MD, Richardson A. The increase in the induction of IL-2 expression with caloric restriction is correlated to changes in the transcription factor NFAT. Cell Immunol. 1997;180:10–19. doi: 10.1006/cimm.1997.1155. [DOI] [PubMed] [Google Scholar]
- Palacios C, Joshipura K, Willett W. Nutrition and health: guidelines for dental practitioners. Oral Dis. 2009;15:369–381. doi: 10.1111/j.1601-0825.2009.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona R, Requena JR, Portero-Otin M, Prat J, Thorpe SR, Bellmunt MJ. Carboxymethylated phosphatidylethanolamine in mitochondrial membranes of mammals--evidence for intracellular lipid glycoxidation. Eur J Biochem. 1998;255:685–689. doi: 10.1046/j.1432-1327.1998.2550685.x. [DOI] [PubMed] [Google Scholar]
- Park SY, Choi GH, Choi HI, Ryu J, Jung CY, Lee W. Calorie restriction improves whole-body glucose disposal and insulin resistance in association with the increased adipocyte-specific GLUT4 expression in Otsuka Long-Evans Tokushima fatty rats. Arch Biochem Biophys. 2005;436:276–284. doi: 10.1016/j.abb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- Peck MD, Babcock GF, Alexander JW. The role of protein and calorie restriction in outcome from Salmonella infection in mice. JPEN J Parenter Enteral Nutr. 1992;16:561–565. doi: 10.1177/0148607192016006561. [DOI] [PubMed] [Google Scholar]
- Pischon N, Heng N, Bernimoulin JP, Kleber BM, Willich SN, Pischon T. Obesity, inflammation, and periodontal disease. J Dent Res. 2007;86:400–409. doi: 10.1177/154405910708600503. [DOI] [PubMed] [Google Scholar]
- Reynolds MA, Dawson DR, Novak KF, Ebersole JL, Gunsolley JC, Branch-Mays GL, Holt SC, Mattison JA, Ingram DK, Novak MJ. Effects of caloric restriction on inflammatory periodontal disease. Nutrition. 2009;25:88–97. doi: 10.1016/j.nut.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann N Y Acad Sci. 2001;928:305–315. doi: 10.1111/j.1749-6632.2001.tb05660.x. [DOI] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Sakamoto M. Obesity and periodontitis. N Engl J Med. 1998;339:482–483. doi: 10.1056/NEJM199808133390717. [DOI] [PubMed] [Google Scholar]
- Salas-Salvado J, Bullo M, Garcia-Lorda P, Figueredo R, Del Castillo D, Bonada A, Balanza R. Subcutaneous adipose tissue cytokine production is not responsible for the restoration of systemic inflammation markers during weight loss. Int J Obes (Lond) 2006;30:1714–1720. doi: 10.1038/sj.ijo.0803348. [DOI] [PubMed] [Google Scholar]
- Santos MS, Lichtenstein AH, Leka LS, Goldin B, Schaefer EJ, Meydani SN. Immunological effects of low-fat diets with and without weight loss. J Am Coll Nutr. 2003;22:174–182. doi: 10.1080/07315724.2003.10719291. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Higashi Y, Nakagawa K, Kimura M, Noma K, Hara K, Matsuura H, Goto C, Oshima T, Chayama K. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am J Hypertens. 2002;15:302–309. doi: 10.1016/s0895-7061(01)02322-6. [DOI] [PubMed] [Google Scholar]
- Scheiwiller E, Guler HP, Merryweather J, Scandella C, Maerki W, Zapf J, Froesch ER. Growth restoration of insulin-deficient diabetic rats by recombinant human insulin-like growth factor I. Nature. 1986;323:169–171. doi: 10.1038/323169a0. [DOI] [PubMed] [Google Scholar]
- Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- Selman C, Kerrison ND, Cooray A, Piper MD, Lingard SJ, Barton RH, Schuster EF, Blanc E, Gems D, Nicholson JK, Thornton JM, Partridge L, Withers DJ. Coordinated multitissue transcriptional and plasma metabonomic profiles following acute caloric restriction in mice. Physiol Genomics. 2006;27:187–200. doi: 10.1152/physiolgenomics.00084.2006. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 Interactions with p300 Modulate Receptor Activator of NF-{kappa}B Ligand (RANKL) Activation of NF-{kappa}B Signaling and Inhibit Osteoclastogenesis in Bone-derived Cells. J Biol Chem. 2011;286:11492–11505. doi: 10.1074/jbc.M110.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibolet O, Alper R, Avraham Y, Berry EM, Ilan Y. Immunomodulation of experimental colitis via caloric restriction: role of Nk1.1+ T cells. Clin Immunol. 2002;105:48–56. doi: 10.1006/clim.2002.5260. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39:285–296. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. Unlocking the secrets of longevity genes. Sci Am. 2006;294:48–51. 54–57. doi: 10.1038/scientificamerican0306-48. [DOI] [PubMed] [Google Scholar]
- Skrha J, Kunesova M, Hilgertova J, Weiserova H, Krizova J, Kotrlikova E. Short-term very low calorie diet reduces oxidative stress in obese type 2 diabetic patients. Physiol Res. 2005;54:33–39. doi: 10.33549/physiolres.930584. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. "Checkerboard" DNA-DNA hybridization. Biotechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- Sola E, Jover A, Lopez-Ruiz A, Jarabo M, Vaya A, Morillas C, Gomez-Balaguer M, Hernandez-Mijares A. Parameters of inflammation in morbid obesity: lack of effect of moderate weight loss. Obes Surg. 2009;19:571–576. doi: 10.1007/s11695-008-9772-8. [DOI] [PubMed] [Google Scholar]
- Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A, Matsui T. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299:C1256–C1266. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- Stapleton PP, Fujita J, Murphy EM, Naama HA, Daly JM. The influence of restricted calorie intake on peritoneal macrophage function. Nutrition. 2001;17:41–45. doi: 10.1016/s0899-9007(00)00502-5. [DOI] [PubMed] [Google Scholar]
- Stepkowski SM, Chen H, Daloze P, Kahan BD. Rapamycin, a potent immunosuppressive drug for vascularized heart, kidney, and small bowel transplantation in the rat. Transplantation. 1991;51:22–26. doi: 10.1097/00007890-199101000-00002. [DOI] [PubMed] [Google Scholar]
- Stojanovic S, Sprinz H, Brede O. Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch Biochem Biophys. 2001;391:79–89. doi: 10.1006/abbi.2001.2388. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Sung B, Park S, Yu BP, Chung HY. Modulation of PPAR in aging, inflammation, and calorie restriction. J Gerontol A Biol Sci Med Sci. 2004;59:997–1006. doi: 10.1093/gerona/59.10.b997. [DOI] [PubMed] [Google Scholar]
- Swindell WR. Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genomics. 2009;10:585. doi: 10.1186/1471-2164-10-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49:491–516. doi: 10.1016/j.cden.2005.03.001. v. [DOI] [PubMed] [Google Scholar]
- Ugochukwu NH, Figgers CL. Dietary caloric restriction improves the redox status at the onset of diabetes in hepatocytes of streptozotocin-induced diabetic rats. Chem Biol Interact. 2007;165:45–53. doi: 10.1016/j.cbi.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Uskokovic A, Arambasic J, Bogojevic D, Ivanovic-Matic S, Mihailovic M, Dinic S, Grigorov I. Differences between molecular mechanisms involved in the regulation of haptoglobin gene expression during the acute phase response and dietary restriction. Folia Biol (Praha) 2009;55:107–115. [PubMed] [Google Scholar]
- Vega VL, De Cabo R, De Maio A. Age and caloric restriction diets are confounding factors that modify the response to lipopolysaccharide by peritoneal macrophages in C57BL/6 mice. Shock. 2004;22:248–253. doi: 10.1097/01.shk.0000133590.09659.a1. [DOI] [PubMed] [Google Scholar]
- Viguerie N, Poitou C, Cancello R, Stich V, Clement K, Langin D. Transcriptomics applied to obesity and caloric restriction. Biochimie. 2005;87:117–123. doi: 10.1016/j.biochi.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Viljanen AP, Karmi A, Borra R, Parkka JP, Lepomaki V, Parkkola R, Lautamaki R, Jarvisalo M, Taittonen M, Ronnemaa T, Iozzo P, Knuuti J, Nuutila P, Raitakari OT. Effect of caloric restriction on myocardial fatty acid uptake, left ventricular mass, and cardiac work in obese adults. Am J Cardiol. 2009;103:1721–1726. doi: 10.1016/j.amjcard.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Floyd ZE, Qin J, Liu X, Yu Y, Zhang XH, Wagner JD, Cefalu WT. Modulation of skeletal muscle insulin signaling with chronic caloric restriction in cynomolgus monkeys. Diabetes. 2009;58:1488–1498. doi: 10.2337/db08-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman A. Prevention of chronic diseases: WHO global strategy on diet, physical activity and health. Food Nutr Bull. 2003;24:281–284. doi: 10.1177/156482650302400306. [DOI] [PubMed] [Google Scholar]
- Wheeler ML, Delahanty L, Wylie-Rosett J. Diet and exercise in noninsulin-dependent diabetes mellitus: implications for dietitians from the NIH Consensus Development Conference. J Am Diet Assoc. 1987;87:480–485. [PubMed] [Google Scholar]
- Whitmore C. Type 2 diabetes and obesity in adults. Br J Nurs. 2010;19:880, 882–886. doi: 10.12968/bjon.2010.19.14.49041. [DOI] [PubMed] [Google Scholar]
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994;17:30–36. doi: 10.2337/diacare.17.1.30. [DOI] [PubMed] [Google Scholar]
- Witters LA. The blooming of the French lilac. J Clin Invest. 2001;108:1105–1107. doi: 10.1172/JCI14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jiang C, Shen Q, Hu Y. Systematic gene expression profile of hypothalamus in calorie-restricted mice implicates the involvement of mTOR signaling in neuroprotective activity. Mech Ageing Dev. 2009;130:602–610. doi: 10.1016/j.mad.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Wycherley TP, Brinkworth GD, Noakes M, Buckley JD, Clifton PM. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10:1062–1073. doi: 10.1111/j.1463-1326.2008.00863.x. [DOI] [PubMed] [Google Scholar]
- Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults--a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2009;64:90–95. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY) 2010;2:361–368. doi: 10.18632/aging.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen GC, Chen YC, Chang WT, Hsu CL. Effects of polyphenolic compounds on tumor necrosis factor-alpha (TNF-alpha)-induced changes of adipokines and oxidative stress in 3T3-L1 adipocytes. J Agric Food Chem. 2011;59:546–551. doi: 10.1021/jf1036992. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick CD, Stevens RB. Trends in Americans' food-related time use: 1975–2006. Public Health Nutr. 2010;13:1064–1072. doi: 10.1017/S1368980009992138. [DOI] [PubMed] [Google Scholar]