Abstract
Leukotriene C4 is an important mediator in the development of inflammatory reactions and ischaemia. Previous studies have shown that leukotriene C4 is able to modulate the function of dendritic cells (DCs) and induce their chemotaxis from skin to lymph node. In this study, we decided to evaluate the modulation exerted by leukotriene C4 on DCs, depending on their status of activation. We showed for the first time that leukotriene C4 stimulates endocytosis both in immature and lipopolysaccharide (LPS) -activated DCs. Moreover, it suppressed the interleukin-12p70 (IL-12p70) release, but induces the secretion of IL-23 by DCs activated with LPS and promotes the expansion of T helper type 17 (Th17) lymphocytes. Furthermore, blocking the release of IL-23 reduced the percentages of CD4+ T cells producing IL-17 in a mixed lymphocyte reaction. Ours results suggest that leukotriene C4 interferes with the complete maturation of inflammatory DCs in terms of phenotype and antigen uptake, while favouring the release of IL-23, the main cytokine involved in the maintenance of the Th17 profile.
Keywords: endocytosis, interleukin-23, inflammatory dendritic cells, leukotriene C4, T helper type 17 lymphocytes
Introduction
Dendritic cells (DCs) are highly specialized antigen-presenting cells with a unique capability to activate naive T lymphocytes and initiate the adaptive immune response, as well as induce peripheral tolerance.1,2 In peripheral tissues, immature DCs sense the microenvironment and after their encounter with inflammatory stimuli or pathogens, DCs are activated; a process that is accompanied by several physiological changes and that ends with the maturation of DCs.3–5
Once initiated the process of DCs maturation, the expression of CD80, CD86 and MHC class II molecules increases.1–4 The DCs migrate to the draining lymph nodes, as a result of the up-regulation of CCR7, which renders them responsive to CCL19 and CCL21 chemokines that direct their migration to the T-cell areas of lymph nodes.6 Finally, the mature DCs present the antigen to naive CD4+ and CD8+ T lymphocytes. The maturational status can be modulated by different stimuli.5 The impact of microbial products through Toll-like receptor leads to DCs that produce interleukin-12 (IL-12)/IL-23 and prime T helper type 1 (Th1)/Th17 responses.7,8 In contrast, in the absence of inflammatory signals, ‘semi-mature’ DCs produce IL-10, which primes a regulatory T-cell response.9 However, mediators other than cytokines and pathogens have a great impact on the physiology of DCs. Prostaglandin E2 acting on mature DCs induces the differentiation of CD4+ T cells in a Th2 profile.10,11 Also, histamine activates murine DCs through the increase of endocytosis and cross-presentation of extracellular antigens.12
Leukotriene C4 (LTC4), a member of the cysteinyl leukotriene family (CysLT), is a potent pro-inflammatory lipid mediator, produced by inflammatory cells such as mast cells, eosinophils, basophils and macrophages.13,14 It is a potent spasmogen and vasoconstrictor, promotes mucus secretion, and together with histamine is a known immunomodulatory agent of allergic and inflammatory reactions.15–17 The pharmacological effects of CysLT are conducted through two types of membrane receptors – CysLTR1 and CysLTR2 – which are coupled to protein-G.18 Remarkably, these receptors were primarily described at the level of lung mucosa and intestinal mucosa at the ileum and colon.19 In many diseases affecting lung and intestinal mucosa, such as asthma and interstitial cystitis, the use of montelukast, a selective antagonist of CysLTR1, minimizes the effects of these pathologies, probably through the inhibition of cytosolic Ca2+.20–22
It is known that LTC4 induces the chemotaxis of DCs from the skin.23 Zymosan, a Toll-like receptor 2 agonist, but not lipopolysaccharide (LPS), a classic Toll-like receptor 4 agonist, stimulates the production of CysLT by DCs.24,25 Despite these observations, their impact on cytokine production by DCs is unclear. In spite of the close relationship between mast cells and DCs in mucosal epithelium and skin, little progress has been made regarding the impact of CysLT on the genesis of DCs.
In the present study, we analysed the effects of LTC4 on the phenotype and function of murine inflammatory DCs.26 In particular, we studied the differential expression of CysLT1 and CysLT2 receptors in immature and LPS-activated DCs. Our results show that LTC4 interferes with the maturation of DCs triggered by LPS, in terms of phenotype and antigen uptake. In this context, LTC4 induces the release of IL-23 by inflammatory DCs, favouring the expansion of Th17 cells.
Materials and methods
Mice
All experiments were carried out using 2-month-old virgin female C57BL/6 mice raised at the National Academy of Medicine, Buenos Aires, Argentina. They were housed six per cage and kept at 20 ± 2° under an automatic 12 hr light–dark schedule. Animal care was in accordance with institutional guidelines.
Generation of DCs from bone marrow cultures
The procedure used in this study was as described by Inaba et al.27 with some minor modifications. Briefly, bone marrow was flushed from the long bones of the limbs using 2 ml RPMI-1640 (Gibco, Invitrogen, Carlsbad, CA) with a syringe and 25-gauge needle. Red cells were lysed with ammonium chloride. After washing, cells were suspended at a concentration of 1 × 106 cells/ml in 70% RPMI-1640 medium supplemented with 10% fetal calf serum (FCS; Gibco), and 5·5 × 10−5 mercaptoethanol (Sigma, St Louis, MO) (mouse complete medium) and 30% J588-GM cell line supernatant. The cultures were fed every 2 days by gently swirling the plates, aspirating 50% of the medium, and adding back fresh medium with J588-GM cell line supernatant. At day 9 of the culture, > 85% of the harvested cells expressed MHC class II, CD40 and CD11c, but not Gr-1 (not shown).
Culture conditions
The standard medium used in this study was bicarbonate-buffered RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FCS, 50 U/ml penicillin, 50 μg/ml streptomycin, 0·1 mm non-essential amino acids, and 5·5 × 10−5 mercaptoethanol (all from Invitrogen) (complete medium).
Reagents
Horseradish peroxidase (HRP), dextran (DX, 40 000 molecular weight), Zymosan (Zy, from Saccharomyces cerevisiae), LPS from Escherichia coli (0111:B4), were from Sigma Chemical Co. (St Louis, MO). SB-202190 [p38 mitogen-activated protein kinase (MAPK)], PD-98059 [extracellular signal-regulated kinase (ERK)/MAP kinase Kinase (MEK) MAPK], were from Promega Corporation (Madison, WI). The DX and Zy were conjugated with FITC, as described previously.28
Flow cytometry
Cells staining were performed using the following monoclonal antibodies (mAbs): FIYC-conjugated anti-CD11c, anti-CD40-FITC, anti-I-Ad conjugated with phycoerythrin (PE), GR1-PE and CD86-PE (Pharmingen, San Diego, CA). Cell surface antigen expression was evaluated by single staining, and analysis was performed using a FACS flow cytometer and cellquest software (Becton Dickinson, San Jose, CA).
Endocytosis by FACS analysis
After different treatments, DCs were suspended in medium RPMI-1640 at 37°. FIYC-DX was added at the final concentration of 100 μg/ml. The cells were washed four times with cold PBS containing 1% FCS and were analysed on a FACS flow cytometer (Becton Dickinson). The background (cells pulsed at 0°) was always subtracted.
Endocytosis of HRP
Endocytosis of HRP was performed as previously described.29 Briefly, DCs were suspended in complete medium; HRP was added at the final concentration of 150 μg/ml HRP, and cells were cultured for 30 min at 37°. Then, DCs were collected, washed four times in PBS containing 1% FCS and four times in PBS alone, then were lysed with 0·05% Triton X-100 in 10 mm Tris–HCl buffer, for 30 min, and the enzyme activity of the lysate was measured using O-phenylenediamine and H2O2 as substrates with reference to a standard curve, at 492 nm. The amount of HRP taken up by DCs was determined as the difference between HRP activities in disrupted and non-disrupted cells. The HRP activity in non-disrupted DCs was always < 15% compared with disrupted cells.
Expression of cysteinyl receptors
Total RNA was extracted from lung (positive control for CysLT1 receptor), gut tissues (positive control for CysLT2 receptor) and mouse immature and LPS-treated DCs, using Trizol reagent (Gibco-Life Technologies). The reverse transcription reaction contained 3 μg total RNA and was performed using the Moloney-murine leukaemia virus reverse transcriptase enzyme (Promega). The primers were provided by Invitrogen: forward primers for the CysLTR1 and CysLTR2: CAA CGA ACT ATC CAC CTT CAC C and CCA AGG TCA CAA GAG GGT GT, respectively. Reverse primers for the CysLTR1 and CysLTR2: AGC CTT CTC CTA AAG TTT CC AC and GAG TTG ACA GAG GCG AGG AC, respectively. A GeneAmp PCR system (Perkin-Elmer/Applied Biosystems, Foster City, CA) was used. The PCR products were separated on a 1·5% agarose gel, stained with ethidium bromide, and visualized by a UV transilluminator.
Western blot analysis
Murine DCs were suspended in complete medium (2 × 106/500 μl) were prewarmed for 30 min at 37°. The DCs were treated without or with 1 μg/ml LPS for 20 min at 37°. Then cells were washed and treated with or without 0·01 μm LTC4 for 5 min at 37°. The reaction was stopped by adding cold PBS, the mixture was centrifuged and pellets were resuspended at 3 × 106 cells/ml in Western sample buffer (100 mm Tris–HCl pH 6·8; 4% SDS, 0·2% Bromophenol-Blue, 20% glycerol, 200 mm dithiothreitol) and frozen at – 80°. Before the analysis, lysates were thawed, heated for 3 min to 96° and finally homogenized with a sonicator and 5 × 104 cells (10 μl extract) per lane were separated onto 10% SDS–PAGE followed by electroblotting. The membranes were blocked in PBS + 5% milk powder for 2 hr, and then incubated with the following primary antibodies in blocking buffer + 0·1% Tween-20 overnight at 4°: anti-phospho-ERK1/2 (Thr202/Tyr204, 1 : 1000; Cell Signaling Technology, Boston, MA), anti-phospho-p38K (1 : 1000; Cell Signaling). After washing, secondary antibodies were applied in blocking buffer for 2 hr at room temperature: anti-rabbit or anti-mouse-HRP mAb (1 : 3000; Cell Signaling). Membranes were washed and specific bands were developed by enhanced chemiluminescence (Amersham Biosciences, Uppsala, Sweden). Membranes were stripped and reproved with a rabbit mAb against murine β-actin (Cell Signaling Technology).
Mixed leucocyte reaction
Splenocytes were obtained from the spleen of naive BALB/c mice and cultured at 1·5 × 105 cells/well in 96-well microplates with 5 × 104 immature DCs or LPS-stimulated DCs from C57BL/6 mice, either treated (0·01 μm) or untreated with LTC4. The cells were cultivated for 4 days in RPMI-1640 containing 10% FCS, 10 mm HEPES buffer and 5·5 × 10−5 m mercaptoethanol (Sigma). At day 4 of culture, cells were pulsed for 18 hr with [3H]thymidine (1 μCi/well; DuPont, AR). Then, cells were harvested using a cell harvester (Perkin-Elmer Inc.) and the amount of [3H]thymidine incorporation was determined in a β-scintillation counter. Intracellular staining for cytokine production was performed after stimulation of co-culture for 24 hr with PMA (10 ng/ml), ionomycin (1 μg/ml) with or without IL-23p19 (10 μg/ml) (ebiosciences, San Diego, CA) in the presence of brefeldin A for the last 6 hr. Finally, CD4+ IL-17A+ interferon-γ (IFN-γ)+ cells were analysed by flow cytometry.
Intracytoplasmic cytokine staining
Two or three-colour analysis was performed using flow cytometry, DCs were cultured without or with LPS (1 μg/ml) for 20 min at 37°. After washing, DCs were treated with or without 0·01 μm LTC4 for 30 min at 37° in complete medium in the presence of brefeldin A (5 μg/ml). After 18 hr, cells were fixed in 4% paraformaldehyde and permeabilized with saponin (0·1% in PBS). The permeabilized cells were incubated with a PE-conjugated anti-IL-12p40 antibody (BD Pharmingen, Trento, NJ) in PBS 0·5% BSA or similarly labelled isotype-matched control antibodies for 30 min. The stained cells were washed with saponin buffer twice and resuspended in isoflow. In some cases, intracytoplasmic cytokines were evaluated in co-cultures of mixed lymphocyte reaction (MLR) and permeabilized cells were incubated with PE-conjugated anti-IL17A and FITC-conjugated anti-IFN-γ antibodies (BD Pharmingen). In all cases, the surface staining with FITC-conjugated anti-CD11c (DCs) or Peridinin chlorophyll protein-conjugated anti-CD4 antibodies (BD Pharmingen) was performed before to permeabilization. The staining was analysed by flow cytometry on FACS using cellquest software (BD Biosciences, San Jose, CA).
Cytokines determination
The cytokine levels in supernatants of DCs were measured by ELISA. Assays for IL-12p70, p40, IL-23, IL-6, tumour necrosis factor-α (TNF-α), IFN-γ (eBiosciences) and IL-17 (Quantikine; R&D Systems, Bs. AS, AR) were performed according to the manufacturer's protocols. The limits of detection were: 15 pg/ml (IL-12p70; p35/p40), 30 pg/ml (IL-23; p19/p40), 10 pg/ml (IL-12p40), 8 pg/ml (TNF-α), 4 pg/ml (IL-6), 15 pg/ml (IFN-γ) and 5 pg/ml (IL-17).
Statistics
The significance between means was assessed by Student's paired t-test. P ≤ 0·05 was determined to indicate statistical significance.
Results
Exogenous LTC4 modulate phenotypical expression from LPS-activated DCs
We decided to evaluate whether LTC4 is able to modulate the central molecules expressed by DCs that are involved in the activation of T lymphocytes.3,4 In the first place, we studied the concentrations of LTC4 able to modulate the expression of the MHC class II molecules. To analyse this point, DCs were cultured in the presence of different concentrations of LTC4 (10−5–10−9 m) at 37°. After 18 hr, its expression was analysed by flow cytometry. As shown in Fig. 1(a), LTC4 increased in a dose-dependent manner, the expression of MHC class II on immature DCs was more significant at 10−8 m, so the trials were conducted using this concentration. Then, considering that LTC4 is released during inflammatory responses,17,30 we studied the effect of LTC4 (10−8 m) on the phenotype of immature DCs and LPS-stimulated DCs. Interestingly, after 18 hr of culture, LTC4 strongly inhibited the expression of CD86 and CD40 molecules (Fig. 1b,c,f) when DCs were activated with 1 μg/ml LPS, whereas the lipid mediator had no effect on immature DCs. However, in the case of the class II molecules, LTC4 had antagonistic effects depending on the activation status of DCs, increasing its expression in immature DCs and inhibiting in LPS-treated DCs (Fig. 1d,f). As shown in Fig. 1(g), although MHC class II decreased its expression in LPS-activated DCs, LTC4 had the ability to prime T lymphocytes, because it induced a low but significant increase in the allostimulatory response mediated by activated DCs. This effect was also observed in immature DCs, which correlates with the increased expression of class II molecules by LTC4.
Figure 1.
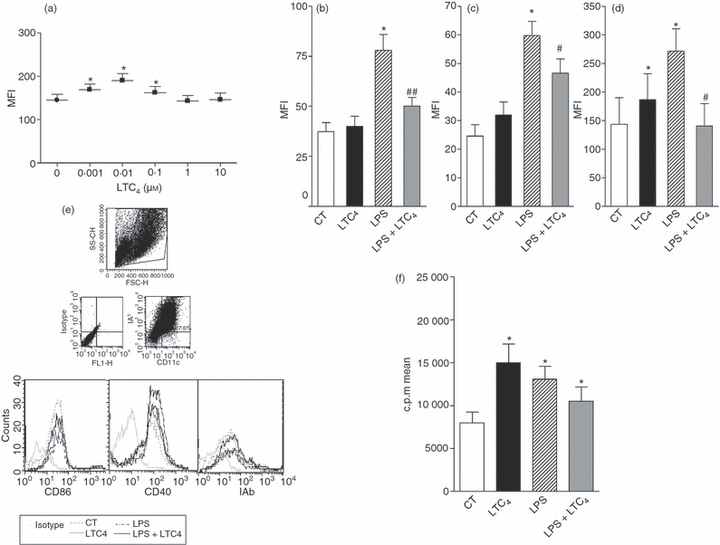
Leukotriene C4 (LTC4) inhibits CD86 and MHC class II expression on lipopolysaccharide (LPS) -matured dendritic cells (DCs). (a) DCs (1.5 × 106/ml) were cultured for 18 hr at 37° with different concentrations of LTC4. Then, MHC class II molecule expression was analysed by flow cytometry. (b,c,d) CD40; CD86 and MHC class II expression, DCs were evaluated after the incubation for 30 min at 37° in presence (LPS) or not (Ct) of LPS (1 μg/ml). Finally, cells were incubated overnight at 37° with or without LTC4 (0.01 μm). (e) Representative histograms of six or seven experiments, for MHC class II, CD40 and CD86 molecules, respectively. Results are expressed as mean fluorescence intensity (MFI) and represent the arithmetic mean ± SEM of six or seven experiments. (f) DCs (1.5 × 106/ml) were cultured with or without LPS (1 μg/ml) for 30 min at 37°, finally cells were treated or not with LTC4 (0.01 μm, 30 min at 37°). Then, DCs were washed and co-cultured with freshly isolated allogeneic splenocytes for 5 days at a ratio 1 : 10. Thymidine incorporation was measured on day 5 by a 16-hr pulse with [3H]thymidine (1 μCi/well). Results are the mean ± SEM of five experiments. Asterisk represents statistical significance (*P < 0.05) versus CT; and # represents significance (#P ≤ 0.05; ##P ≤ 0.01) versus LPS.
Leukotriene C4 counteracts the effect of LPS on DCs endocytosis
Immature DCs are specialized to sense the microenvironment and when stress or infection are detected they incorporate the antigen through phagocytosis or endocytosis.28,29,31,32 We aimed to determine whether LTC4 was able to affect the antigen uptake of immature and activated DCs. To this end, cells were treated or not with LPS (1 μg/ml) for 30 min at 37°, then DCs were incubated without or with 10−8 m LTC4 for 30 min at 37°. Finally, cells were washed and incubated in the presence of Zy (10 particles/DC) coupled to FITC for 30 min at room temperature or DX-FITC (100 μg/ml) for 40 min at 37°. The phagocytosis controls were supplied by DCs treated with cytochalasin B, a disruptor of actin microfilaments, 33 previous to their incubation with Zy-FITC. For DX endocytosis, the control of reaction was provided by DCs incubated with the antigen at 4°, because this is a temperature-dependent phenomenon. In addition, we analysed the uptake of HRP. For this, after treatment with LTC4 (0·01 μm) of both DCs and LPS-stimulated DCs, these were cultured with 150 μg/ml HRP for 40 min at 37°. Subsequently, cells were washed several times with cold PBS and permeabilized by addition of 0·5% Triton X-100 in PBS for 30 min at room temperature. The control was provided by DCs treated with HRP but not permeabilized. Finally, the enzymatic activity was measured in supernatants of reaction by addition of the substrate [alpha-phenylendiamine (OPD)] and read at 492 nm. In Fig. 2(a), we demonstrated that LTC4 increased the phagocytosis of Zy-FITC by immature DCs but had no effect in LPS-activated DCs. In contrast, as shown in Fig. 2(b,c), uptake of DX and HRP was increased by LTC4 in both immature and LPS-stimulated DCs. This result would indicate that LTC4 prevents complete maturation of DCs by classical stimuli such as LPS, because it restores their endocytic capacity.
Figure 2.
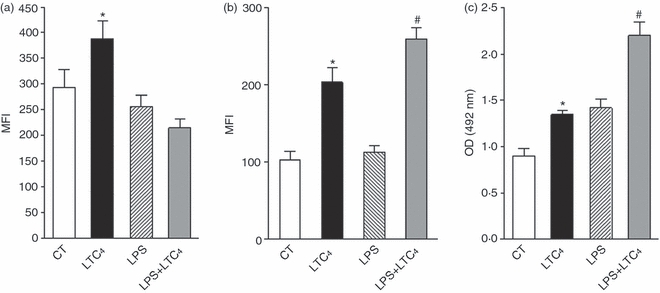
Leukotriene C4 (LTC4) differentially stimulates antigen-uptake in immature and mature dendritic cells (DCs). (a,b) The DCs (1.5 × 106/ml) were cultured for 30 min at 37° with or without lipopolysaccharide (LPS; 1 μg/ml), then cells were stimulated in the presence or not of 0.01 μm LTC4 for 30 min at 37°. Then, cells were incubated with Zy-FITC (a) for 40 min at room temperature or DX-FITC (b) (100 μg/ml) for 1 hr at 37°, and antigen uptake was measured by flow cytometry. Results are expressed as mean fluorescence intensity (MFI) and represent the arithmetic mean ± SEM of six experiments. In (c), DCs or LPS-treated DCs (1.5 × 106/ml) were cultured with or without LTC4 (0.01 μm) for 30 min at 37°. Then, HRP (150 μg/ml) was added and the HRP uptake by DCs was evaluated as described in the Materials and methods. Results are expressed as optical density (OD) at 492 nm and represent the arithmetic mean ± SEM of five experiments. The results are expressed as MFI or OD and represent the arithmetic mean ± SEM of five or six experiments. Asterisk represents statistical significance (*P ≤ 0.05) versus controls and # represents significance (#P ≤ 0.05) versus LPS.
Leukotriene C4 strongly inhibits the induction of a Th1 profile and increases IL-23 production by LPS-activated DCs
Taking into account the fact that LTC4 imposes changes in DCs that prevent their maturation we decided to evaluate their impact on the genesis of the adaptive response, through the analysis of the cytokines induced. With this aim, immature and activated DCs were cultured for 18 hr at 37° in presence or not of LTC4 (10–8 m). After incubation, culture supernatants were collected and we evaluated cytokines by ELISA. As shown in Fig. 3(a), LTC4 increased the production of TNF-α in immature DCs but was unable to reverse its release induced by LPS. Interestingly, LTC4 completely abolished the induction of IL-12p70 in LPS-stimulated DCs (Fig. 3b), indicating an antagonistic effect of LPS. Therefore, LTC4 inhibits the induction of a Th1 profile by T CD4+ naive lymphocytes, by acting on activated DCs.34,35
Figure 3.
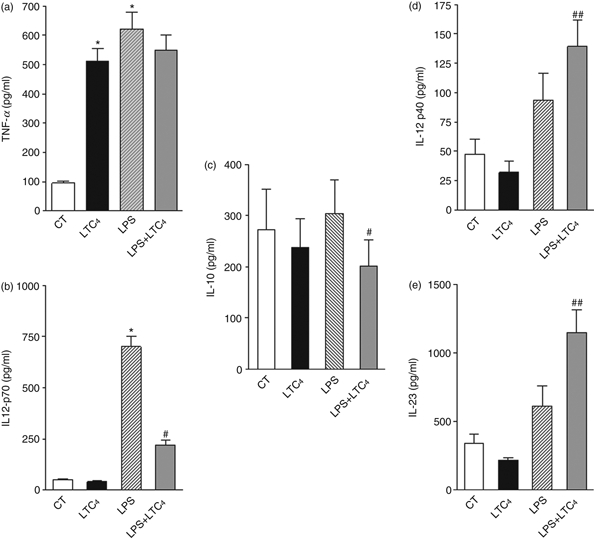
Leukotriene C4 (LTC4) inhibits the T helper type 1 (Th1) profile by lipopolysaccharide (LPS) matured dendritic cells (DCs). The DCs were incubated for 30 min in the presence (LPS, 1 μg/ml) or absence (CT) of LPS at 37°. After washing, DCs were treated with or without 0.01 μm LTC4 at 37°, overnight, Then we collected the supernatants and we quantified the levels of tumour necrosis factor-α (TNF-α) (a), interleukin-12p70 (IL-12p70) (b), IL-10 (c), IL-12p40 (d) and IL-23 (e) by ELISA. Bars represent the cytokine concentrations (pg/ml) and represent the mean ± SEM of six experiments. Asterisk represents statistical significance (*P ≤ 0.05) versus CT and # represents significance (#P ≤ 0.05; ##P ≤ 0.01) versus LPS.
Moreover, to further investigate the effect of LTC4 we decided to evaluate whether LTC4 could favour a tolerogenic state;36,37 however, when we analysed the release of IL-10 in culture supernatants, we showed inhibition of this cytokine in LPS-treated DCs (Fig. 3c), whereas it was not modulated on immature DCs.
Finally, as demonstrated in Fig. 3(d), LTC4 significantly stimulated the production of IL-12p40 by LPS-stimulated DCs. Taking into account that p40 is a chain shared by the cytokines IL-12 and IL-23 and the finding that IL-12p70 was strongly inhibited by LTC4, we decided to evaluate the presence of IL-23 in supernatants of DCs. As shown in Fig. 4(e), LTC4 increased the release of IL-23 in LPS-stimulated DCs, a cytokine associated with the maintenance of Th17 profiles.38,39
Figure 4.
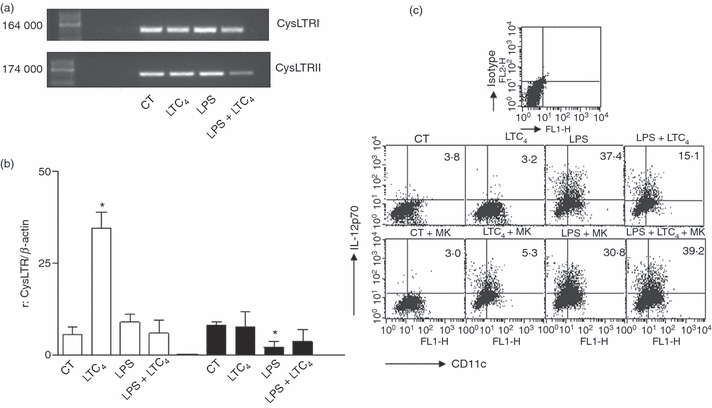
Immature and mature dendritic cells (DCs) express cysteinyl leukotriene receptors 1 (CysLTR1) and CysLTR2. The expression of CysLTR1 and CysLTR2 was evaluated by RT-PCR. RNAs from immature and lipopolysaccharide (LPS) -stimulated DCs, were extracted after 18 hr of culture in the presence or absence of leukotriene C4 (LTC4; 0.01 μm). RNAs used as positive controls were from lung to the CysLTR1 and tissues of intestinal mucosa to the CysLTR2. (a) Representative gels of RT-PCR from six independent experiments are shown. (b) Shows the histogram bars for the ratio values obtained from the normalization of the diameters of each band compared with β-actin. The results are expressed as ratio and represent the arithmetic mean ± SEM of six experiments. Asterisk represents statistical significance (*P ≤ 0.05) versus controls. (b) DCs and lipopolysaccharide (LPS) -stimulated DCs (1 μg/ml for 30 min at 37°) were incubated (1 × 106 cells/ml) for 20 min at 37° without or with 1 μm montelukast. Then, DCs were untreated or treated with LTC4 (0.01 μm) for 18 hr at 37°. Brefeldin A (5 μg/ml) was added during the last 6 hr of culture to inhibit the release of cytokines into the supernatant. Finally, the percentages of interleukin-12p70-positive cells were evaluated by intracytoplasmic staining. We showed a representative dot plot obtained by cytometry.
Murine DCs express CysLTR1 and CysLTR2
The CysLTs exert their effects in several tissues through their action on CysLT1 and CysLT2 receptors.18 Expression of CysLTR1 has been demonstrated in murine DCs.40 Our objective was to evaluate the expression of both receptors in immature and LPS-stimulated DCs by reverse transcription (RT-) PCR. For that, DCs were incubated without or with LPS (1 μg/ml) at 37°, after 30 min we added or not 10–8 m LTC4 and cells were cultured overnight at 37° and finally we analysed the expression of both receptors using RT-PCR. The RT-PCR amplification yielded DNA fragments of the expected size for both CysLTR1 and CysLTR2 (Fig. 4a). By analysis of bands compared with β-actin, we found similar expression for both receptors in immature and LPS-stimulated DCs (Fig. 4b), an interesting fact was that, LTC4 treatment of immature DCs up-regulated the expression of CysLTR1 mRNA. This could suggest that the effects of LTC4 are mediated through the CysLTR1. However, when we analysed DX uptake and cytokine secretion in the presence of montelukast (MK-571), an antagonist of CysLTR1, we found that DX endocytosis only decreased the mean fluorescence intensity in immature DCs by 25–30% (control: 78·2 ± 8·1; LTC4: 165·5 ± 12·4 versus MK-571: 91 ± 15·1; MK-571 + LTC4: 108 ± 21·0, mean ± SEM, n = 3, P < 0·05). However, in LPS-stimulated DCs the blockage with MK did not affect this function (data not shown). Additional MK treatment significantly increased the production of IL-12p70 by LPS-activated DCs (Fig. 4c), suggesting a central role of CysLTR1 as inhibitor of Th1 responses.
Leukotriene C4 activates MAPK depending on the activation status of DCs
The activation of MAPK plays a central role in DCs function.39 It has been shown that LPS and CysLT induce the activation of ERK1/2 and p38.41,42 Taking this into account, we decided to analyse the activation of ERK1/2 and p38 MAPK. Western blots of lysates from DCs cultured without or with LPS (1 μg/ml) for 30 min at 37° were incubated in the presence or not of LTC4 (10–8 m) for 5 min and finally were probed with antibodies against MAPK. Figure 5(a,c) illustrates that LTC4 only triggers the activation of p38 in immature DCs; on the contrary with LPS stimulation the lipid mediator was not able to affect activation of this pathway induced by LPS on DCs. Interestingly, LTC4 led to the phosphorylation of ERK1/2 MAPK on LPS-activated DCs (Fig. 5b,c) suggesting that, these pathways would be responsible for LTC4 modulation of DC function.
Figure 5.
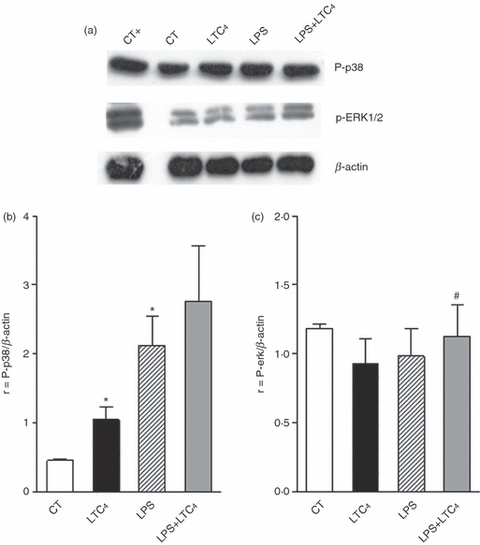
Leukotriene C4 (LTC4) activates ERK1/2 and p38 MAPK in dendritic cells (DCs). (a) DCs (3 × 106 cells per 500 μl complete medium) were prewarmed for 30 min at 37°. Cells were incubated in the presence or absence of LPS (1 μg/ml) for 30 min at 37° and then treated or not with 0.01 μm LTC4 for 5 min at 37°. The samples were analysed by Western blotting as described in the Materials and methods. Pervanadate-treated DCs (0.1 mm orthovanadate plus 0.3 mm H2O2 for 10 min at 37°) were used as positive phosphorylation controls. Western blots are representative of five experiments. (b) and (c) Histograms of ratio obtained by quantitative densitometric analysis of P-p38 and ERK1/2; respectively, normalized with β-actin densitometric units. The bars represent the mean ± SEM of five experiments. Asterisk represents statistical significance (*P ≤ 0.05) versus CT and # represents significance (#P ≤ 0.05) versus LPS.
To evaluate this point, we decided to analyse DCs function in the presence of SB and PD, known inhibitors of p38 and ERK1/2 phosphorylation, respectively. For this, immature and LPS-stimulated DCs were cultured in the presence of SB or PD (50 μm) for 20 min at 37°, after this time cells were cultured in the presence or absence of LTC4 (10−8 m) for 30 min at 37°. Finally, we studied the endocytosis of DX-FITC. As shown in Fig. 6(a), the blockade of p38 inhibited DX uptake in LPS-activated DCs, suggesting that the activation of this MAPK is an essential mechanism for LTC4-induced up-regulation of LPS endocytosis.
Figure 6.
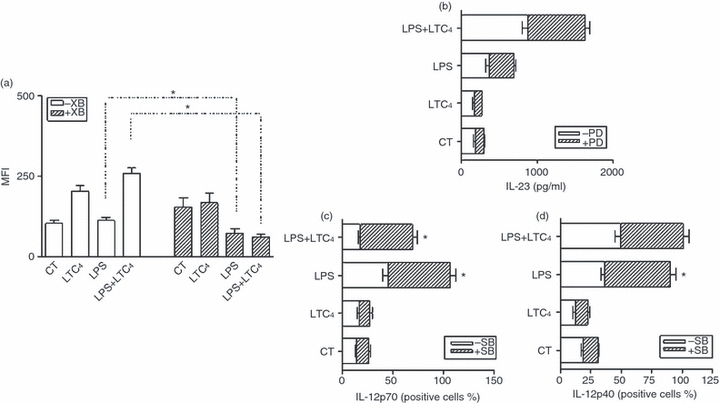
p38 MAPK is involved in regulating endocytosis by leukotriene C4 (LTC4) on lipopolysaccharide (LPS) -stimulated dendritic cells (DCs). The DCs and LPS-stimulated DCs (1 μg/ml for 30 min at 37°) were incubated (2.5 × 106 cells/ml) for 20 min at 37° without inhibitors or in the presence of 50 μm SB202190. Then, the cells were exposed to LTC4 (0.01 μm) for 30 min at 37°. After this time, DCs were washed and cultured for an additional 40 min in the presence of dextran (DX) -FITC (100 μg/ml) at 37°. (a) Results are expressed as mean fluorescence intensity (MFI) and represent the arithmetic mean ± SEM of four experiments. (b,c) and (d) DCs and LPS-stimulated DCs (1 μg/ml for 30 min at 37°) were incubated (2.5 × 106 cells/ml) for 20 min at 37° without inhibitors or in the presence of 50 μm PD98059 or 50 μm SB202190. Then, DCs were untreated or treated with LTC4 (0.01 μm) for 18 hr at 37°, in some cases, brefeldin A (5 μg/ml) was added during the last 6 hr of culture to inhibit the release of cytokines into the supernatant. (b) Culture supernatants were collected and levels of interleukin-23 (IL-23) were quantified by ELISA. Results are expressed as the cytokine concentrations (pg/ml) and represent the mean ± SEM of four experiments. The percentages of positive cells for IL-12p70 (c) and IL-12p40 (d) are shown and represent the arithmetic mean ± SEM of three experiments. Asterisk represents statistical significance *P< 0.05 for DCs pretreated or not with inhibitors and then exposed to LTC4.
On the other hand, when we evaluated the effect of these inhibitors in culture supernatants, we found that release of IL-23 was independent of the blockade of ERK1/2, as shown in Fig. 6(b); the presence of PD, an antagonist of ERK1/2 MAPK, did not inhibit its production in activated DCs, as expected because in these conditions this pathway was activated by LTC4. Interestingly, the use of SB significantly increased the release of IL-12p70, whereas IL-12p40 was not affected (Fig. 6c,d). These results allow us to conclude that other activation pathways may be involved in the induction of cytokines. However, it should be noted that, under the influence of LTC4 impacting on activated-DCs, p38 plays an essential role in the control of Th1 polarization.
LTC4 promotes the expansion of Th17 lymphocytes by LPS-stimulated DCs
To determine whether LTC4 is capable of defining a Th17 profile by activated DCs, we decided to analyse this point in an MLR. The DCs from C57BL/6 mice were stimulated or not with LPS (1 μg/ml), then cells were untreated or treated with LTC4 (0·01 μm) for 30 min at 37°. Finally, DCs were extensively washed and co-cultured with splenocytes from BALB/c mice. Immature DCs were used as controls. As shown in Fig. 7(a,b), the treatment of LPS-stimulated DCs with LTC4 increased by 50% the percentages of CD4+ T lymphocytes producing IL-17 compared with those induced by LPS alone. These results have an inverse correlation with the proportions of CD4+ T lymphocytes producing IFN-γ. Similar results were obtained to evaluate both cytokines in the supernatants of MLR (Fig. 7c). As treatment of LPS-activated DCs with LTC4 affected the IL-12/IL-23 balance, we investigated whether IL-23 held a central role in mediating the increase of IL-17. For this, co-cultures of DCs and splenocytes were performed in the presence of neutralizing antibodies. The neutralization of IL-23 by an anti-IL-23p19 reduced by more than 20% the percentages of CD4+ IL-17+ cells (Fig. 7d). Hence, IL-23 seems to be an important mediator for the expansion of CD4 T lymphocytes in a Th17 profile.
Figure 7.
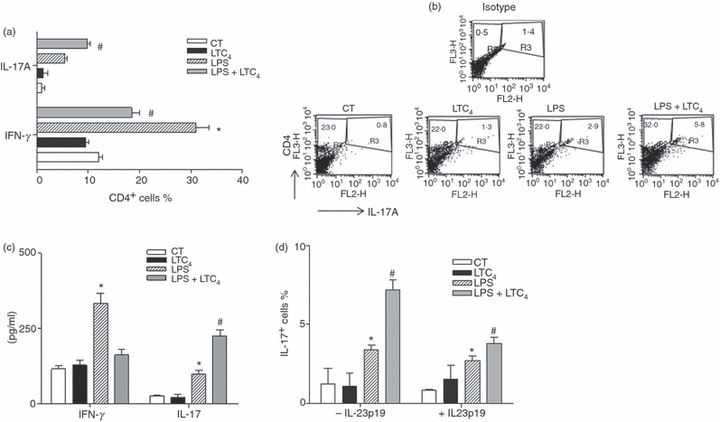
Leukotriene C4 (LTC4) induces the genesis of a T helper type 17 (Th17) profile by lipopolysaccharide (LPS) -stimulated dendritic cells (DCs). DCs obtained from C57BL/6 mice were incubated for 30 min in the presence (LPS, 1 μg/ml) or not (CT) of LPS at 37°. After washing, DCs were treated with or without 0.01 μm LTC4 at 37°. Thirty minutes later; cells were washed and co-cultured with allogeneic lymphocytes obtained from BALB/c mice (5 × 104 DCs/well versus 2.5 × 105 splenocytes/well) at 37° for 24 hr in the presence of PMA (10 ng/ml), ionomycin (1 μg/ml) and 5 μg/ml of brefeldin A to inhibit the cytokine release of supernatants. The percentages of positive CD4 T cells for interleukin-17 (IL-17) and interferon-γ (IFN-γ) (a) are shown and represent the arithmetic mean ± SEM of three experiments. In (b) the representative dot plots to IL-17 and IFN-γ CD4+ lymphocytes is shown. (c) Supernatants were recovered after 36 hr of mixed lymphocyte reaction, IL-17 and IFN-γ concentrations were quantified by ELISA. (d) The percentages of positive CD4 T cells for IL-17 and IFN-γ were analysed in the mixed lymphocyte reaction in the presence of the neutralizing antibody anti IL-23p19 (1 μg/ml) during the 24 hr of co-culture. The bars represent the mean values ± SEM of four independent experiments. Asterisk represents statistical significance (*P< 0.05) versus CT and # respresents significance (#P ≤ 0.05) versus LPS.
Discussion
Cysteinyl LTC4 is a potent lipid mediator of inflammatory reactions, such as asthma, arthritis, gastritis and ischaemia.43,44 It modulates the chemotaxis of DCs from the skin to lymph nodes,23 the only antigen-presenting cell capable of activating naive T lymphocytes.3,4 Previous studies aimed at analysing the effect of LTC4 showed increases in the production of IL-10 by allergen-pulsed DCs, favouring their capacity to increase lung eosinophilia and IL-5 production in a model of murine asthma. This effect involves the CysLTR1, which seems to contribute to the severity of inflammatory responses.45,46
In the present study we observed that DCs and LPS-activated DCs express the two subtypes of cysteinyl receptors. In most systems CysLTR1 was described as responsible for most of inflammatory effects,45–48 but no previous studies have examined the expression of both receptors in murine DCs. Real-time PCR demonstrated that the DCs not only express the CysLTR1, primarily expressed in smooth muscle, eosinophils and other immune cells and generally associated with the induction of bronchospasm and vasoconstriction,18,19 but also the CysLTR2,19 expressed mainly in the heart, prostate, brain, adrenal cells, endothelium and lung, but it is expressed at lower levels on leucocytes, and is more associated with the remodelling of the fibrotic process.19 Several groups have demonstrated the modulation of CysLT receptors by cytokines and inflammatory stimuli.49,50 Thivierge et al.25 demonstrated that human monocytes express both CysLT1 and CysLT2 receptors similarly and their differentiation in DCs inhibits the expression of CysLT1, whereas their maturation with 200 ng/ml LPS increases CysLTR2 expression. In contrast, upon activation of DCs by LPS (1 μg/ml) no variations in the expression of CysLRT1 were observed but there is a greater reduction of CysLRT2. These differences may be the result of the source of DCs as well as of concentrations, methodology and time of LPS stimulation used. Interestingly, incubation with exogenous LTC4 of immature DCs potently up-regulated the expression of CysLTR1, indicating that LTC4 could exert a regulatory mechanism on receptor expression. In this sense, it has been demonstrated in several tissues that the expression of both receptors can be regulated by CysLT release.18,50 The use of montelukast did not allow us to block the production of IL-23, indicating that it could be modulated by the action of LTC4 through the CysLTR2. This point could not be evaluated; because there is still no specific receptor antagonist.
Immature DCs constitutively macropinocytose extracellular fluid,51 and also express a large variety of receptors mediating endocytosis and phagocytosis of antigens and pathogens.5 Previously it was demonstrated that CysLTs are able to induce the phagocytosis of opsonized bacteria through the Fcγ receptors.52 Here, we showed that LTC4 induces the phagocytosis of Zy and also stimulates Dextran and HRP endocytosis by immature DCs. Interestingly, despite the phenotypic changes and antigen capture that produced LTC4 in activated DCs, which might correlate with the alteration of their function as antigen-presenting cells, their capacity to activate naive T lymphocytes remained intact.2–4 Although the LTC4 antagonizes the effect of LPS on the expression of class II molecules and CD86, its expression is greater than that shown by immature DCs. Our hypothesis is that through this mechanism, the LTC4 allows DCs to improve their ability to sense the environment without compromising their capacity to activate an effector response.
The activation of MAPK, including ERK1/2, c-Jun N-terminal kinase and p38 MAPK play an important role in many cellular processes, including differentiation, cellular proliferation, apoptosis and immune response.53,54 The p38 pathway is associated with cytokine induction and inflammation and is strongly activated by inflammatory stimuli.54 Binding of CysLT with their receptors triggers the phosphorylation of MAPK.18,19 Hashimoto et al.55 demonstrated that IL-10 production in human DCs stimulated with Zy was dependent on ERK and p38 MAPK activation. Also, the phagocytosis of opsonized particles by macrophages cultured with LTD4 or LTC4 was associated with p38 activation.56 Our results indicate that LTC4 activates p38 MAPK. Indeed, their inhibition by SB-303080 abrogates the uptake of DX by DCs. Also, ERK1/2 was only activated in LTC4-stimulated DCs. In spite of the previous studies,18,19,52 however, the fact that the blockade of p38 and ERK1/2 MAPK was not able to abolish either IL-12p40 or IL-23 production supports the theory that other pathways could be involved. Consistent with these results, Yang et al.53 reported that inhibition of p38 MAPK can induce Th1 responses through the production by DCs of IL-12p40 and IL-12p70. Therefore, we believe that p38 MAPK phosphorylation acts as a regulatory mechanism of genesis of Th1 profiles. It is known that nuclear factor-κB activation triggered by LPS is controlled by a series of kinases and phosphatases. Chang et al.57 demonstrated that the serine-threonine protein phosphatase A2 (PPA2) binds inhibitor of κB kinase, a subunit of nuclear factor-κB, mechanism which prevents the production of IL-23. Future experiments will define whether the LTC4 is capable of preventing the binding or synthesis of PPA2.
We report that LTC4 abolishes completely in DCs the secretion of IL-12p70, the biological chain of IL-12, triggered by LPS, but enhances p40, the common chain to IL-12/IL-23. The partial or complete reversal of production of IL-12p70 by LPS-activated DCs has been linked to stimuli as diverse as prostaglandins, histamine, alkaloids and phenolic products.58–61 In relation to CysLT, in terms of cytokines, the results are contradictory. Machida et al.40 described in Derf-pulsed DCs from bone marrow precursors how antagonists of CysLTR1 led to the enhancement of IL-12p40 while IL-10 was inhibited. On the other hand, in allergen-pulsed DCs from spleen there was strong inhibition of both IL-10 and IL-12p70 in the presence of CysLTR1 antagonists.62 These differences can be explained by the origin of the DCs used in each study; however, the main difference would be the nature of the stimuli used, we evaluated the effect of LTC4 in DCs activated with LPS, a classic Toll-like receptor 4 agonist, which triggers a Th1 profile compared with the allergens that trigger Th2 responses.
The strong inhibition of IL-12p70 release, together with the increased production of IL-23, represent a suitable microenvironment induced by LTC4 acting on inflammatory DCs resulting in the expansion of Th17 cells, as demonstrated by the higher proportions of IL-17+ lymphocytes compared with the IFN-γ+ lymphocytes expanded in vitro. Despite the fact that in MLR the neutralization of IL-23 did not completely abrogate the percentages of CD4+ IL-17+ cells, this cytokine seems to play a major role in the induction of the Th17 response, at least in mice. The Th17 lymphocytes63 can be induced by IL-23 in the presence of IL-6 and IL-1β in mice. In agreement with our results, previous reports also described the induction of Th17 profiles through the release of IL-23 by inflammatory DCs.64,65 That DCs are inflammatory as derived from bone marrow precursors26 is probably critical for the induction of CD4+ cells producing IL-17 against lipid mediators such as prostaglandin E2 and LTC4.
It is known that Th17 cells mediate protection against extracellular pathogens via neutrophil recruitment,66 but also play a central role in immunopathology.67 Ours results open the way to further studies on the potential role of LTC4 in inflammatory disorders such as gastritis, cystic fibrosis,68,69 inflammatory pathologies associated with a greater recruitment of neutrophils in which the levels of LTC4 and its receptors are excessively increased.19–22 In conclusion, here we provide evidence that ‘maturity’ of DCs and the stimulus that causes it, is critical for the balance of the effector profile induced by LTC4. Therefore, LTC4 prevents the complete maturation of DCs but induces the production of IL-23, resulting in the preferential development of Th17 cells. These results could partially explain the mechanism triggered by LTC4 in inflammatory disorders.
Acknowledgments
We thank Beatriz Loria and Edith Mabel Horvat for their technical assistance. This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), School of Medicine, Buenos Aires University, and Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
Glossary
Abbreviations:
- CysLTR
cysteinyl receptors
- DCs
dendritic cells
- DX
dextran
- HRP
horseradish peroxidase
- LTC4
leukotriene C4
- Zy
zymosan
Disclosures
The authors have no conflicts of interest.
References
- 1.Lutz MB, Kurts C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol. 2009;32:2325–30. doi: 10.1002/eji.200939548. [DOI] [PubMed] [Google Scholar]
- 2.Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–47. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 4.Reis e Sousa C. Activation of dendritic cells: translating innate into adaptative immunity. Curr Opin Immunol. 2006;16:21–5. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Sabatté J, Maggini J, Nahmod K, et al. Interplay of pathogens, cytokines and other stress signals in the regulation of dendritic cell function. Cytokine Growth Factor Rev. 2007;18:5–17. doi: 10.1016/j.cytogfr.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Ricart BG, John B, Lee D, Hunter CA, Hammer DA. Dendritic cells distinguish individual chemokine signals through CCR7 and CXCR4. J Immunol. 2011;186:53–61. doi: 10.4049/jimmunol.1002358. [DOI] [PubMed] [Google Scholar]
- 7.Lombardi V, Van Overtvelt L, Horiot S, Moingeon P. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-gamma, and IL-17A by naive CD4+ T cells. J Immunol. 2009;182:3372–9. doi: 10.4049/jimmunol.0801969. [DOI] [PubMed] [Google Scholar]
- 8.Paustian C, Caspell R, Johnson T, Cohen PA, Shu S, Xu S, Czerniecki BJ, Koski GK. Effect of multiple activation stimuli on the generation of Th1-polarizing dendritic cells. Hum Immunol. 2011;72:24–31. doi: 10.1016/j.humimm.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Rossetti M, Gregori S, Roncarolo MG. Granulocyte-colony stimulating factor drives the in vitro differentiation of human dendritic cells that induce anergy in naïve T cells. Eur J Immunol. 2010;40:3097–106. doi: 10.1002/eji.201040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIlroy A, Caron G, Blanchard S, Frémaux I, Duluc D, Delneste Y, Chevailler A, Jeannin P. Histamine and prostaglandin E up-regulate the production of Th2-attracting chemokines (CCL17 and CCL22) and down-regulate IFN-gamma-induced CXCL10 production by immature human dendritic cells. Immunology. 2006;117:507–16. doi: 10.1111/j.1365-2567.2006.02326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivas-Carvalho A, Meraz-Ríos MA, Santos-Argumedo L, Bajaña S, Soldevila G, Moreno-García ME, Sánchez-Torres C. CD16+ human monocyte-derived dendritic cells matured with different and unrelated stimuli promote similar allogeneic Th2 responses: regulation by pro- and anti-inflammatory cytokines. Int Immunol. 2004;16:1251–63. doi: 10.1093/intimm/dxh127. [DOI] [PubMed] [Google Scholar]
- 12.Amaral MM, Davio C, Ceballos A, Salamone G, Cañones C, Geffner J, Vermeulen M. Histamine improves antigen uptake and cross-presentation by dendritic cells. J Immunol. 2007;179:3425–33. doi: 10.4049/jimmunol.179.6.3425. [DOI] [PubMed] [Google Scholar]
- 13.Rinaldo-Matthis A, Haeggström JZ. Structures and mechanisms of enzymes in the leukotriene cascade. Biochimie. 2010;92:676–81. doi: 10.1016/j.biochi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Austen KF, Maekawa A, Kanaoka Y, Boyce JA. The leukotriene E4 puzzle: finding the missing pieces and revealing the pathobiologic implications. J Allergy Clin Immunol. 2009;124:406–14. doi: 10.1016/j.jaci.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaidyanathan S, Williamson P, Clearie K, Morrison A, Lipworth B. Nasal AMP and histamine challenge within and outside the pollen season in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2011;127:173–8. doi: 10.1016/j.jaci.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Muraki M, Imbe S, Sato R, Ikeda Y, Yamagata S, Iwanaga T, Tohda Y. Inhaled montelukast inhibits cysteinyl-leukotriene-induced bronchoconstriction in ovalbumin-sensitized guinea-pigs: the potential as a new asthma medication. Int Immunopharmacol. 2009;9:1337–41. doi: 10.1016/j.intimp.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Poulin S, Thompson C, Thivierge M, Véronneau S, McMahon S, Dubois CM, Stankova J, Rola-Pleszczynski M. Cysteinyl-leukotrienes induce vascular endothelial growth factor production in human monocytes and bronchial smooth muscle cells. Clin Exp Allergy. 2011;41:204–17. doi: 10.1111/j.1365-2222.2010.03653.x. [DOI] [PubMed] [Google Scholar]
- 18.Singh RK, Gupta S, Dastidar S, Ray A. Cysteinyl leukotrienes and their receptors: molecular and functional characteristics. Pharmacology. 2010;85:336–49. doi: 10.1159/000312669. [DOI] [PubMed] [Google Scholar]
- 19.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–10. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- 20.Cho SH. Pharmacogenomic approaches to asthma treatment. Allergy Asthma Immunol Res. 2010;2:177–82. doi: 10.4168/aair.2010.2.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tintinger GR, Feldman C, Theron AJ, Anderson R. Montelukast: more than a cysteinyl leukotriene receptor antagonist? Scientific World Journal. 2010;10:2403–13. doi: 10.1100/tsw.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimbori C, Shiota N, Okunishi H. Effects of montelukast, a cysteinyl-leukotriene type 1 receptor antagonist, on the pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Eur J Pharmacol. 2011;650:424–30. doi: 10.1016/j.ejphar.2010.09.084. [DOI] [PubMed] [Google Scholar]
- 23.Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C4 transporter MRP1 regulates CCL19 (MIP-3β, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–68. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 24.Jozefowski S, Biedroń R, Bobek M, Marcinkiewicz J. Leukotrienes modulate cytokine release from dendritic cells. Immunology. 2005;116:418–28. doi: 10.1111/j.1365-2567.2005.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thivierge M, Stankova J, Rola-Pleszczynski M. Cysteinyl-leukotriene receptor type 1 expression and function is down-regulated during monocyte-derived dendritic cell maturation with zymosan: involvement of IL-10 and prostaglandins. J Immunol. 2009;83:6778–87. doi: 10.4049/jimmunol.0901800. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577–84. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
- 27.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–7. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz MB, Assmann CU, Girolomoni G, Ricciardi-Castagnoli P. Different cytokines regulate antigen uptake and presentation of a precursor dendritic cell line. Eur J Immunol. 1996;26:586–9. doi: 10.1002/eji.1830260313. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropynocitosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: down regulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ono E, Taniguchi M, Higashi N, et al. Increase in salivary cysteinyl-leukotriene concentration in patients with aspirin-intolerant asthma. Allergol Int. 2011;60:37–43. doi: 10.2332/allergolint.09-OA-0166. [DOI] [PubMed] [Google Scholar]
- 31.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Yong-Jun L, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka Y, Taneichi M, Kasai M, Kakiuchi T, Uchida T. Liposome-coupled antigens are internalized by antigen-presenting cells via pinocytosis and cross-presented to CD8 T cells. PLoS One. 2010;5:e15225. doi: 10.1371/journal.pone.0015225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahlin S, Hed J, Rundquist I. Differentiation between attached and ingested immune complexes by a fluorescence quenching cytofluorometric assay. J Immunol Methods. 1983;60:115–24. doi: 10.1016/0022-1759(83)90340-x. [DOI] [PubMed] [Google Scholar]
- 34.Conzelmann M, Wagner AH, Hildebrandt A, et al. IFN-γ activated JAK1 shifts CD40-induced cytokine profiles in human antigen-presenting cells toward high IL-12p70 and low IL-10 production. Biochem Pharmacol. 2010;80:2074–86. doi: 10.1016/j.bcp.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 35.Jensen SS, Gad M. Differential induction of inflammatory cytokines by dendritic cells treated with novel TLR-agonist and cytokine based cocktails: targeting dendritic cells in autoimmunity. J Inflamm. 2010;7:37–49. doi: 10.1186/1476-9255-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregori S. Dendritic cells in networks of immunological tolerance. Tissue Antigens. 2011;77:89–99. doi: 10.1111/j.1399-0039.2010.01615.x. [DOI] [PubMed] [Google Scholar]
- 37.Saito M, Nagasawa M, Takada H, et al. Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J Exp Med. 2011;208:235–49. doi: 10.1084/jem.20100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doisne JM, Soulard V, Bécourt C, et al. Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1– invariant NKT cells to bacteria. J Immunol. 2011;186:662–6. doi: 10.4049/jimmunol.1002725. [DOI] [PubMed] [Google Scholar]
- 39.Jackson AM, Mulcahy LA, Porte J, et al. Role of mitogen-activated protein kinase and PI3K pathways, in the regulation of IL-12-family cytokines in dendritic cells and the generation of T H-responses. Eur Cytokine Netw. 2010;21:319–28. doi: 10.1684/ecn.2010.0219. [DOI] [PubMed] [Google Scholar]
- 40.Machida I, Matsuse H, Kondo Y, et al. Cysteinyl leukotrienes regulate dendritic cell functions in a murine model of asthma. J Immunol. 2004;172:1833–8. doi: 10.4049/jimmunol.172.3.1833. [DOI] [PubMed] [Google Scholar]
- 41.Liu YY, Cai WF, Yang HZ, et al. Bacillus Calmette–Guérin and TLR4 agonist prevent cardiovascular hypertrophy and fibrosis by regulating immune microenvironment. J Immunol. 2008;180:7349–57. doi: 10.4049/jimmunol.180.11.7349. [DOI] [PubMed] [Google Scholar]
- 42.Yuan YM, Fang SH, Qian XD, et al. Leukotriene D4 stimulates the migration but not proliferation of endothelial cells mediated by the cysteinyl leukotriene cyslt (1) receptor via the extracellular signal-regulated kinase pathway. J Pharmacol Sci. 2009;109:285–92. doi: 10.1254/jphs.08321fp. [DOI] [PubMed] [Google Scholar]
- 43.Bisgaard H. Pathophysiology of the cysteinyl leukotrienes and effects of leukotriene receptor antagonists in asthma. Allergy. 2001;66:7–11. doi: 10.1034/j.1398-9995.56.s66.2.x. [DOI] [PubMed] [Google Scholar]
- 44.Riccioni G, Zanasi A, Vitulano N, Mancini B, D'Orazio N. Leukotrienes in atherosclerosis: new target insights and future therapy perspectives. Mediators Inflamm. 2009;2009:737282–8. doi: 10.1155/2009/737282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ihaku D, Cameron L, Suzuki M, Molet S, Martin J, Hamid Q. Montelukast, a leukotriene receptor antagonist, inhibits the late airway response to antigen, airway eosinophilia, and IL-5-expressing cells in Brown Norway rats. J Allergy Clin Immunol. 1999;104:1147–54. doi: 10.1016/s0091-6749(99)70006-0. [DOI] [PubMed] [Google Scholar]
- 46.Hisada T, Salmon M, Nasuhara Y, Chung KF. Cysteinyl-leukotrienes partly mediate eotaxin-induced bronchial hyperresponsiveness and eosinophilia in IL-5 transgenic mice. Am J Respir Crit Care Med. 1999;160:571–5. doi: 10.1164/ajrccm.160.2.9810101. [DOI] [PubMed] [Google Scholar]
- 47.Kiwamoto T, Ishii Y, Morishima Y, et al. Blockade of cysteinyl leukotriene-1 receptors suppresses airway remodelling in mice overexpressing GATA-3. Clin Exp Allergy. 2011;41:116–28. doi: 10.1111/j.1365-2222.2010.03571.x. [DOI] [PubMed] [Google Scholar]
- 48.Wu AY, Chik SC, Chan AW, Li Z, Tsang KW, Li W. Anti-inflammatory effects of high-dose montelukast in an animal model of acute asthma. Clin Exp Allergy. 2003;33:359–66. doi: 10.1046/j.1365-2222.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- 49.Magnusson C, Mezhybovska M, Lörinc E, Fernebro E, Nilbert M, Sjölander A. Low expression of CysLT1R and high expression of CysLT2R mediate good prognosis in colorectal cancer. Eur J Cancer. 2010;46:826–35. doi: 10.1016/j.ejca.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Magnusson C, Ehrnström R, Olsen J, Sjölander A. An increased expression of cysteinyl leukotriene 2 receptor in colorectal adenocarcinomas correlates with high differentiation. Cancer Res. 2007;67:9190–8. doi: 10.1158/0008-5472.CAN-07-0771. [DOI] [PubMed] [Google Scholar]
- 51.Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol. 2002;291:487–94. doi: 10.1078/1438-4221-00157. [DOI] [PubMed] [Google Scholar]
- 52.Silva RC, Landgraf MA, Hiyane MI, Pacheco-Silva A, Câmara NO, Landgraf RG. Leukotrienes produced in allergic lung inflammation activate alveolar macrophages. Cell Physiol Biochem. 2010;26:319–26. doi: 10.1159/000320555. [DOI] [PubMed] [Google Scholar]
- 53.Yang Z, Zhang X, Darrah PA, Mosser DM. The regulation of Th1 responses by the p38 MAPK. J Immunol. 2010;185:6205–13. doi: 10.4049/jimmunol.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol. 2005;83:1–14. doi: 10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto K, Ichiyama T, Hasegawa M, Hasegawa S, Matsubara T, Furukawa S. Cysteinyl leukotrienes induce monocyte chemoattractant protein-1 in human monocyte/macrophages via mitogen-activated protein kinase and nuclear factor-kappaB pathways. Int Arch Allergy Immunol. 2009;149:275–82. doi: 10.1159/000199724. [DOI] [PubMed] [Google Scholar]
- 56.Campos MR, Serezani CH, Peters-Golden M, Jancar S. Differential kinase requirement for enhancement of Fc gammaR-mediated phagocytosis in alveolar macrophages by leukotriene B4 vs. D4. Mol Immunol. 2009;46:1204–11. doi: 10.1016/j.molimm.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 57.Chang JH, Voorhees TJ, Liu J, Zhao Y, Chang C-H. Interleukin-23 production in dendritic cells is negatively regulated by protein phosphatase 2A. PNAS. 2009;107:8340–5. doi: 10.1073/pnas.0914703107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim GY, Kim KH, Lee SH, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-κB as potential targets. J Immunol. 2005;174:8116–24. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 59.Kang BY, Chung SW, Cho D, Kim TS. Involvement of p38 mitogen-activated protein kinase in the induction of interleukin-12 p40 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid. Biochem Pharmacol. 2002;63:1901–10. doi: 10.1016/s0006-2952(02)00982-6. [DOI] [PubMed] [Google Scholar]
- 60.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–9. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 61.Li CY, Chao LK, Wang SC, et al. Honokiol inhibits LPS-induced maturation and inflammatory response of human monocyte-derived dendritic cells. J Cell Physiol. 2011;226:2338–49. doi: 10.1002/jcp.22576. [DOI] [PubMed] [Google Scholar]
- 62.Okunishi K, Dohi M, Nakagome K, Tanaka R, Yamamoto K. A novel role of cysteinyl leukotrienes to promote dendritic cell activation in the antigen-induced immune responses in the lung. J Immunol. 2004;173:6393–402. doi: 10.4049/jimmunol.173.10.6393. [DOI] [PubMed] [Google Scholar]
- 63.Kyburz D, Corr M. Th17 cells generated in the absence of TGF-β induce experimental allergic encephalitis upon adoptive transfer. Expert Rev Clin Immunol. 2011;7:283–5. doi: 10.1586/eci.11.7. [DOI] [PubMed] [Google Scholar]
- 64.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–35. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, Ganea D. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23–IL-17 axis. J Immunol. 2007;178:8138–47. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 66.Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int Immunol. 2011;23:317–26. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Nitto D, Sarra M, Cupi ML, Pallone F, Monteleone G. Targeting IL-23 and Th17-cytokines in inflammatory bowel diseases. Curr Pharm Des. 2010;16:3656–60. doi: 10.2174/138161210794079164. [DOI] [PubMed] [Google Scholar]
- 68.Stelmach I, Korzeniewska A, Stelmach W, Majak P, Grzelewski T, Jerzynska J. Effects of montelukast treatment on clinical and inflammatory variables in patients with cystic fibrosis. Ann Allergy Asthma Immunol. 2005;95:372–80. doi: 10.1016/S1081-1206(10)61156-8. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed A, Holton J, Vaira D, Smith SK, Hoult JR. Eicosanoid synthesis and Helicobacter pylori associated gastritis: increase in leukotriene C4 generation associated with H. pylori colonization. Prostaglandins. 1992;44:75–86. doi: 10.1016/0090-6980(92)90109-7. [DOI] [PubMed] [Google Scholar]


