Abstract
Indirect resource competition and interference are widely occurring mechanisms of interspecific interactions. We have studied the seasonal expression of these two interaction types within a two-species, boreal small mammal system. Seasons differ by resource availability, individual breeding state and intraspecific social system. Live-trapping methods were used to monitor space use and reproduction in 14 experimental populations of bank voles Myodes glareolus in large outdoor enclosures with and without a dominant competitor, the field vole Microtus agrestis. We further compared vole behaviour using staged dyadic encounters in neutral arenas in both seasons. Survival of the non-breeding overwintering bank voles was not affected by competition. In the spring, the numbers of male bank voles, but not of females, were reduced significantly in the competition populations. Bank vole home ranges expanded with vole density in the presence of competitors, indicating food limitation. A comparison of behaviour between seasons based on an analysis of similarity revealed an avoidance of costly aggression against opponents, independent of species. Interactions were more aggressive during the summer than during the winter, and heterospecific encounters were more aggressive than conspecific encounters. Based on these results, we suggest that interaction types and their respective mechanisms are not either–or categories and may change over the seasons. During the winter, energy constraints and thermoregulatory needs decrease direct aggression, but food constraints increase indirect resource competition. Direct interference appears in the summer, probably triggered by each individual’s reproductive and hormonal state and the defence of offspring against conspecific and heterospecific intruders. Both interaction forms overlap in the spring, possibly contributing to spring declines in the numbers of subordinate species.
Keywords: Rodents, Aggression, Seasonality, Space use, Winter biology
Introduction
Competition is commonly considered to be the primary explanation for observed patterns in ecology and evolutionary theory (for reviews: Connell 1980, 1983; Schoener 1983; Gurevitch et al. 1992; Schluter 2001; Eccard and Ylönen 2003a). However, the role of interspecific competition may depend on the type of competitive interaction (Morris 1999). One type, exploitative competition, involves indirect negative interactions arising from the use of a common resource (e.g. Case and Gilpin 1974). In contrast, interference, an other interaction type, involves direct negative interactions arising from territoriality, overgrowth, predation or chemical competition (Schoener 1983), where consumers alter other’s ability to exploit the resource at any level of abundance (e.g. Vance 1984).
Classical theories on interspecific competition have focused on resource exploitation, neglecting the theoretical implications of interference (Amarasekare and Nisbet 2001; Amarasekare 2002). This neglect contrasts with the ubiquity of interference competition in nature. For example, territoriality between individuals of different species and other aggressive behaviours (Walls 1990; Kennedy and White 1996), allelopathy (e.g. Nilsson 1994), overgrowth (Connell 1961) and the killing of young (Leving and Franks 1982; Polis et al. 1989) occur in a wide variety of taxa, from invertebrates to mammals, including many invasive species (Case et al. 1994; Huenneke and Thomson 1995; Harris 2006).
However, the direction of behavioural dominance can differ among life history stages of the same species-pairs (Walls 1990), and the type of competition can change with changes in resource abundances and reproductive state (Harris 2006). Many environments are seasonal, and seasons differ in terms of resource availability. Most organisms reduce breeding activities during the season when resource availability declines (winter or periodical dry seasons), while ecological studies often focus on the reproductive season since it is important for population growth. However, information on over-winter survival and the onset of spring breeding is also essential in the study of population dynamics as determinants of the seasonal propagule of new breeding populations (e.g. Eccard and Ylönen 2001). In addition, little is currently known on the nature of and effects of interspecific interactions during non-breeding season.
In animal communities, species often avoid the detrimental effects of competition by segregation—either in time or in space (Rosenzweig 1995; Morris 1999). Consequently, the fitness costs of coexistence in mixed species communities that may have historically led to segregation are difficult to study, prevented by the “ghost of competition past” (Connell 1980). Experimental studies forcing situations of coexistence of probable competitor species offer a tool to study both mechanisms of competition and density-dependent processes (Eccard and Ylönen 2003a). For a number of years our group has studied competitive interactions over several breeding seasons in a system with two microtine vole species, the bank vole (Myodes glareolus) and the field vole (Mirotus agrestis) (Eccard and Ylönen 2002, 2003a, b, 2007; Eccard et al. 2002). In these studies, we observed that the presence of field voles decreased space use and the survival of bank voles. The nature of the interspecific competition between species was evidenced by the type of life history trait affected: litter size and body condition and sensitivity to food competition were not affected, while bank vole mortality increased with the density of field voles (Eccard and Ylönen 2002, 2007)—although only for territorial breeders. Year-born breeders suffered a greater reduction in survival compared to over-wintering breeders (Eccard and Ylönen 2003b) or year-born immatures (Eccard et al. 2002). Taken together, the results of these studies indicate that the detrimental effects of interaction were mainly due to interference among field voles and the youngest cohort of adult breeding bank voles.
In nature these two clearly competing vole species coexist in sympatry, but we know very little about the mechanisms and dynamics of their coexistence. Our earlier studies may have shed some light on the interactions during the breeding season, but interaction types may change during the non-reproductive season, which is far longer than the breeding season in our latitudes, due to changes in many factors, such as individual reproductive state and energy needs during periods of low temperature and resource limitation.
In the study reported here we investigated seasonal changes in the behaviour, space use, survival and reproduction of bank vole females with or without the presence of field voles. Data on survival, breeding and space use were gathered in overwintering populations in two independent years, and data on behavioural interactions were collected during one winter and one summer within the study period.
Experimental populations were settled in large outdoor enclosures. The main focus of our behavioural studies was the expression of and allocation to different interactive behaviours between opponent species and between seasons. We hypothesised that seasonal differences in reproductive status and social systems would result in different interaction types. During the winter, the interactions should be limited by low survival rates and high energetic needs. In contrast, during the summer, the interactions should be direct and aggressive, since individuals should aim at improving reproductive success and their own survival as well as that of their offspring without energetic restrictions. The predictions for the different individual measures are summarised in Table 1 (see also for sources). Space use, for example, should be larger if animals compete indirectly for resources because the range for gathering food increases, but it should be smaller if animals interfere aggressively and try to avoid each other—but without resource competition. Survival should decrease in the case of aggressive interference among breeding animals because of the stressful aggressive interactions, but this should not affect the non-breeders since they do not engage in aggression. If species compete for food, survival should decrease for all functional categories independent of their breeding state since all species need food. Condition measures, such as the size of the adult or offspring, should differ if animals compete for food, but they should not differ if animals interfere and try to avoid each other. Behavioural aggression among species should be observable only between adults in the breeding season in the case of interspecific interference, but non-breeders should not be affected. In the case of resource competition, the aggressive behaviour of both categories should not change.
Table 1.
Expected effects of different interspecific interaction types on individual measures of the bank vole life history variables monitored
| Measure | Effect by interactive mechanism | |||
|---|---|---|---|---|
| Direct interference | Indirect resource competition | |||
| Breeding population | Non-breeding population | Breeding population | Non-breeding population | |
| Space use | Decrease (1, 2, 3) | No effect (H) | Increase (6, H) | Increase (H) |
| Survival | Decrease (1, 2, 3) | No effect (1, 4) | Decrease (H) | Decrease (H) |
| Reproduction (breeding)/maturation (not-breeding) | Decrease (1, 2, 3) | Decrease (H) | Decrease (H) | Decrease (H) |
| Offspring size, litter size | Not affected (1–4) | – | Decrease (H) | – |
| Adult size, condition | Not affected (1–3) | No effect (4) | Decrease (H) | Decrease (H) |
| Aggression | Increase (H) | No effect (H) | No effect (H) | No effect (H) |
Materials and methods
Species
Myodes and Microtus voles are common genera of the Northern hemisphere. Microtus species are mostly found in grassland habitats, whereas Myodes species are generally considered to be woodland inhabitants. However, on islands where only one genus is present, it is often found in the habitat usually occupied by the other genus (Cameron 1964). The bank vole Myodes glareolus prefers forest edges, old fields and grassland over spruce forests in the absence of competitors (Myllymäki 1977; Hansson 1983; Ylönen et al. 1988). In fragmented boreal landscapes, considerable habitat overlap occurs between bank voles and Microtus agrestis, the field vole, especially in clearcuts and abandoned fields (Henttonen et al. 1977; Myllymäki 1977). Habitat overlap increases at high population densities (Grant 1969; Iverson and Turner 1972; Henttonen and Hansson 1984). As the food niches of the two species overlap and the potential amount of winter food should decrease over the winter, competition for resources is likely to become more severe (Larsson and Hansson 1977).
Enclosures and populations
Data were collected on voles kept in eight outdoor enclosures at the Konnevesi Research Station, Central Finland (62°37′N, 26°20′E) during the 1998/1999 and 1999/2000 winters and the summers of 1999, 2000 and 2001. The enclosures were 50 × 50 m (0.25 ha) in size each and consisted of old field habitat with willow and alder bushes. Snow covered the study area from the beginning of December until the end of April. In each enclosure 25 multiple capture live traps (Uglan, Grahn AB, Sweden) were distributed in a regular grid with a trap distance 10 m. Traps were sheltered by snow chimneys (40 × 40 × 50 cm) made of galvanised metal sheets that were in place before the first snow fall so that voles were trappable in the emerging subnivean environment.
In the first winter, four enclosures were assigned to the control treatment (bank voles only) and four enclosures to the competition treatment (bank voles and field voles). In the second winter, two enclosures were assigned to the bank vole control treatment (bank voles only), two to the field vole control treatment (field voles only, not further reported here because of a low sample size) and four to the competition treatment.
The experimental set-up used was additive (Connell 1983), i.e. populations were compared in the presence and absence of competitors. This particular set-up was necessary to determine whether or not the species actually affect each other, and if yes, which variables are affected. The set-up does not allow the researcher to distinguish between effects of intra- and interspecific competition, which would better be tested in a replacement series (for review: Connell 1983) in which the absolute density has to be kept constant. By applying logistic restrictions we had to limit ourselves to the former research question and respective additive set-up. In species with different social systems, such as territorial bank voles and kin-clustering field voles, we suspected that density in terms of individuals or biomass would have less effect than density in terms of occupied space, a concept that has not yet been sufficiently tested. However, these questions were beyond the scope of this winter experiment (but see also Eccard and Ylönen 2007).
Winter populations were created in October by releasing five to eight adult but immature bank vole females and three to five bank vole males to each enclosure. This density of immature females (i.e. 20–30 females/ha in October) was moderate compared to the 42 females/ha counted in October of a peak year in an enclosed grassland population in Finland that was allowed to fluctuate and grow freely over many seasons. The maximum density of mature females in that population had been 20 females/ha during June (Ylönen et al. 1988). The bank voles used in our study were offspring from wild captured voles kept in a laboratory colony and ear-tagged to allow individual recognition. They were monitored during November and December, and if found missing were replaced by new animals. Ten additional field voles were also released to the competition enclosures in October.
Winter populations were live-trapped for one or two nights once a month from December to February and weekly from March onwards until mid May. Oats were used as bait, and the traps were lined with hay for insulation. Upon capture, identity of the vole, trap location, weight and reproductive state were recorded. Immediately after inspection, the voles were released at the capture site. After each trapping series, the remaining bait was removed.
Bank vole survival was easy to monitor due to their high trappability (Ylönen and Viitala 1991). Field voles were more trap-shy than bank voles and tended to lose their ear tags. We reconstructed their identity (and subsequently their density) by combining data on recaptures, location data, sex and individual weights. All animals were removed from the enclosures after the experiment. The numbers of field voles in each enclosure varied from five to 15.
Breeding state was determined by an examination of external characteristics. In the wintering condition, males have abdominal testes and females have closed vaginas. When entering breeding condition, male testes become scrotal and visible; in females, an open vagina indicates the onset of oestrus. Pregnancy in females was detected visually or by weight increase.
The sizes of the home range were estimated by calculating the ranges as minimum convex polygons using “Ranges V” (Kenward and Hodder 1998) for each individual’s capture locations. With trap–grid data, the absolute number of possible locations is low, and animals are trapped at artificial points of interest. The absolute value of the home range size therefore has little ecological meaning compared to values obtained by radio-tracking data. However, by using a constant estimator of spatial behaviour, comparisons among treatments are possible. Individual home ranges were averaged over populations by sex and breeding state of the individual (i.e. for each population we had four values: non-breeding females, non-breeding males, breeding females, breeding males) and analysed with repeated measures for the effects of sex and breeding state as a within-population effect, and for the effects of density of bank voles per population (we used the mid-experiment density of vole individuals of both sexes and both species in March as a representative covariate for density) as between-subject effects. We did not use spatial data from enclosures in which the population became extinct early in the experiment because low numbers of captures decrease the estimates of the home range size (Kenward and Hodder 1998).
We used the population as the unit of observation and therefore calculated the proportions surviving, proportions reproducing and population means of space use variables.
Behaviour: dyadic encounter trials in the field
Female bank voles’ behaviour, either with a bank vole or a field vole in the same arena, was observed in staged dyadic encounter trials by a single observer (Fey) in populations during the 1999/2000 winter and in (different) populations during summer of 2001. Staged dyadic encounters are an approved tool to approach to the aggressive behaviour of voles and lemmings (Harper and Batzli 1997). We targeted focal animals by setting the most commonly used traps for these individual animals early in the morning and then checking the traps after 2–4 h. Upon capture, an opponent was presented to the focal female at the point of capture in a clean arena (macrolon standard cage 42 × 28 cm with an extension of wall height to 30 cm) for 10 min. The opponent was either an unknown female bank vole or an unknown female field vole which, for both species, was taken from the laboratory colony. We conducted 90 staged dyadic encounters with bank vole females from the enclosed populations (43 in winter and 47 in summer) so that each female was tested at least twice against either a conspecific or heterospecific opponent. In winter, with few animals and a long experimental duration, some individuals were tested several times. We averaged encounters over the individual to avoid pseudo-replication. For analyses between enclosures, we averaged values of individuals for each population over season and opponent type (i.e. two values per population: bank voles tested with conspecific bank vole, bank vole tested with heterospecific field vole). Each population was also assigned to a treatment (control vs. competition enclosure).
After each 10-s-interval all behaviours in that interval were recorded, resulting in data representing the number of intervals with a certain behaviour type for each dyadic encounter. We distinguished between 11 behavioural types (Ims 1987). The most common behaviour was sitting, which occurred in all encounters, followed by explore, approach, touch and threat in one-half or more of encounters. Extreme amicable behaviours, such as huddle, or aggressive ones, such as attack, fight, flee and chase, were rare and seen in fewer than 21% of encounters (for descriptives see Table 2).
Table 2.
Descriptive statistics for 11 behaviours of bank vole females observed in 90 staged dyadic encounters against bank vole and field vole opponents
| Behaviour | Number of encounters >0 | Mean count | Minimum | Maximum |
|---|---|---|---|---|
| Sit | 90 | 55.7 | 42 | 60 |
| Explore | 73 | 10.1 | 0 | 40 |
| Approach | 60 | 3.0 | 0 | 19 |
| Threat | 59 | 3.3 | 0 | 25 |
| Touch | 49 | 1.1 | 0 | 8 |
| Avoid | 43 | 1.6 | 0 | 11 |
| Attack | 19 | 0.3 | 0 | 3 |
| Flee | 19 | 0.4 | 0 | 4 |
| Fight | 11 | 0.3 | 0 | 11 |
| Huddle | 2 | 0.1 | 0 | 7 |
| Chase | 2 | 0.0 | 0 | 1 |
Behavioural types are ordered by frequency of appearance. In contrast to the statistical analysis in the text, which is based on population means, the descriptive statistics presented in the table are based on individuals (mean results for animals that were tested more than once)
Behavioural data were characterised by the high prevalence of zeros, because not all types of behaviors occurred in every encounter. Data were thus highly skewed to the right and consequently lacked normality (Table 2). We analysed the behavioural data using a non-parametric multivariate approach, based on ordination of the data.
In this analysis of similarity (ANOSIM), a similarity matrix is compiled, calculating a similarity index for each pair of samples. As a measure of similarity we used the Bray–Curtis Similarity Index (Clarke et al. 2006) of the transformed [log (x + 1)] data. We statistically compared a priori defined groups [here season (winter or summer), opponent (bank vole or field vole), and treatment of enclosure (control or competition)] by calculating a non-parametric analysis of similarities permutation test (ANOSIM; Clarke 1993). In the case of group separation (significant differences between groups), we determined the discrimination variables with the similarity percentage (SIMPER) routine. Non-metric multidimensional scaling (nMDS) based on the Bray–Curtis similarity matrix was used to visualise the relationship of samples. All multivariate analyses (ANOSIM, nMDS and SIMPER) were done using PRIMER 6.1.12 (Primer-E Ltd, Plymouth, UK).
Results
Bank vole populations over winter
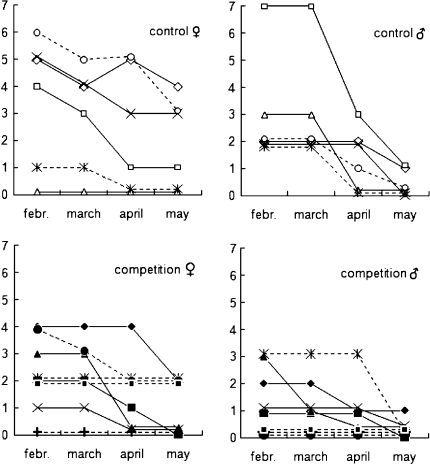
Over the winter the number of viable bank vole populations (containing at least one female) fell, independent of treatment, from six to four in the control treatment and from eight to four in the competition treatment (Fisher’s exact test between treatments in May p = 0.627). However, the dynamics of this shrinking was different in the populations of both treatments and was related to the sex of the animal and the treatment [Fig. 1; 14 populations of two treatments; repeated measures ANOVA over 4 months (February–May; inner-subject effects: month F 3,36 = 21.1, p < 0.001; sex F 1,12 = 2.7, p = 0.250; interaction sex × month × treatment F 3,36 = 3.2, p = 0.034; between-subjects effect: treatment F 1,12 = 3.8, p = 0.075)]. The number of males per population from February to April [mean 2.0–1.0 ± 1.8–1.1 standard deviation (SD)] did not differ from that of females (2.8–1.8 ± 1.9–1.4; paired t test t < 1.7, p > 0.119) but was lower than the number of females in May (males 0.2 ± 0.4, females 1.4 ± 1.4, t = 3.3, p = 0.006). The number of males in competition populations in February, April and May (1.3, 0.8 and 0.1 ± 1.3, 1.0 and 0.4, respectively) was similar as that of the controls (3.0, 1.3 and 0.3 ± 2.0, 1.2, 0.5, respectively; independent t test t = −2.0, −0.9 and −0.9; p = 0.069, 0.350 and 0.386) but was lower in competition populations during March (1.0 ± 1.1) compared to the controls (3.0 ± 2.0; independent t test t = −2.4 p = 0.032). The number of females did not differ among treatments in any of the months (competition populations: mean 1.0–2.3; controls: 1.8–3.5; independent t tests separate for months t > −1.2, p > 0.245).
Fig. 1.
Number of bank vole males and females per population in enclosures over winter and spring. Control populations consisted of one species; the competition population shared the enclosure with heterospecific field voles. Solid lines populations of the first winter, broken lines independent populations of the second winter
At the end of the winter, the timing of maturation in bank voles was independent of treatment and first recorded in late March (late March, 11th–13th calendar week, females t = 0.7, p = 0.507; males t = 1.7, p = 0.120). A comparison of only enclosures where both sexes were present until April revealed that the proportion of breeding females was lower in the two competition enclosures (0 and 50%) compared to the four control enclosures (100, 100, 100 and 50%, respectively). The timing of breeding in the remaining competition enclosure (early May, 18th calendar week) was in line with that in the four control enclosures (17th–19th calendar week).
Space use
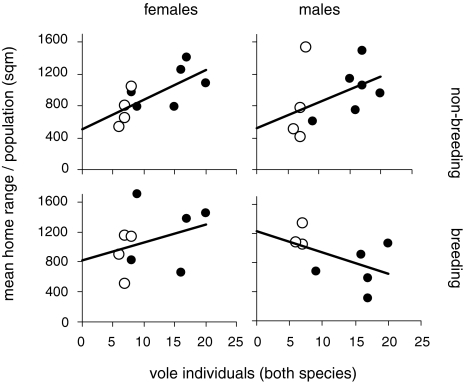
During the winter, home ranges increased with increasing number of vole individuals (both species) per population [a linear mixed model with sex and breeding state as repeat measures within an enclosure was used, which allows gaps in the data set (leading to uneven degrees of freedom) since not all functional categories always existed in each of the populations; fixed effects: sex F 1,25.2 = 0.8, p = 0.370; breeding F 1,23.4 = 0.5, p = 0.479; covariate density F 1,22.6 = 7.1, p = 0.014; Fig. 2]. Home ranges were not different between sexes or breeding states.
Fig. 2.
Space use of female (left) and male (right) bank voles in winter (non-breeding condition) and in spring (breeding condition) expressed as 100% minimum convex polygons based on population means calculated from individuals data obtained from live trapping. Open circles Control populations with bank vole populations only, filled circles competition populations with both species. Each symbol in each panel represents one population. Note that not all populations existed long enough (Fig. 1) to obtain sufficient data for all categories
The treatment effect was analysed in a separate model since treatment and total density were not independent (Fig. 2). Home range increased with the competition treatment for females but not for males (same repeat structure as above; fixed effects: sex F 1,28.2 = 0.3, p = 0.592; breeding F 1,26.7 = 0.5, p = 0.501; treatment F 1,22.1 = 0.1, p = 0.843; interaction sex × treatment F 1,22.1 = 6.4, p = 0.019). The home ranges of non-breeding females and breeding females measured 755 ± 214 and 920 ± 302 m2, respectively, without competitors, but with competitors, non-breeding and breeding females used 1,044 ± 251 and 1,200 ± 443 m2, respectively [simple treatment effects for females: breeding (repeat) F 1,12.9 = 1.2, p = 0.288; treatment F 1,13.6 = 4.9, p = 0.044). The home ranges of non-breeding and breeding males were 802 ± 507 and 1,147 ± 155 m2, respectively, without competitors, but with competitors, non-breeding and breeding males used 995 ± 311 and 701 ± 290 m2, respectively [simple treatment effects for males: breeding (repeat) F 1,14.1 = 0.1, p = 0.803; treatment F 1,10.7 = 2.1, p = 0.172].
For comparison with the winter data obtained in this study, briefly refer to our earlier results on space use in the summer, where the presence of field voles always decreased space use. In late summer, space use of reproducing females, which was initially 828 ± 86 m2 without competitors, decreased by 33% in the presence of 5–12 field voles (Fig. 1 in Eccard and Ylönen 2002; data from 16 independent populations within 1 year, analyses by age groups in Eccard and Ylönen 2003a, b). In addition, when competitor density increased to different densities, the space use of bank vole females decreased linearly from 870 m2 without competitors to 330 m2 in the presence of 30 field voles (Fig. 3b in Eccard and Ylönen 2002, 2007; n = 14 populations).
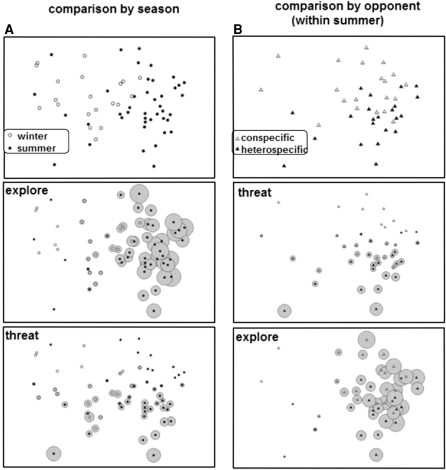
Fig. 3.
Non-metric multidimensional scaling ordinations (nMDS) of Bray–Curtis similarities of the behavioural data of bank vole females in staged dyadic encounters with other bank voles (conspecific, circles) or field voles (heterospecific, squares) during the winter (open symbols) and summer (filled symbols). Statistically separable groups (see text) were framed by hand (dashed line season; solid line opponent within summer). Each symbol represents behaviour averaged over the combination of one population with one opponent species. In nMDS, axes are arbitrary and without dimension. Note that not all populations existed long enough to obtain sufficient behavioural data (Fig. 1), while other populations are represented twice, differing by opponent types
Behaviour
The behaviour of bank vole females in the dyadic encounter trials of this study differed significantly between seasons and according to the opponent species [two-way crossed ANOSIM for values averaged over populations: Global R (season) = 0.61, p = 0.001; Global R (opponent) = 0.216, p = 0.015]. The two-dimensional ordination was good (second stress value = 0.07). Figure 3 shows that behavioural patterns of overwintering females were distinct from those in the summer and that within the summer, patterns from heterospecific encounters can be distinguished from conspecific encounters. There was no effect of the competition, therefore this factor was removed from the analyses. Qualitatively, the differences between seasons were expressed in a reduction of aggressive behaviours in winter encounters compared to summer encounters. The behavioural differences within seasons were expressed (1) as a decrease in the occurrence of behavioural categories explore, approach and avoid, which represents a decrease of activity in the winter; (2) as a decrease in the behaviours threat and avoid, both representing a decrease in defensive behaviour; (3) as an increase in touch, an amicable behaviour that was more often observed during the winter than in the summer (Table 3, Fig. 4a).
Table 3.
SIMPER (similarity percentage) analysis of behaviours of bank vole females in staged dyadic encounters against conspecific females and heterospecific (field vole females) opponents
| Behaviour | Discrimination by season | Discrimination by opponent | ||||
|---|---|---|---|---|---|---|
| Average abundance | Contribution (%) to dissimilarity | Average abundance | Contribution (%) to dissimilarity | |||
| Winter | Summer | Bank vole | Field vole | |||
| Explore | 1.2 | 2.5 | 25 | 2 | 2 | 17 |
| Approach | 0.5 | 1.4 | 17 | 1.1 | 1.1 | 11 |
| Threat | 0.9 | 1.3 | 11 | 0.7 | 1.6 | 22 |
| Touch | 0.8 | 0.5 | 9 | 0.7 | 0.5 | 11 |
| Avoid | 0.2 | 1.1 | 15 | 0.5 | 1 | 13 |
| Cumulative contribution | 77 | 74 | ||||
Results based on population means, presenting the variables mostly responsible for differences between season and opponent groups. Only behaviours are shown which contribute more than 10% to dissimilarity. Inclusion limit: major contribution (>70% of cumulative contribution to dissimilarity)
Fig. 4.
Frequency of the threat and explore behavioural categories in their order of contribution to dissimilarity (SIMPER analysis, Table 2) to dyadic encounters of bank vole females in different seasons (a) or against opponents of different species (b). Each symbol represents the behaviour of one animal, at a position according to the non-metric, multidimensional scaling plot of the Bray–Curtis similarity indices (upper panels). Larger bubbles indicate a higher frequency of the respective behavioural category in the four lower panels
The ANOSIM procedure cannot test for interaction of factors, but close study of Fig. 3 suggests an interaction between season and opponent. We therefore tested for simple effects by investigating opponent effects separately in both seasons. In the summer, we found a clear effect of the opponent species [ANOSIM: Global R (opponent) = 0.262, p = 0.018), whereas the behavioural pattern in the winter was not affected by opponent species [Global R (opponent) = −0.067, p = 0.57]. Qualitatively, the differences in summer were mainly due to increase in the defensive variables threat and avoid in encounters with field voles; further, explorative activity was higher in encounters with heterospecifics than with conspecifics (Table 3, Fig. 4b). These results suggest an increase of defensive behaviour of bank voles in encounters with field voles, probably induced by the higher aggression of field voles.
Discussion
In this study we focused on seasonal variation in interaction types and their fitness consequences in a boreal small rodent community. Over the winter, the species seemed to coexist for a long time period, but individual survival was dependent both on sex and on treatment. The survival of bank vole females was not affected by competition with field voles, which was a surprising observation since the survival of adult females in previous summer studies had always been negatively affected by the presence of competitors (Eccard and Ylönen 2002, 2003a, b, 2007). On the other hand, in one of these earlier studies, the survival of juvenile and immature females in the summer had not been affected by the presence of field voles (Eccard et al. 2002). In nature, the overwintering bank vole females are already adult but in an immature breeding state. Thus, their interaction with field voles may be more comparable to that of juvenile females during the late summer. Without the need to defend breeding territories, immature bank vole females became socially tolerant intraspecifically, but they also seemed to be able to coexist with the dominant field vole.
The survival of bank vole males in earlier studies had been reduced by field vole competition in March (Fig. 1), i.e. with the onset of their maturation (Eccard and Ylönen 2001). As the survival rate can decrease through increased contract rates with superior conspecifics (Smyth 1968; Gilbert et al. 1986), we assume that in our study the increased activity of males associated to the onset of breeding activity also increased contact rates to superior heterospecifics with consequences for mortality. Intra- and interspecific interference seemed to work in a very similar manner on the male mortality of bank voles irrespective of total density. Thus, these effects would also probably be inseparable in a constant-density set-up (i.e. the same density in different combinations of either con- or heterospecifics).
During the winter, the bank vole females in this study increased their space use with vole density, both hetero- and conspecifics (Fig. 2). Space use patterns can be resource based, with the range increasing due to food scarcity and the resultant need to secure sufficient resources for the individual (Ims 1987). The density-dependent increase of space use observed in this study suggests an intra- as well as interspecific resource competition for food during the winter (for expectations see Table 1). Field voles are regarded as being herbivorous, but as fresh plant material depletes during the winter, food overlap and consequently competition with the more granivorous bank vole (Hansson 1983) appear to increase. However, the slope of the home range increase per vole seemed to differ for intraspecific and interspecific interactions during the non-breeding season (Fig. 2), suggesting that an addition of one bank vole would have had a larger increasing effect on the home range than the addition of one field vole. It would appear that the food niche overlap is larger with conspecifics than between species. In a constant-density set-up we would therefore expect the home range increase to be larger with conspecifics than with heterospecifics.
The space use patterns which had been observed in previous summer experiments were fundamentally different from those observed in the present study. In these earlier studies, the home ranges decreased with increasing density of animals (Eccard and Ylönen 2002, 2003b, 2007), suggesting interference at traps as points of interest. The occupation of traps by dominant field voles had probably prevented the subordinate bank voles from entering traps, and this had subsequently been considered to be evidence of decreased home ranges. In support of this hypothesis, the space use patterns of males in this study (Fig. 2) did show any density dependence, or even a decrease in range with increasing density, contrary to females and non-breeding males. Since male voles come into the breeding state earlier than females in the spring (Eccard and Ylönen 2001), there may have been a change from winter to summer behaviour already visible in this study. In summary, seasonal space use patterns suggest that we are observing resource competition in the non-breeding season but interference effects during the breeding season (Table 1).
Our comparison of behavioural data from both seasons suggests that in the winter interactions are less aggressive between the species, as indicated by the lower abundance of explore, approach, avoid and threat behaviour but the higher abundance of touch behaviour (Table 2). The general appearance of more amicable behaviours indicate an increase in social tolerance, which is necessary to facilitate communal nesting for thermoregulatory reasons. Bank vole females are tolerant to conspecifics during the winter (Ylönen and Viitala 1985, 1991), but territorial towards conspecific females in the breeding season (Bujalska 1985; Koskela et al. 1997). Our behavioural data (Figs. 3, 4) indicate that the same seasonal differences can be seen in the interaction between the species. During the winter, aggressive interactions towards both con- and heterospecific opponents decreased, most probably in order to save energy. Social tolerance during the winter is suggested to be mediated by a decrease in the levels of reproductive hormones (Beery et al. 2008), enabling mixed-sex communal nesting for huddling and thermoregulation. The same physiological mechanism may also contribute to the observed decrease in interspecific aggression during the non-breeding season.
In the summer, however, we found that the effect of an opponent’s species on behaviour in encounters was stronger than that in winter. Encounters with field vole opponents differed from the winter behaviour pattern to a greater extent than encounters with bank vole opponents (Fig. 3), indicating that aggression levels between species are higher than between conspecific females. In support of this possibility, the threat behaviour, which is, despite its name, a rather defensive behaviour displayed by animals being approached by an opponent (Ims 1987; Koskela et al. 1997), was most abundant in interactions with field voles in the summer, indicating aggression also from the opponents’ side. This finding is in accordance with the general picture of dominance ranks between Myodes and Microtus species in boreal vole communities (Henttonen and Hansson 1984).
Bank voles from control populations were naïve towards unknown heterospecifics. In the behavioural analyses we found no impact of competition treatment on the population level, but we did find clear effects of the individual opponent’s species. This indicates that behavioural interaction towards heterospecific competitors is rather innate than learned and is dependent on the seasonal breeding state, as predicted in Table 1.
In this winter study a lower proportion of bank vole females started breeding in competition populations compared to the controls, although this result should be considered with caution as the sample size was very limited. In contrast, in our earlier summer experiments, the proportion of breeders from the adult and sexually mature bank vole female populations point in the opposite direction—with an increase under the competition treatment (Eccard and Ylönen 2007). This result is probably due to a higher mortality in the competition populations and a resulting selection for the strongest breeding females of the subordinate species. An earlier experiment on juvenile, maturing bank vole breeders showed that the breeding proportion in this age group decreased through interspecific competition (Eccard et al. 2002). Similarly to our argument on survival, wintering bank voles, or any other vole species, are adults in terms of age, but overwinter in a sexually immature state. Maturation in bank voles is intraspecifically density dependent in the spring (Eccard and Ylönen 2001) and summer populations (Bujalska 1985; Prevot-Julliard et al. 1999). In our study, we found that the presence of field voles during the winter apparently reduced sexual maturation of bank voles in the spring. Larger and dominant field voles may suppress the maturation and breeding of bank vole females within a dominance hierarchy in a manner similar to that of stronger conspecifics. This has been suggested to be the case between bank voles and the closely related grey-sided voles, Myodes rufocanus (Kaarsalo and Wallgren 1991). A second explanation for the observed reduction in the breeding rate of bank voles under competition may have been resource depletion at the end of winter.
In conclusion, we were able to experimentally verify a seasonal change in interaction type between two sympatric vole species and between seasons, as evidenced by differences in spacing behaviour and interactive behaviour. Our results indicate that the nature of interactions between the species differs among seasons. Furthermore, the two interaction types may overlap with grave consequences for the subordinate species. Indirect food competition dominates the type of interaction in the winter. A reduction of aggression in the winter due to physiological and hormonal changes in the autumn, energetic constraints and/or the lack of territoriality associated with the bank vole’s breeding system may allow coexistence of species over winter despite reduced resource levels. In comparison, direct aggressive interference dominates interactions during the summer. In the spring, aggressive behaviours were found to increase while resource levels continued to be limited. This is the time window in each individual vole’s life when both resource-based competition, due to decreased food resources, and interference competition, due to physiological changes at the onset of breeding, act simultaneously. Thus, both interaction types may act during the onset of breeding after winter and may contribute to the often observed strong late-winter mortality in boreal rodent populations. A further general conclusion that can be drawn from our study is that interspecific interactions are not constant in given systems and thus should not be treated as either–or categories in competition theory.
Acknowledgments
We thank the staff of the Konnevesi Research Station for maintaining the facilities and caring for animals, as well as researchers and field helpers. Many thanks to Chris Madden and Jork Meyer, who helped us through the winter. Lars Korslund is acknowledged for valuable comments on the paper. The study was supported by the Academy of Finland.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Amarasekare P. Interference competition and species coexistence. Proc R Soc Lond B. 2002;269:2541–2550. doi: 10.1098/rspb.2002.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasekare P, Nisbet RM. Spatial heterogeneity, source-sink dynamics, and the local coexistence of competing species. Am Nat. 2001;158:572–584. doi: 10.1086/323586. [DOI] [PubMed] [Google Scholar]
- Beery AK, Loo TJ, Zucker I. Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Horm Behav. 2008;54:153–159. doi: 10.1016/j.yhbeh.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujalska G. Regulation of female maturation in Clethrionomys species, with special reference to an island population of C. glareolus. Ann Zool Fenn. 1985;22:331–342. [Google Scholar]
- Cameron AW. Competitive exclusion between the rodent genera Microtus and Clethrionomys. Evolution. 1964;18:630–634. doi: 10.2307/2406215. [DOI] [Google Scholar]
- Case TJ, Gilpin ME. Interference competition and niche theory. Proc Natl Acad Sci USA. 1974;71:3073–3077. doi: 10.1073/pnas.71.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case TJ, Bolger DT, Petren K. Invasions and competitive displacement among house geckos in the tropical Pacific. Ecology. 1994;75:464–477. doi: 10.2307/1939550. [DOI] [Google Scholar]
- Clarke KR. Non-parametric multivariate analyses of changes in community structures. Aust J Ecol. 1993;18:117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- Clarke KR, Somerfield PJ, Chapman MG. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J Exp Mar Biol Ecol. 2006;330:55–80. doi: 10.1016/j.jembe.2005.12.017. [DOI] [Google Scholar]
- Connell JH. The effects of competition, predation by Thais lapillus, and other factors on natural populations of the barnacle Balanus balanoides. Ecol Monogr. 1961;31:61–104. doi: 10.2307/1950746. [DOI] [Google Scholar]
- Connell JH. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos. 1980;35:131–138. doi: 10.2307/3544421. [DOI] [Google Scholar]
- Connell JH. On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am Nat. 1983;122:661–696. doi: 10.1086/284165. [DOI] [Google Scholar]
- Eccard JA, Ylönen H. Initiation of breeding after winter in bank voles: effects of food and population density. Can J Zool—Rev Can Zool. 2001;79:1743–1753. doi: 10.1139/z01-133. [DOI] [Google Scholar]
- Eccard JA, Ylönen H. Direct interference or indirect exploitation? An experimental study of fitness costs of interspecific competition in voles. Oikos. 2002;99:580–590. doi: 10.1034/j.1600-0706.2002.11833.x. [DOI] [Google Scholar]
- Eccard JA, Ylönen H. From population size fluctuations to individuals’ fitness: research on interspecific competition in small rodents. Evol Ecol. 2003;17:423–440. doi: 10.1023/A:1027305410005. [DOI] [Google Scholar]
- Eccard JA, Ylönen H. Who bears the costs of interspecific competition in an age structured population? Ecology. 2003;84:3284–3293. doi: 10.1890/02-0220. [DOI] [Google Scholar]
- Eccard JA, Ylönen H. Costs of coexistence along a gradient of competitor densities: an experiment with arvicoline rodents. J Anim Ecol. 2007;76:65–71. doi: 10.1111/j.1365-2656.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- Eccard JA, Klemme I, Horne TJ, Ylönen H. Effects of competition and season on survival and maturation of young bank vole females. Evol Ecol. 2002;16:85–99. doi: 10.1023/A:1016387318107. [DOI] [Google Scholar]
- Gilbert BS, Krebs CJ, Talarico C, Cichowski DB. Do Clethrionomys rutilus females suppress maturation of juvenile females? J Anim Ecol. 1986;55:543–552. doi: 10.2307/4737. [DOI] [Google Scholar]
- Grant PR. Experimental studies of competitive interaction in a two-species system. I. Microtus and Clethrionomys species in enclosures. Can J Zool. 1969;47:1059–1082. doi: 10.1139/z69-168. [DOI] [Google Scholar]
- Gurevitch J, Morrow LL, Wallace A, Walsh JS. A meta-analysis of competition in field experiments. Am Nat. 1992;140:539–572. doi: 10.1086/285428. [DOI] [Google Scholar]
- Hansson L. Competition between rodents in successional stages of taiga forests: Microtus agrestis vs. Clethrionomys glareolus. Oikos. 1983;40:258–266. doi: 10.2307/3544590. [DOI] [Google Scholar]
- Harper SJ, Batzli GO. Are staged dyadic encounters useful for studying aggressive behaviour of arvicoline rodents? Can J Zool. 1997;75:1051–1058. doi: 10.1139/z97-126. [DOI] [Google Scholar]
- Harris DB. Space invaders? A search for patterns underlying the coexistence of alien black rats and Galapagos rice rats. Oecologia. 2006;149:276–288. doi: 10.1007/s00442-006-0447-7. [DOI] [PubMed] [Google Scholar]
- Henttonen H, Hansson L. Interspecific relations between small rodents in European boreal and subarctic environments. Acta Zool Fenn. 1984;172:61–65. [Google Scholar]
- Henttonen H, Kaikusalo A, Tast J, Viitala J. Interspecific competition between small rodents in subarctic and boreal ecosystems. Oikos. 1977;29:581–590. doi: 10.2307/3543596. [DOI] [Google Scholar]
- Huenneke LF, Thomson JK. Potential interference between a threatened endemic thistle and an invasive nonnative plant. Conserv Biol. 1995;9:416–425. doi: 10.1046/j.1523-1739.1995.9020416.x. [DOI] [Google Scholar]
- Ims RA. Responses in spatial organisation and behaviour to manipulations of the food resources in the vole Chethrionomys rufocanus. J Anim Ecol. 1987;56:585–596. doi: 10.2307/5070. [DOI] [Google Scholar]
- Iverson SL, Turner BN. Winter Coexistence of Clethrionomys gapperi and Microtus pennsylvanicus in a Grassland Habitat. Am Midl Nat. 1972;88:440–445. doi: 10.2307/2424368. [DOI] [Google Scholar]
- Kaarsalo K, Wallgren H. Changes in fecundity of thorthern red vole females caused by conspecific males, males or females of gray-sided voles, and weasels. Acta Physiol Scand. 1991;143:A60. [Google Scholar]
- Kennedy ED, White DW. Interference competition from house wrens as a factor in the decline of Bewicks wrens. Conserv Biol. 1996;10:281–284. doi: 10.1046/j.1523-1739.1996.10010281.x. [DOI] [Google Scholar]
- Kenward RE, Hodder KH. Red squirrels (Sciurus vulgaris) released in conifer woodland: the effects of source habitat, predation and interactions with grey squirrels (Sciurus carolinensis) J Zool. 1998;244:23–32. doi: 10.1111/j.1469-7998.1998.tb00003.x. [DOI] [Google Scholar]
- Koskela E, Mappes T, Ylonen H. Territorial behaviour and reproductive success of bank vole Clethrionomys glareolus females. J Anim Ecol. 1997;66:341–349. doi: 10.2307/5980. [DOI] [Google Scholar]
- Larsson TB, Hansson L. Vole diet on experimentally managed afforestation areas in northern Sweden. Oikos. 1977;28:242–249. doi: 10.2307/3543977. [DOI] [Google Scholar]
- Leving S, Franks N. Patterns of nest dispersion in a tropical ground ant community. Ecology. 1982;63:338–394. doi: 10.2307/1938951. [DOI] [Google Scholar]
- Morris DW. A haunting legacy from isoclines: mammal coexistence and the ghost of competition. Oikos. 1999;80:375–384. [Google Scholar]
- Myllymäki A. Interactions between the field vole Microtus agrestis and its microtine competitors in Central-Scandinavian populations. Oikos. 1977;29:570–580. doi: 10.2307/3543595. [DOI] [Google Scholar]
- Nilsson MC. Separation of allelopathy and resource competition by the dwarf shrub Empetrum hermaphroditum Hagerup. Oecologia. 1994;98:1–7. doi: 10.1007/BF00326083. [DOI] [PubMed] [Google Scholar]
- Polis GA, Myers CA, Holt RD. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst. 1989;20:297–330. doi: 10.1146/annurev.es.20.110189.001501. [DOI] [Google Scholar]
- Prevot-Julliard AC, Henttonen H, Yoccoz NG, Stenseth NC. Delayed maturation in female bank voles: optimal decision or social constraint? J Anim Ecol. 1999;68:684–697. doi: 10.1046/j.1365-2656.1999.00307.x. [DOI] [Google Scholar]
- Rosenzweig ML. Species diversity in space and time. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Schluter DW. Interspecific interactions: ecological character displacement. In: Fox CW, Roff DA, Fairbairn DJ, editors. Evolutionary ecology: concepts and cases studies. Oxford: Oxford University Press; 2001. pp. 265–276. [Google Scholar]
- Schoener TW. Field experiments on interspecific competition. Am Nat. 1983;122:240–285. doi: 10.1086/284133. [DOI] [Google Scholar]
- Smyth G. The effects of the removal of individuals from a population of bank voles Clethrionomys glareolus. J Anim Ecol. 1968;37:167–183. doi: 10.2307/2717. [DOI] [Google Scholar]
- Vance RR. Interference competition and the coexistence of 2 competitors on a single limiting resource. Ecology. 1984;5:1349–1357. doi: 10.2307/1939115. [DOI] [Google Scholar]
- Walls SC. Interspecific competition in postmetamorphic salamanders: interspecific differences in aggression by coexisting species. Ecology. 1990;71:307–314. doi: 10.2307/1940270. [DOI] [Google Scholar]
- Ylönen H, Viitala J. Social organisation of an enclosed winter population of the bank vole Cletrionomys glareolus. Ann Zool Fenn. 1985;22:353–358. [Google Scholar]
- Ylönen H, Viitala J. Social overwintering and food distribution in the bank vole Clethrionomys glareolus. Holarctic Ecol. 1991;14:131–137. [Google Scholar]
- Ylönen H, Kojola T, Viitala J. Changing female spacing behaviour and demography in an enclosed breeding population of Clethrionomys glareolus. Holarctic Ecol. 1988;11:286–292. [Google Scholar]