Abstract
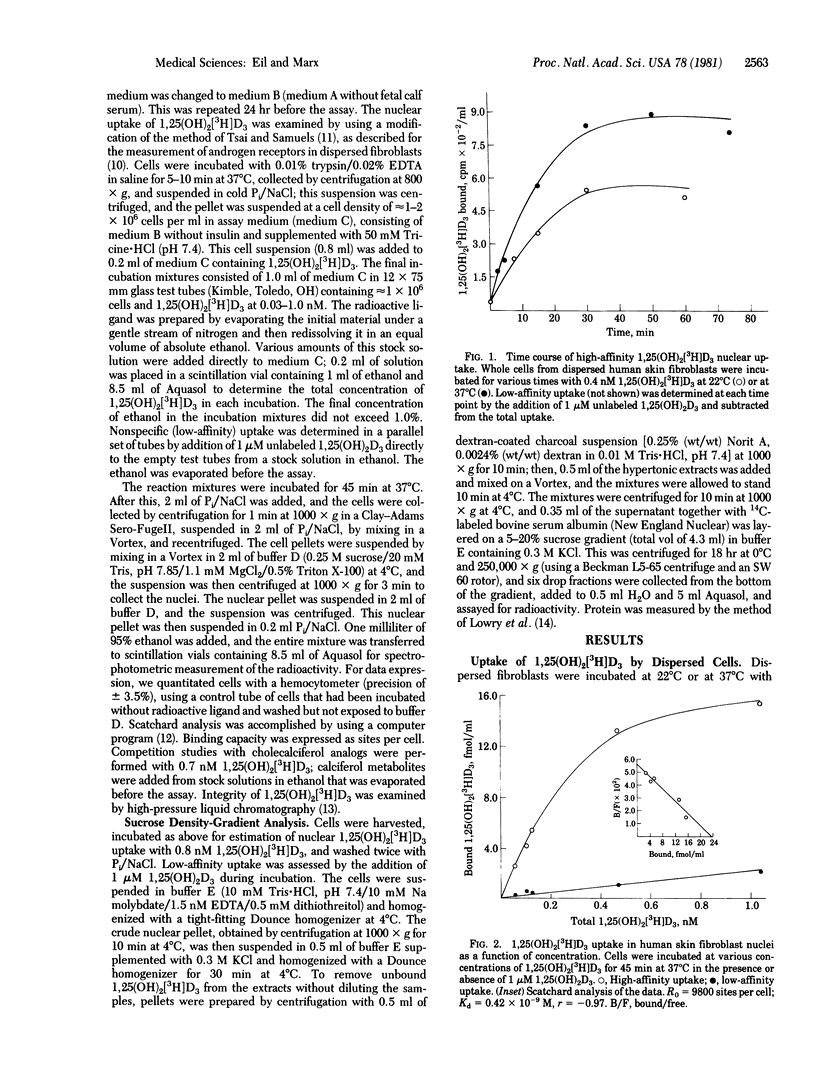
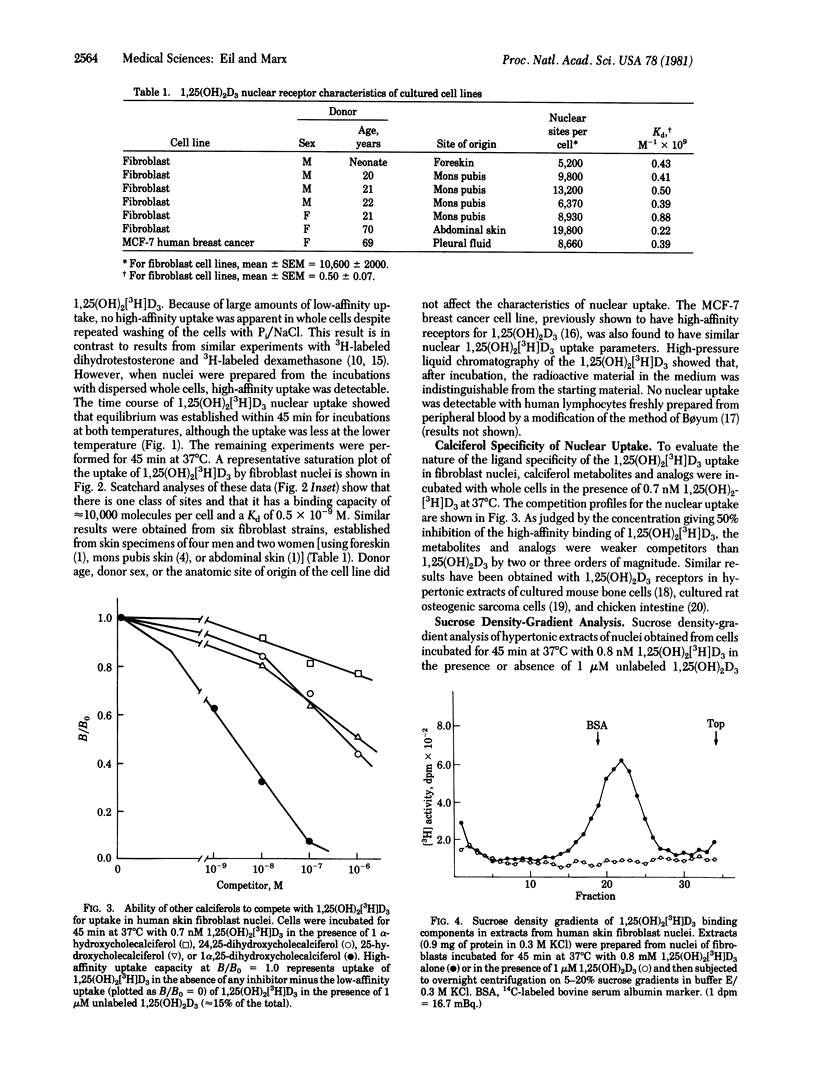
Because of the relative inaccessibility of known calciferol target tissues (i.e., intestine and bone), we examined fibroblasts derived from normal human skin and grown in tissue culture as a means of evaluating the interaction of 1,25-dihydroxycholecalciferol [1,25(OH)2D3] and its effector system. When dispersed, intact cells were used, nuclear uptake of 1,25-dihydroxy[23,24(n)3-H]cholecalciferol [1,25(OH)2[3H]D3) was temperature-dependent, optimal at 45 min at 37 degrees C, and saturable. In competition experiments with other calciferols, the 1,25(OH)2[3H]D3 uptake showed specificity indistinguishable from that reported for 1,25(OH)2D3 receptors from calciferol target tissues. Analysis of 1,25(OH)2[3H]D3 nuclear uptake in fibroblast strains from six normal adults (four male, two female) yielded an average binding capacity (R0) of 10,600 +/- 2,000 (SEM) nuclear sites per cell and an apparent dissociation contant (Kd) of 0.50 +/- 0.07 (SEM) x 10(-9) M. Donor sex, donor age, or anatomic site of origin of the cell line did not affect the characteristics of uptake. Similar nuclear uptake was demonstrable with cultured MCF-7 cells (derived from human breast cancer) when assayed in the same fashion. When hypertonic extracts of nuclei obtained from skin fibroblasts incubated with 1,25(OH)2[3H]D3 were subjected to centrifugation on sucrose gradients, a single peak of radioactivity sedimented at approximately 3 S; when excess 1,25(OH)2D3 was coincubated during the cellular uptake phase, this 3S peak was not observed. Molybdate was an essential buffer component for receptor stabilization during cell fractionation and sedimentation analysis. In summary, by using fibroblasts cultured from normal human skin, we have identified a process of nuclear uptake of 1,25(OH)2[3H]D3 with the affinity, saturability, and specificity characteristics of a steroid hormone--receptor interaction. This method should be useful in studying 1,25(OH)2D3 recept physiology in cells from normal persons as well as in cells from patients who have disorders in the responsiveness of calciferol target tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken S. C., Lippman M. E. A simple computer program for quantitation and Scatchard analysis of steroid receptor proteins. J Steroid Biochem. 1977 Jan;8(1):77–94. doi: 10.1016/0022-4731(77)90221-7. [DOI] [PubMed] [Google Scholar]
- Bernal J., Refetoff S., DeGroot L. J. Abnormalities of triiodothyronine binding to lymphocyte and fibroblast nuclei from a patient with peripheral tissue resistance to thyroid hormone action. J Clin Endocrinol Metab. 1978 Dec;47(6):1266–1272. doi: 10.1210/jcem-47-6-1266. [DOI] [PubMed] [Google Scholar]
- Brooks M. H., Bell N. H., Love L., Stern P. H., Orfei E., Queener S. F., Hamstra A. J., DeLuca H. F. Vitamin-D-dependent rickets type II. Resistance of target organs to 1,25-dihydroxyvitamin D. N Engl J Med. 1978 May 4;298(18):996–999. doi: 10.1056/NEJM197805042981804. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. I. Association of 1 alpha,25-dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem. 1974 Feb 25;249(4):1251–1257. [PubMed] [Google Scholar]
- Brumbaugh P. F., Hughes M. R., Haussler M. R. Cytoplasmic and nuclear binding components for 1alpha25-dihydroxyvitamin D3 in chick parathyroid glands. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4871–4875. doi: 10.1073/pnas.72.12.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. L., Hirst M. A., Feldman D. A receptor-like binding macromolecule for 1 alpha, 25-dihydroxycholecalciferol in cultured mouse bone cells. J Biol Chem. 1979 Aug 25;254(16):7491–7494. [PubMed] [Google Scholar]
- Christakos S., Norman A. W. Studies on the mode of action of calciferol. XVIII. Evidence for a specific high affinity binding protein for 1,25 dihydroxyvitamin D3 in chick kidney and pancreas. Biochem Biophys Res Commun. 1979 Jul 12;89(1):56–63. doi: 10.1016/0006-291x(79)90942-2. [DOI] [PubMed] [Google Scholar]
- Colston K., Hirt M., Feldman D. Organ distribution of the cytoplasmic 1,25-dihydroxycholecalciferol receptor in various mouse tissues. Endocrinology. 1980 Dec;107(6):1916–1922. doi: 10.1210/endo-107-6-1916. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. The vitamin D system in the regulation of calcium and phosphorus metabolism. Nutr Rev. 1979 Jun;37(6):161–193. doi: 10.1111/j.1753-4887.1979.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Eil C., Lippman M. E., Loriaux D. L. A dispersed-whole cell method for the determination of androgen receptors in human skin fibroblasts. Steroids. 1980 Apr;35(4):389–404. doi: 10.1016/0039-128x(80)90140-3. [DOI] [PubMed] [Google Scholar]
- Eisman J. A., Martin T. J., MacIntyre I., Moseley J. M. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979 Dec 22;2(8156-8157):1335–1336. doi: 10.1016/s0140-6736(79)92816-2. [DOI] [PubMed] [Google Scholar]
- Feldman D., Chen T., Hirst M., Colston K., Karasek M., Cone C. Demonstration of 1,25-dihydroxyvitamin D3 receptors in human skin biopsies. J Clin Endocrinol Metab. 1980 Dec;51(6):1463–1465. doi: 10.1210/jcem-51-6-1463. [DOI] [PubMed] [Google Scholar]
- Gallagher J. C., Riggs B. L., Eisman J., Hamstra A., Arnaud S. B., DeLuca H. F. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest. 1979 Sep;64(3):729–736. doi: 10.1172/JCI109516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E., Wilson J. D. The syndromes of androgen resistance. N Engl J Med. 1980 Jan 24;302(4):198–209. doi: 10.1056/NEJM198001243020404. [DOI] [PubMed] [Google Scholar]
- Hackney J. F., Gross S. R., Aronow L., Pratt W. B. Specific glucocorticoid-binding macromolecules from mouse fibroblasts growing in vitro. A possible steroid receptor for growth inhibition. Mol Pharmacol. 1970 Sep;6(5):500–512. [PubMed] [Google Scholar]
- Haddad J. G., Birge S. J. Widespread, specific binding of 25-hydroxycholecalciferol in rat tissues. J Biol Chem. 1975 Jan 10;250(1):299–303. [PubMed] [Google Scholar]
- Haussler M. R., McCain T. A. Basic and clinical concepts related to vitamin D metabolism and action (first of two parts). N Engl J Med. 1977 Nov 3;297(18):974–983. doi: 10.1056/NEJM197711032971804. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Jose M., Yamada S., DeLuca H. F. A specific high-affinity binding macromolecule for 1,25-dihydroxyvitamin D3 in fetal bone. Science. 1977 Sep 9;197(4308):1086–1088. doi: 10.1126/science.887939. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Yamada S., Schnoes H. K., DeLuca H. F. Specific cytosol-binding protein for 1,25-dihydroxyvitamin D3 in rat intestine. J Biol Chem. 1977 Jul 10;252(13):4501–4505. [PubMed] [Google Scholar]
- Kream B. E., Yamada S., Schnoes H. K., DeLuca H. F. Specific cytosol-binding protein for 1,25-dihydroxyvitamin D3 in rat intestine. J Biol Chem. 1977 Jul 10;252(13):4501–4505. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laouari D., Pavlovitch H., Deceneux G., Balsan S. A vitamin D-dependent calcium-binding protein in rat skin. FEBS Lett. 1980 Mar 10;111(2):285–289. doi: 10.1016/0014-5793(80)80811-8. [DOI] [PubMed] [Google Scholar]
- Liberman U. A., Samuel R., Halabe A., Kauli R., Edelstein S., Weisman Y., Papapoulos S. E., Clemens T. L., Fraher L. J., O'Riordan J. L. End-organ resistance to 1,25-dihydroxycholecalciferol. Lancet. 1980 Mar 8;1(8167):504–506. doi: 10.1016/s0140-6736(80)92763-4. [DOI] [PubMed] [Google Scholar]
- Manolagas S. C., Haussler M. R., Deftos L. J. 1,25-Dihydroxyvitamin D3 receptor-like macromolecule in rat osteogenic sarcoma cell lines. J Biol Chem. 1980 May 25;255(10):4414–4417. [PubMed] [Google Scholar]
- Marx S. J., Spiegel A. M., Brown E. M., Gardner D. G., Downs R. W., Jr, Attie M., Hamstra A. J., DeLuca H. F. A familial syndrome of decrease in sensitivity to 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 1978 Dec;47(6):1303–1310. doi: 10.1210/jcem-47-6-1303. [DOI] [PubMed] [Google Scholar]
- Peterson E. W., Ghazarian J. G., Garancis J. C. Mixed-function oxidases of 25-hydroxycholecalciferol in isolated chick kidney glomeruli: evidence for nuclear localization. Calcif Tissue Int. 1981;33(1):19–25. doi: 10.1007/BF02409408. [DOI] [PubMed] [Google Scholar]
- Rosen J. F., Fleischman A. R., Finberg L., Hamstra A., DeLuca H. F. Rickets with alopecia: an inborn error of vitamin D metabolism. J Pediatr. 1979 May;94(5):729–735. doi: 10.1016/s0022-3476(79)80139-0. [DOI] [PubMed] [Google Scholar]
- Simpson R. U., DeLuca H. F. Characterization of a receptor-like protein for 1,25-dihydroxyvitamin D3 in rat skin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5822–5826. doi: 10.1073/pnas.77.10.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockalosky J. J., Ulstrom R. A., DeLuca H. F., Brown D. M. Vitamin D--resistant rickets: end-organ unresponsiveness to 1,25(OH)2D3. J Pediatr. 1980 Apr;96(4):701–703. doi: 10.1016/s0022-3476(80)80748-7. [DOI] [PubMed] [Google Scholar]
- Stumpf W. E., Sar M., Reid F. A., Tanaka Y., DeLuca H. F. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979 Dec 7;206(4423):1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- Tsai J. S., Samuels H. H. Thyroid hormone action: demonstration of putative nuclear receptors in human lymphocytes. J Clin Endocrinol Metab. 1974 May;38(5):919–922. doi: 10.1210/jcem-38-5-919. [DOI] [PubMed] [Google Scholar]
- Wecksler W. R., Mason R. S., Norman A. W. Specific cytosol receptors for 1,25-dihydroxyvitamin D3 in human intestine. J Clin Endocrinol Metab. 1979 Apr;48(4):715–717. doi: 10.1210/jcem-48-4-715. [DOI] [PubMed] [Google Scholar]


