Abstract
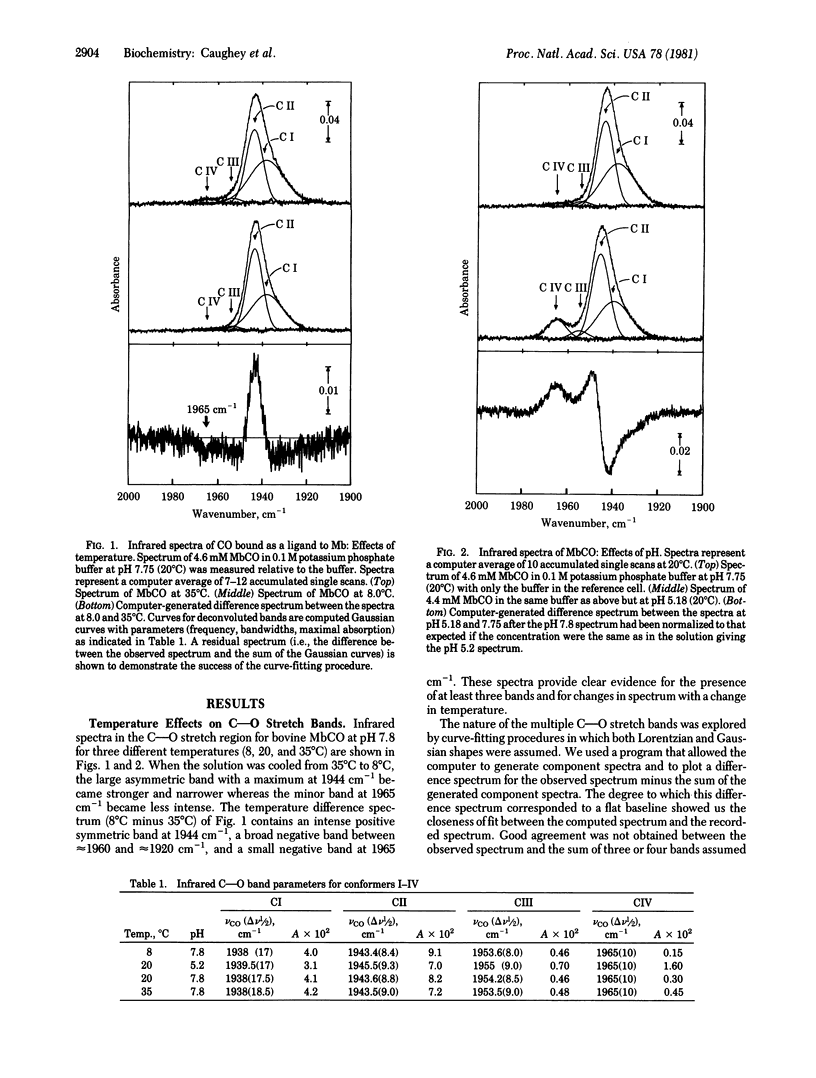
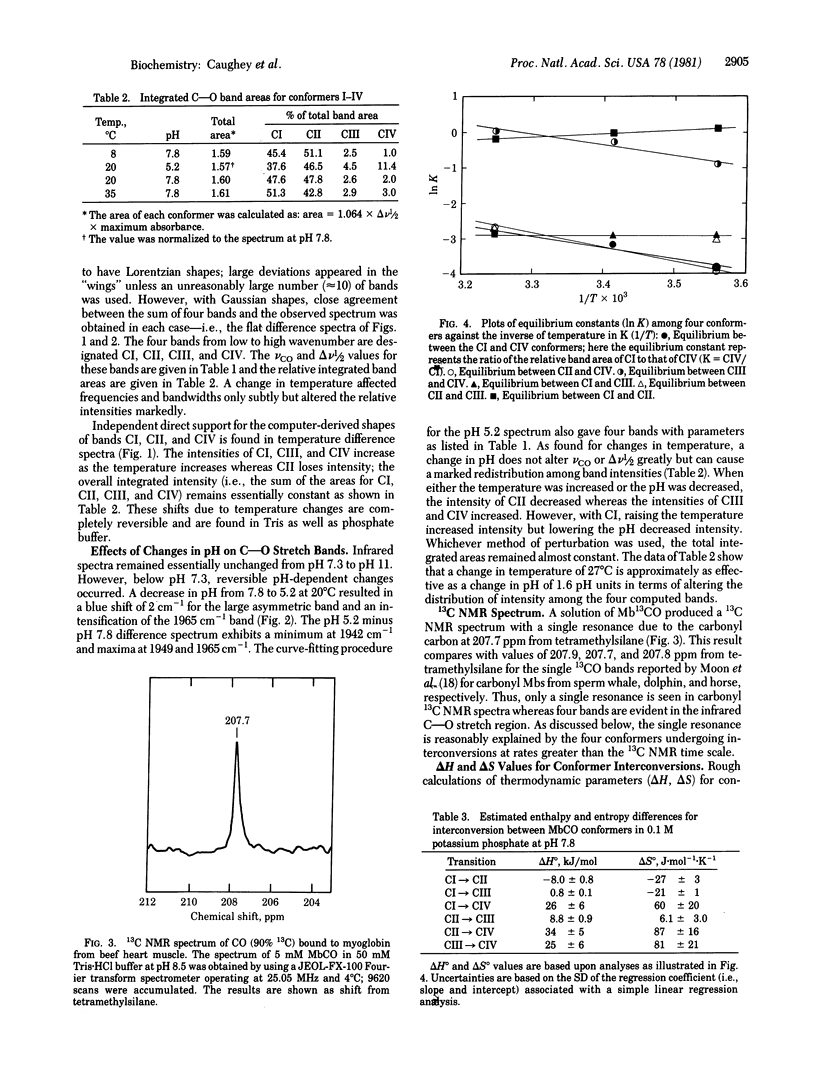
Infrared spectra for the carbon monoxide complex with myoglobin isolated as the oxygenyl species from bovine heart muscle were carefully examined in the C--O stretch region as either the pH or the temperature was varied. Deconvolutions of these spectra into bands of Gaussian shape suggest the presence of four bands near 1938(I), 1944(II), 1954(III), and 1965(IV) cm-1 with halfband widths of about 18, 9, 9, and 10 cm-1, respectively. The relative intensities of the four bands varied with changes in pH or temperature. 13C NMR spectra and other evidence indicate that the four C--O stretch bands arise from four discrete rapidly interconverting conformers: CI, CII, CIII, and CIV. Under conditions of physiological pH and temperature, the relative stabilities are CI approximately CII much greater than CIII approximately CIV. The delta H and delta S values for conformer interconversions are estimated to range from -8 to 34 kJ/mol and -27 to 87 J.mol-1 K-1, respectively; therefore the structures of the conformers may be expected to vary significantly. These findings provide evidence for a highly flexible, dynamic structure at the ligand-binding site of bovine myoglobin, even when ligands are bound.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M. The structure of human carbonmonoxy haemoglobin at 2.7 A resolution. J Mol Biol. 1980 Jan 15;136(2):103–128. doi: 10.1016/0022-2836(80)90308-3. [DOI] [PubMed] [Google Scholar]
- Case D. A., Karplus M. Dynamics of ligand binding to heme proteins. J Mol Biol. 1979 Aug 15;132(3):343–368. doi: 10.1016/0022-2836(79)90265-1. [DOI] [PubMed] [Google Scholar]
- Caughey J. M., Lumb W. V., Caughey W. S. Detection and characterization of nitrous oxide sites in the brain of a dog under halothane-N2O anesthesia. Biochem Biophys Res Commun. 1977 Oct 10;78(3):897–903. doi: 10.1016/0006-291x(77)90507-1. [DOI] [PubMed] [Google Scholar]
- Choc M. G., Caughey W. S. Evidence from infrared and 13C NMR spectra for discrete rapidly interconverting conformers at the carbon monoxide binding sites of hemoglobins A and Zurich. J Biol Chem. 1981 Feb 25;256(4):1831–1838. [PubMed] [Google Scholar]
- Coughey W. S., Alben J. O., McCoy S., Boyer S. H., Charache S., Hathaway P. Differences in the infrared stretching frequency of carbon monoxide bound to abnormal hemoglobins. Biochemistry. 1969 Jan;8(1):59–62. doi: 10.1021/bi00829a009. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Fuchsman W. H., Appleby C. A. CO and O2 complexes of soybean leghemoglobins: pH effects upon infrared and visible spectra. Comparisons with CO and O2 complexes of myoglobin and hemoglobin. Biochemistry. 1979 Apr 3;18(7):1309–1321. doi: 10.1021/bi00574a030. [DOI] [PubMed] [Google Scholar]
- Goto T., Shikama K. Autoxidation of native oxymyoglobin from bovine heart muscle. Arch Biochem Biophys. 1974 Aug;163(2):476–481. doi: 10.1016/0003-9861(74)90504-9. [DOI] [PubMed] [Google Scholar]
- Heidner E. J., Ladner R. C., Perutz M. F. Structure of horse carbonmonoxyhaemoglobin. J Mol Biol. 1976 Jul 5;104(3):707–722. doi: 10.1016/0022-2836(76)90130-3. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Houtchens R. A., Caughey W. S. Structure of carboxymyoglobin in crystals and in solution. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6042–6046. doi: 10.1073/pnas.76.12.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell J. C., Caughey W. S. Infrared spectroscopy of ligands, gases, and other groups in aqueous solutions and tissue. Methods Enzymol. 1978;54:302–323. doi: 10.1016/s0076-6879(78)54021-4. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Dill K., Richards J. H. Magnetic resonance studies of the binding of 13C-labeled carbon monoxide to myoglobins and hemoglobins containing modified hemes. Biochemistry. 1977 Jan 25;16(2):221–228. doi: 10.1021/bi00621a010. [DOI] [PubMed] [Google Scholar]
- Norvell J. C., Nunes A. C., Schoenborn B. P. Neutron diffraction analysis of myoglobin: structure of the carbon monoxide derivative. Science. 1975 Nov 7;190(4214):568–570. doi: 10.1126/science.1188354. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Mathews F. S. An x-ray study of azide methaemoglobin. J Mol Biol. 1966 Oct 28;21(1):199–202. doi: 10.1016/0022-2836(66)90088-x. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]
- Tucker P. W., Phillips S. E., Perutz M. F., Houtchens R., Caughey W. S. Structure of hemoglobins Zürich [His E7(63)beta replaced by Arg] and Sydney [Val E11(67)beta replaced by Ala] and role of the distal residues in ligand binding. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1076–1080. doi: 10.1073/pnas.75.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAZAKI I., YOKOTA K. N., SHIKAMA K. PREPARATION OF CRYSTALLINE OXYMYOGLOBIN FROM HORSE HEART. J Biol Chem. 1964 Dec;239:4151–4153. [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]


