Abstract
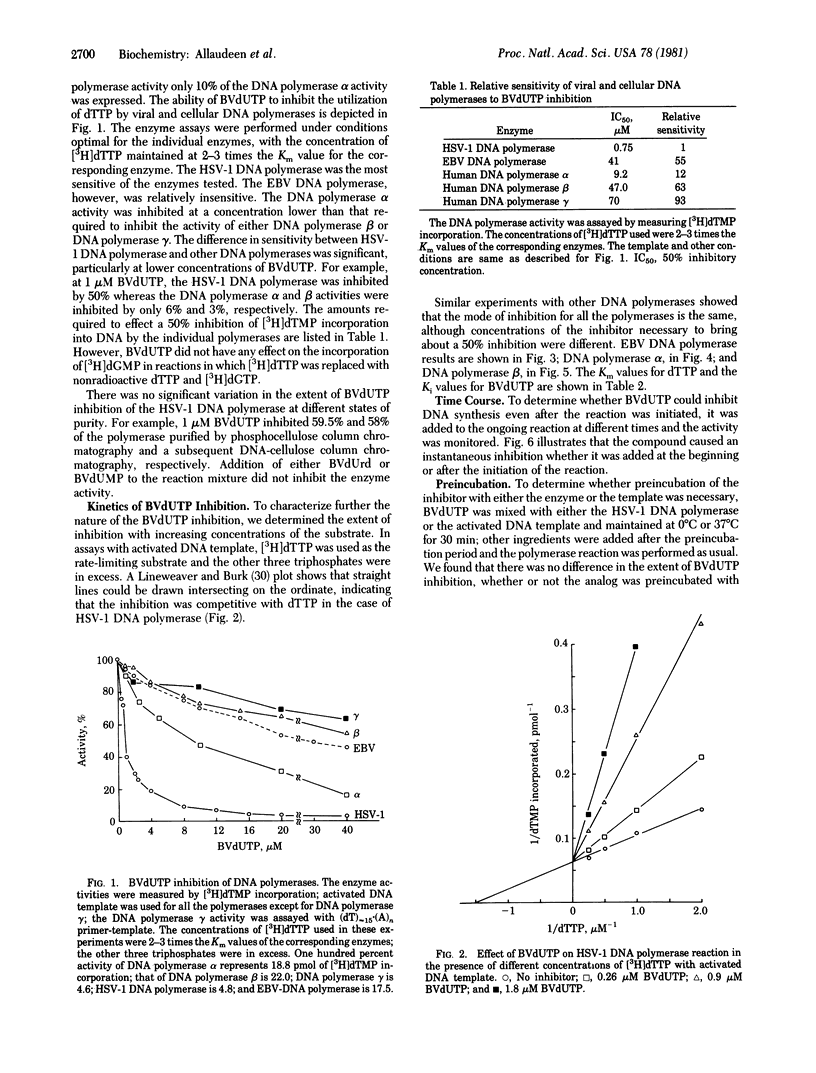
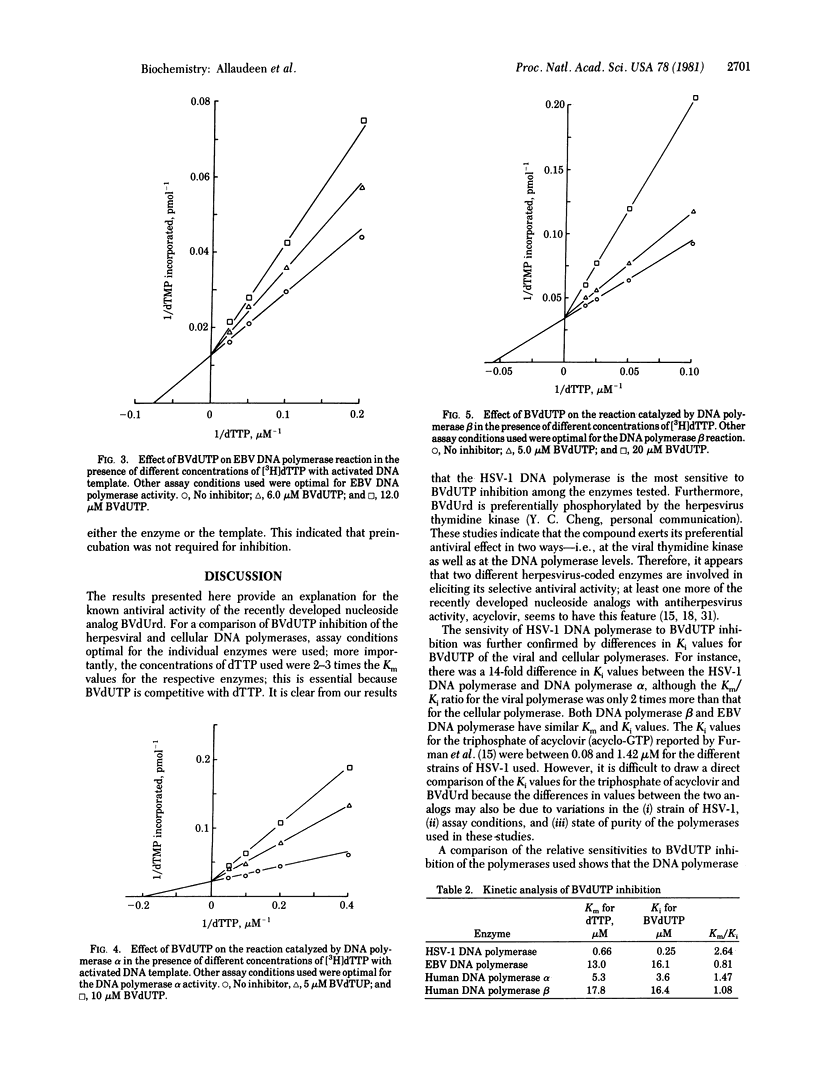
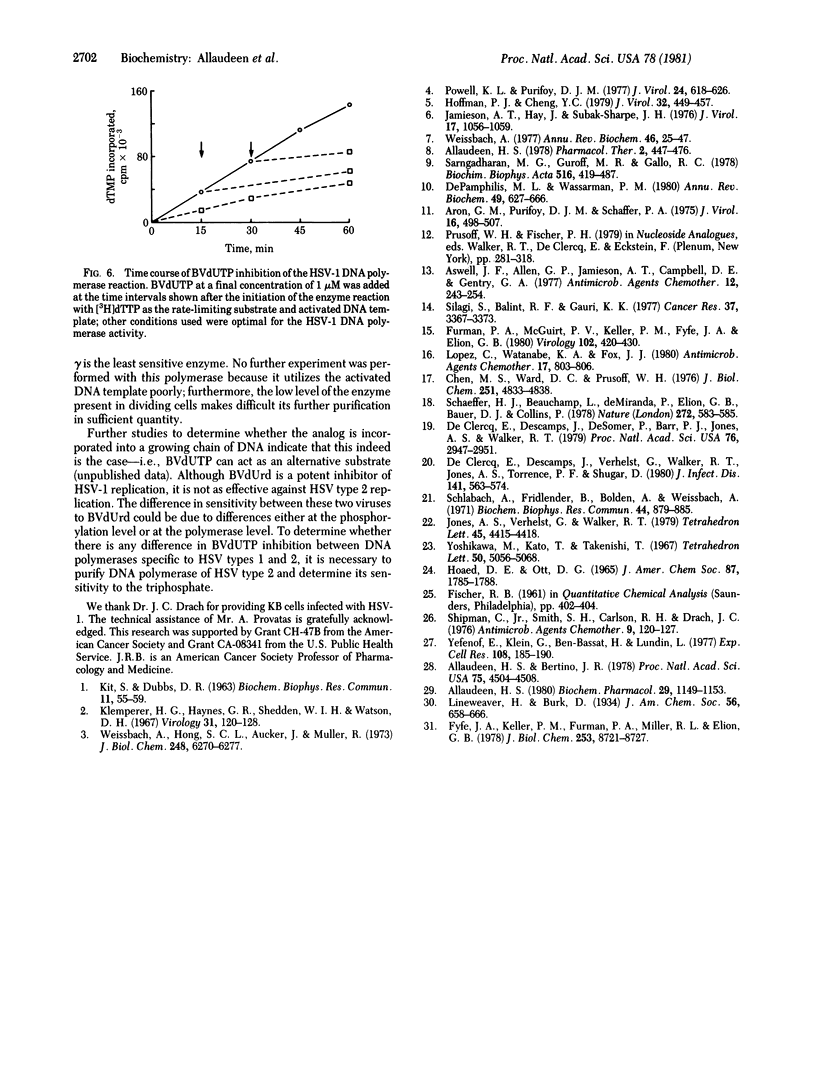
Bromovinyldeoxyuridine (BVdUrd) is a potent antiherpesvirus compound with low cytotoxicity. To gain an insight into its selectivity and mechanism of inhibition, we chemically synthesized the 5'-triphosphate of BVdUrd, BVdUTP, and tested its effect on the activities of DNA polymerases [DNA nucleotidyltransferase (DNA directed), EC 2.7.7.7] of two herpesviruses--i.e., herpes simplex virus type 1 (HSV-1) and Epstein-Barr virus (EBV)--as well as cellular DNA polymerases alpha, beta, and gamma. The effects on the DNA polymerases were determined under assay conditions optimal for the individual polymerases. We found that the BVdUTP was considerably more inhibityory to the utilization of dTTP by the HSV-1 DNA polymerase then by the cellular DNA polymerases. For instance, as little as 1 microM BVdUTP inhibited the utilization of dTTP by HSV-1 DNA polymerase 50%, whereas the same concentration inhibited the DNA polymerase alpha and the DNA polymerase beta activities only 9% and 3%, respectively. The BVdUTP inhibited DNA synthesis by competing with the natural substrate, dTTP. The Km for dTTP and the Ki for the BVdUTP of the HSV-1 DNA polymerase were 0.66 and 0.25 microM, respectively. Kinetic analyses with the DNA polymerases alpha and beta and the EBV DNA polymerase also reflected a similar difference in sensitivity between the HSV-1 enzyme and other enzymes. Increasing the concentration of either the DNA template or the enzyme in the reaction mixture did not bring about a significant change in the extent of inhibition. Preincubation of the inhibitor with the enzyme was not necessary for inhibition. Studies on time course of inhibition revealed that the compound is inhibitory even after the initiation of DNA synthesis. These studies indicate that the ability of BVdUTP to preferentially inhibit the HSV-1 DNA polymerase may contribute towards its selective inhibition of the viral DNA replication in infected cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Bertino J. R. Isolation of a herpesvirus-specific DNA polymerase from tissues of an American patient with Burkitt lymphoma. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4504–4508. doi: 10.1073/pnas.75.9.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaudeen H. S. Inhibition of deoxyribonucleic acid polymerases of human leukemic leukocytes by 2',3'-dideoxythymidine triphosphate. Biochem Pharmacol. 1980 Apr 15;29(8):1149–1153. doi: 10.1016/0006-2952(80)90410-4. [DOI] [PubMed] [Google Scholar]
- Aron G. M., Purifoy D. J., Schaffer P. A. DNA synthesis and DNA polymerase activity of herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1975 Sep;16(3):498–507. doi: 10.1128/jvi.16.3.498-507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswell J. F., Allen G. P., Jamieson A. T., Campbell D. E., Gentry G. A. Antiviral activity of arabinosylthymine in herpesviral replication: mechanism of action in vivo and in vitro. Antimicrob Agents Chemother. 1977 Aug;12(2):243–254. doi: 10.1128/aac.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S., Ward D. C., Prusoff W. H. Specific herpes simplex virus-induced incorporation of 5-iodo-5'-amino-2',5'-dideoxyuridine into deoxyribonucleic acid. J Biol Chem. 1976 Aug 25;251(16):4833–4838. [PubMed] [Google Scholar]
- De Clercq E., Descamps J., De Somer P., Barr P. J., Jones A. S., Walker R. T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., Verhelst G., Walker R. T., Jones A. S., Torrence P. F., Shugar D. Comparative efficacy of antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980 May;141(5):563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Furman P. A., McGuirt P. V., Keller P. M., Fyfe J. A., Elion G. B. Inhibition by acyclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Virology. 1980 Apr 30;102(2):420–430. doi: 10.1016/0042-6822(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. DNase induced after infection of KB cells by herpes simplex virus type 1 or type 2. II. Characterization of an associated endonuclease activity. J Virol. 1979 Nov;32(2):449–457. doi: 10.1128/jvi.32.2.449-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Hay J., Subak-Sharpe J. H. Herpesvirus proteins: induction of nucleoside phosphotransferase activity after herpes simplex virus infection. J Virol. 1976 Mar;17(3):1056–1059. doi: 10.1128/jvi.17.3.1056-1059.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Acquisition of thymidine kinase activity by herpes simplex-infected mouse fibroblast cells. Biochem Biophys Res Commun. 1963 Apr 2;11:55–59. doi: 10.1016/0006-291x(63)90027-5. [DOI] [PubMed] [Google Scholar]
- Klemperer H. G., Haynes G. R., Shedden W. I., Watson D. H. A virus-specific thymidine kinase in BHK-21 cells infected with herpes simplex virus. Virology. 1967 Jan;31(1):120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- Lopez C., Watanabe K. A., Fox J. J. 2'-fluoro-5-iodo-aracytosine, a potent and selective anti-herpesvirus agent. Antimicrob Agents Chemother. 1980 May;17(5):803–806. doi: 10.1128/aac.17.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. Nonstructural proteins of herpes simplex virus. I. Purification of the induced DNA polymerase. J Virol. 1977 Nov;24(2):618–626. doi: 10.1128/jvi.24.2.618-626.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarngadharan M. G., Robert-Guroff M., Gallo R. C. DNA polymerases of normal and neoplastic mammalian cells. Biochim Biophys Acta. 1978 Dec 11;516(4):419–487. doi: 10.1016/0304-419x(78)90019-7. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Schlabach A., Fridlender B., Bolden A., Weissbach A. DNA-dependent DNA polymerases from HeLa cell nuclei. II. Template and substrate utilization. Biochem Biophys Res Commun. 1971 Aug 20;44(4):879–885. doi: 10.1016/0006-291x(71)90793-5. [DOI] [PubMed] [Google Scholar]
- Shipman C., Jr, Smith S. H., Carlson R. H., Drach J. C. Antiviral activity of arabinosyladenine and arabinosylhypoxanthine in herpes simplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in synchronized suspension cultures. Antimicrob Agents Chemother. 1976 Jan;9(1):120–127. doi: 10.1128/aac.9.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silagi S., Balint R. F., Gauri K. K. Comparative effects on growth and tumorigenicity of mouse melanoma cells by thymidine and its analogs, 5-ethyl- and 5-bromodeoxyuridine. Cancer Res. 1977 Sep;37(9):3367–3373. [PubMed] [Google Scholar]
- Weissbach A. Eukaryotic DNA polymerases. Annu Rev Biochem. 1977;46:25–47. doi: 10.1146/annurev.bi.46.070177.000325. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]
- Yefenof E., Klein G., Ben-Bassat H., Lundin L. Differences in the ConA-induced redistribution and agglutination patterns of EBV genome-free and EBV-carrying human lymphoma lines. Exp Cell Res. 1977 Aug;108(1):185–190. doi: 10.1016/s0014-4827(77)80024-4. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Kato T., Takenishi T. A novel method for phosphorylation of nucleosides to 5'-nucleotides. Tetrahedron Lett. 1967 Dec;50:5065–5068. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]


