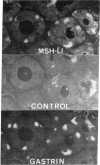
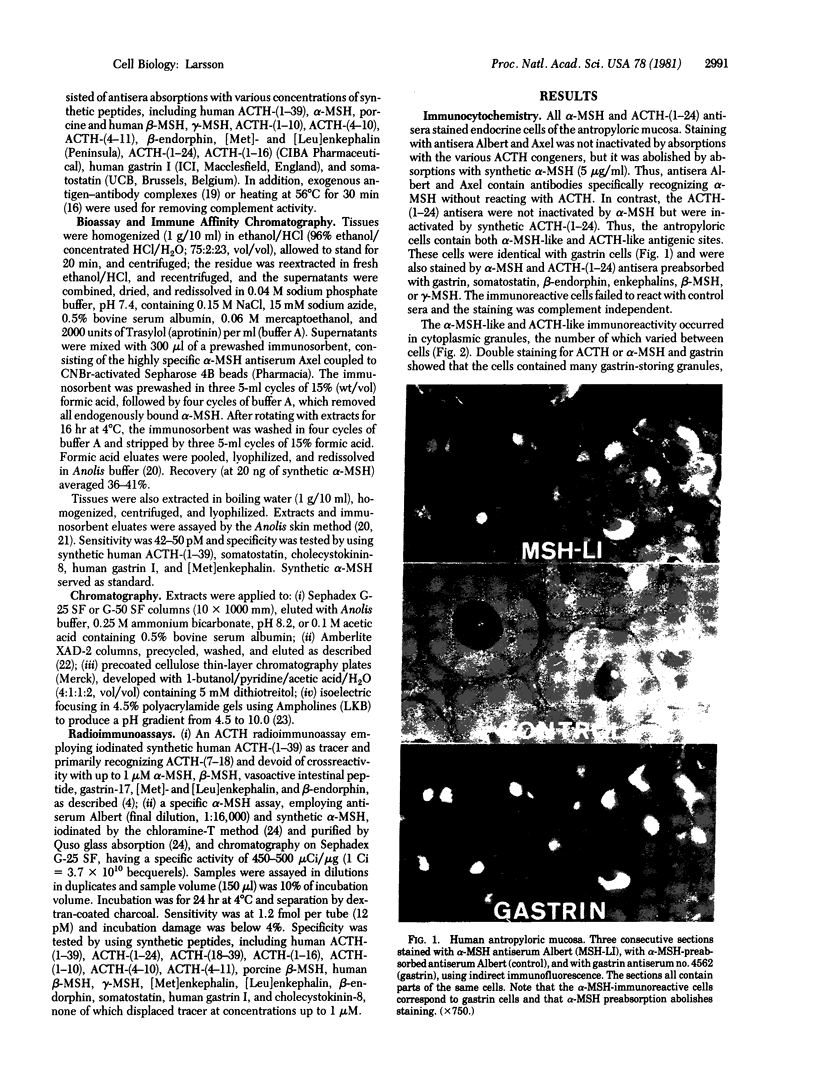
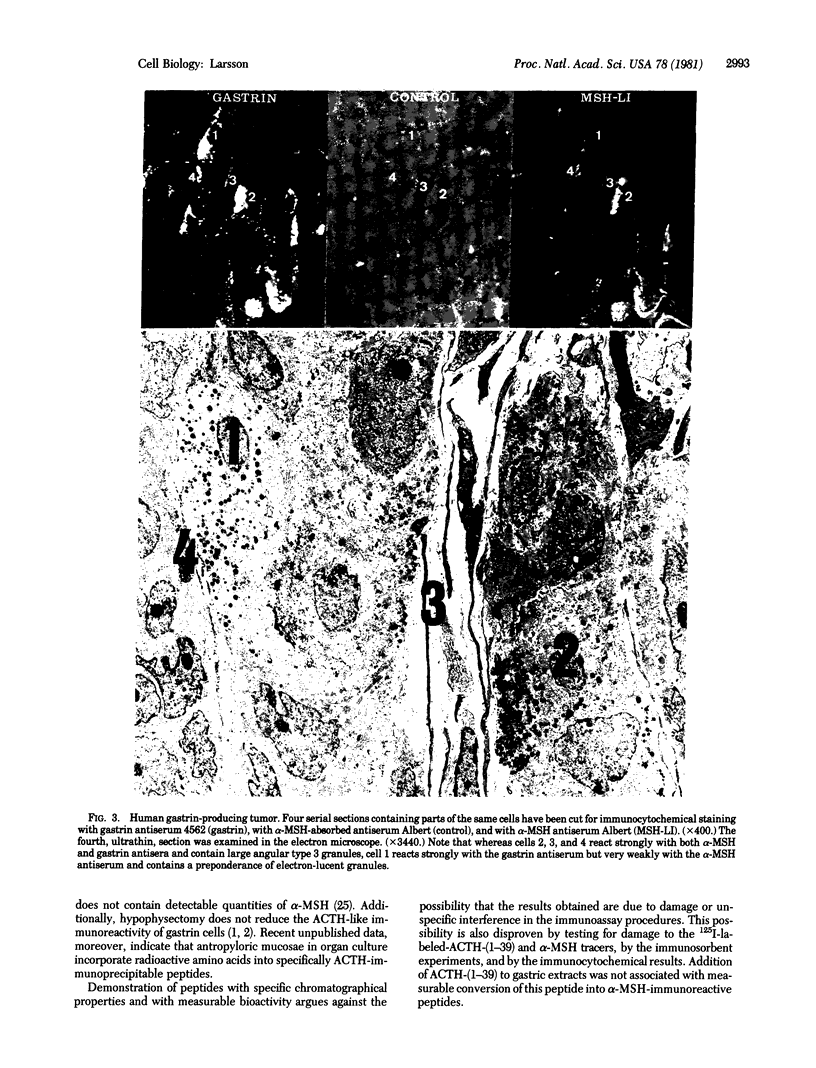
Abstract
Adrenocorticotropin (ACTH)-like and alpha-melanotropin (alpha-MSH)-like peptides have been localized to a subpopulation of cytoplasmic (secretory) granules of human antropyloric gastric cells and of fetal and neoplastic gastrin cells. These granules also store gastrin and belong to the electron-dense variety of gastrin cell granules. Gastrin cells also contain granules of low to medium electron density; these store only gastrin and do not react with ACTH or alpha-MSH antisera. The alpha-MSH immunoreactive peptide was shown also to display alpha-MSH bioactivity by a combined immunosorbent-bioassay technique. This peptide cochromatographs with synthetic alpha-MSH in several systems and is not detected in oxyntic mucosa or in gastric muscle wall. As in the pituitary intermediate lobe, the alpha-MSH-like peptide may be formed by cleavage of ACTH-like peptides also in gastrin cells. These data provide additional evidence for local formation of ACTH/alpha-MSH-related peptides in gastrin cells and suggest a heterogenous peptide make-up of endocrine cell granules.
Full text
PDF

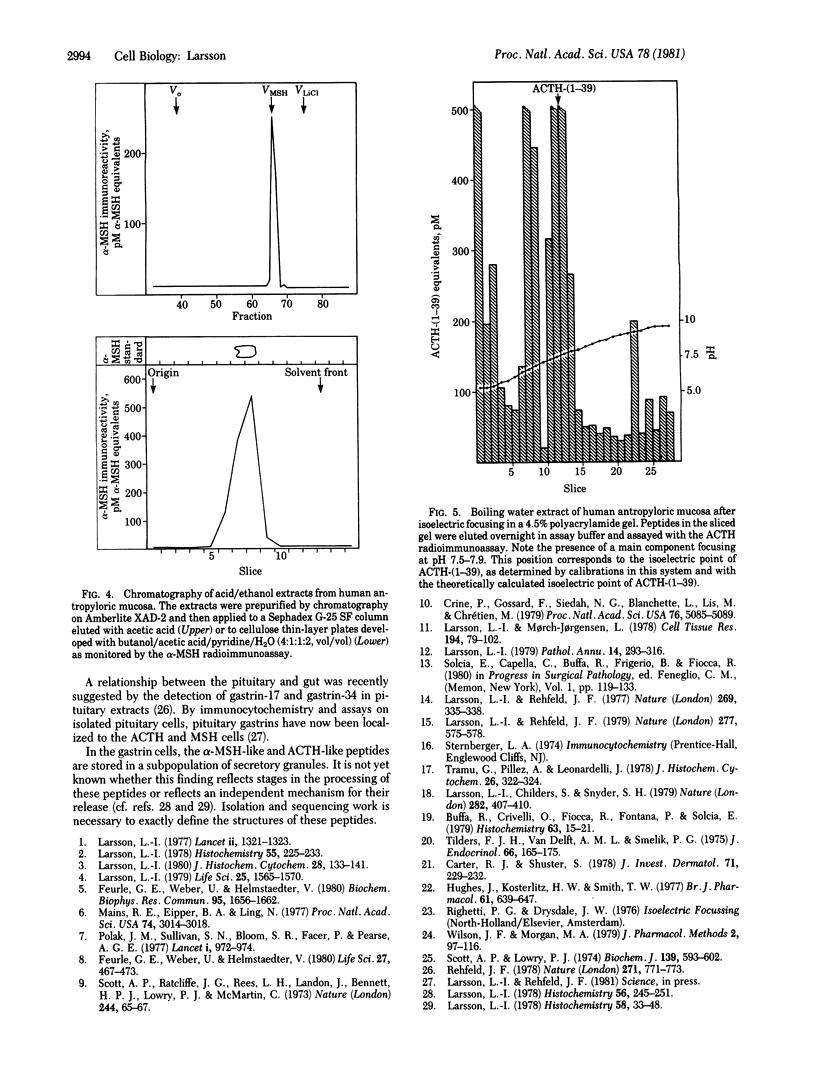
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buffa R., Crivelli O., Fiocca R., Fontana P., Solcia E. Complement-mediated unspecific binding of immunoglobulins to some endocrine cells. Histochemistry. 1979 Sep;63(1):15–21. doi: 10.1007/BF00508008. [DOI] [PubMed] [Google Scholar]
- Carter R. J., Shuster S. Sensitive new in vitro bioassay for melanocyte-stimulating activity using the skin of Anolis carolinensis. J Invest Dermatol. 1978 Oct;71(4):229–232. doi: 10.1111/1523-1747.ep12515091. [DOI] [PubMed] [Google Scholar]
- Crine P., Gossard F., Seidah N. G., Blanchette L., Lis M., Chrétien M. Concomitant synthesis of beta-endorphin and alpha-melanotropin from two forms of pro-opiomelanocortin in the rat pars intermedia. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5085–5089. doi: 10.1073/pnas.76.10.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurle G. E., Weber U., Helmstaedter V. Corticotropin-like substances in human gastric antrum and pancreas. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1656–1662. doi: 10.1016/s0006-291x(80)80089-1. [DOI] [PubMed] [Google Scholar]
- Feurle G. E., Weber U., Helmstaedter V. beta-Lipotropin-like material in human pancreas and pyloric antral mucosa. Life Sci. 1980 Aug 11;27(6):467–473. doi: 10.1016/0024-3205(80)90127-7. [DOI] [PubMed] [Google Scholar]
- Hughes J., Kosterlitz H. W., Smith T. W. The distribution of methionine-enkephalin and leucine-enkephalin in the brain and peripheral tissues. Br J Pharmacol. 1977 Dec;61(4):639–647. doi: 10.1111/j.1476-5381.1977.tb07557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L-I Corticotropin-like peptides in central nerves and in endocrine cells of gut and pancreas. Lancet. 1977 Dec 24;2(8052-8053):1321–1323. doi: 10.1016/s0140-6736(77)90368-3. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. ACTH-like immunoreactivity in the gastrin cell. Independent changes in gastrin and ACTH-like immunoreactivity during ontogeny. Histochemistry. 1978 Jul 12;56(3-4):245–251. doi: 10.1007/BF00495986. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Childers S., Snyder S. H. Met- and Leu-enkephalin immunoreactivity in separate neurones. Nature. 1979 Nov 22;282(5737):407–410. doi: 10.1038/282407a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. Distribution of ACTH-like immunoreactivity in rat brain and gastrointestinal tract. Histochemistry. 1978 Apr 4;55(3):225–233. doi: 10.1007/BF00495761. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. Gastrin and ACTH-like immunoreactivity occurs in two ultrastructurally distinct cell types of rat antropyloric mucosa. Evidence for a non-parallel processing of the peptides during feeding and fasting. Histochemistry. 1978 Nov 24;58(1-2):33–48. doi: 10.1007/BF00489947. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. Immunocytochemical characterization of ACTH-like immunoreactivity in cerebral nerves and in endocrine cells of the pituitary and gastrointestinal tract by using region-specific antisera. J Histochem Cytochem. 1980 Feb;28(2):133–141. doi: 10.1177/28.2.6243680. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Jørgensen L. M. Ultrastructural and cytochemical studies on the cytodifferentiation of duodenal endocrine cells. Cell Tissue Res. 1978 Nov 9;194(1):79–102. doi: 10.1007/BF00209235. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. Pathology of the gastrin cell. Pathol Annu. 1979;14(Pt 1):293–316. [PubMed] [Google Scholar]
- Larsson L. I. Radioimmunochemical characterization of ACTH-like peptides in the antropyloric mucosa. Life Sci. 1979 Oct 29;25(18):1565–1569. doi: 10.1016/0024-3205(79)90438-7. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F. Evidence for a common evolutionary origin of gastrin and cholecystokinin. Nature. 1977 Sep 22;269(5626):335–338. doi: 10.1038/269335a0. [DOI] [PubMed] [Google Scholar]
- Larsson L., Rehfeld J. F. A peptide resembling COOH-terminal tetrapeptide amide of gastrin from a new gastrointestinal endocrine cell type. Nature. 1979 Feb 15;277(5697):575–578. doi: 10.1038/277575a0. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M., Bloom S. R., Sullivan S. N., Facer P., Pearse A. G. Enkephalin-like immunoreactivity in the human gastrointestinal tract. Lancet. 1977 May 7;1(8019):972–974. doi: 10.1016/s0140-6736(77)92277-2. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Localisation of gastrins to neuro- and adenohypophysis. Nature. 1978 Feb 23;271(5647):771–773. doi: 10.1038/271771a0. [DOI] [PubMed] [Google Scholar]
- Scott A. P., Lowry P. J. Adrenocorticotrophic and melanocyte-stimulating peptides in the human pituitary. Biochem J. 1974 Jun;139(3):593–602. doi: 10.1042/bj1390593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. P., Ratcliffe J. G., Rees L. H., Landon J., Bennett H. P., Lowry P. J., McMartin C. Pituitary peptide. Nat New Biol. 1973 Jul 18;244(133):65–67. doi: 10.1038/newbio244065a0. [DOI] [PubMed] [Google Scholar]
- Tilders F. J., van Delft A. M., Smelik P. G. Re-introduction and evaluation of an accurate, high capacity bioassay for melanocyte-stimulating hormone using the skin of Anolis carolinensis in vitro. J Endocrinol. 1975 Aug;66(2):165–175. doi: 10.1677/joe.0.0660165. [DOI] [PubMed] [Google Scholar]
- Tramu G., Pillez A., Leonardelli J. An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem. 1978 Apr;26(4):322–324. doi: 10.1177/26.4.207771. [DOI] [PubMed] [Google Scholar]