Abstract
The Escherichia coli ygjD gene is critical for the universal tRNA modification N6-threonylcarbamoyladenosine, together with two other essential genes, yeaZ and yjeE. This study showed that the transcription of the thr and ilv operons in ygjD mutants was increased through the inhibition of transcription attenuation and that dnaG transcription was reduced.
TEXT
The ygjD gene is essential in Escherichia coli and is conserved among eukaryotes, bacteria, and Archaea (5). The functions of YgjD orthologs have been investigated in many microbial species. In Mannheimia (formerly Pasteurella) haemolytica, the YgjD ortholog was initially identified as an O-sialoglycoprotein endopeptidase (Gcp), suggesting that YgjD family members function as endopeptidases (1). However, the yeast YgjD ortholog, kinase-associated endopeptidase 1 (Kae1), has been reported to be a component of a complex known as KEOPS (kinase, endopeptidase, and other small proteins), which is involved in the regulation of telomere length (3). Kae1 also forms part of the endopeptidase-like kinase chromatin-associated (EKC) complex, which is involved in the transcription of essential genes (12). The YgjD ortholog in “Pyrococcus abyssi” possesses apurinic-endonuclease activity (8), and the YgjD ortholog Qri7 has been implicated in mitochondrial genome maintenance in both Saccharomyces cerevisiae and Caenorhabditis elegans (17).
Recently, YgjD was shown to be essential for the synthesis of the universal tRNA modification N6-threonylcarbamoyladenosine (t6A). This raises the possibility that the previously defined functions of YgjD orthologs may actually be indirect effects of its role in translational regulation (4, 19). t6A, which is present in all tRNAs that pair with ANN codons, comprises a carbonyl group and a threonine attached to the amino group of adenine at position 37, immediately 3′ of the anticodon. The role of t6A is to strengthen the A-U codon-anticodon interaction (15), and it is necessary for the decoding of ANN codons.
In E. coli, YgjD functionally interacts with two additional essential proteins, YeaZ and YjeE, both of which are conserved in eubacteria (6, 19). YeaZ shows homology with YgjD and interacts physically with both YjeE and YgjD, mediating the proteolysis of the latter (6). It has also been suggested that YeaZ and YjeE regulate YgjD activity (6).
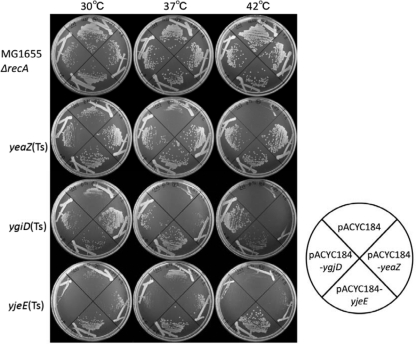
Previous studies characterized genes that are essential in E. coli by constructing mutants with large chromosomal deletions (7, 10, 11). The aim of the present study was to identify only genes that are functionally related to ygjD, yeaZ, and yjeE. The ygjD(Ts), yeaZ(Ts), and yjeE(Ts) mutants, which exhibited negligible growth at the nonpermissive temperature of 42°C, were isolated by random mutagenesis (9) (see Fig. S1 in the supplemental material). An E. coli chromosomal multicopy plasmid library was then screened for genes able to rescue the cell growth defects observed in the temperature-sensitive mutants. Several plasmids that reversed the growth defect seen in the yeaZ(Ts) mutant were isolated, and sequence analysis revealed that the cloned regions showed sequence identity to yjeE. Similarly, genes that corrected the growth defect observed in the ygjD(Ts) mutant showed homology to yeaZ and yjeE. Individual plasmids carrying yeaZ, ygjD, and yjeE were then introduced into the ygjD(Ts), yeaZ(Ts), and yjeE(Ts) mutant strains, and cell growth was examined at different temperatures (30°C, 37°C, and 42°C) (Fig. 1). yjeE strongly suppressed the growth of both the yeaZ(Ts) and ygjD(Ts) mutants; however, yeaZ weakly rescued the growth defect in the ygjD(Ts) mutant, and expression of ygjD corrected the growth defect in the yeaZ(Ts) mutant (Fig. 1). These results confirm that yeaZ, ygjD, and yjeE functionally interact with each other. Using a bacterial two-hybrid system, Handford et al. showed that YeaZ physically interacts with both YjeE and YgjD. Based on these results, they suggested that YeaZ and YjeE are regulators of YgjD (6). In agreement with this, bioinformatic and structural analyses suggested that YgjD and YeaZ heterodimerize through the same interface, and El Yacoubi et al. postulated that YeaZ is essential for the function of YgjD (4). They also showed that the complementation of E. coli ygjD function was dependent on both ygjD and yeaZ from Bacillus subtilis.
Fig. 1.
Multicopy suppression of the yeaZ(Ts), ygjD(Ts), and yjeE(Ts) mutant phenotypes. pACYC184 or one of its derivatives carrying yeaZ, ygjD, or yjeE was introduced into yeaZ(Ts), ygjD(Ts), and yjeE(Ts) mutant strains. These yeaZ, ygjD, and yjeE plasmids carry the E. coli chromosomal regions 1888076 to 1890587, 3203778 to 3208659 (the same result was obtained with the ygjD plasmid carrying the 3206931-to-3208659 region), and 4392209 to 4394317, respectively. Transformants were streaked on Antibiotic Medium 3 and incubated at 30°C, 37°C, or 42°C. The genotypes of the temperature-sensitive mutants are as follows: yeaZ(Ts), ΔyeaZ::Kmr ΔrecA::Tcr/mini-F (Smr)-yeaZ(Ts); ygjD(Ts), ΔygjD::Smr ΔrecA::Tcr/mini-F (Apr)-ygjD(Ts); yjeE(Ts), ΔyjeE::Smr ΔrecA::Tcr/mini-F (Apr)-yjeE(Ts).
The finding that the “P. abyssi” ortholog of YgjD possesses apurinic-endonuclease activity raised the possibility that YgjD also interacts with DNA or RNA. A stock of multicopy plasmids that carry different genes involved in DNA repair/recombination, replication, and transcription were introduced into the yeaZ, ygjD, and yjeE temperature-sensitive mutant strains, and cell growth was examined at semipermissive temperatures. In all three mutants, growth was inhibited by plasmids carrying nusB or rnhA (see Fig. S2 in the supplemental material). NusB is an essential component of the RNA polymerase antitermination complex and is also involved in the transcription termination process at certain sites during normal bacterial growth (18). The rnhA gene encodes RNase HI, one of the RNase H proteins that specifically degrade the RNA component of RNA-DNA hybrids. In E. coli, RNase H removes RNA primers from the Okazaki fragments during the synthesis of the lagging strand and helps to suppress initiation at origins other than the oriC locus (14). The effects of the overexpression of nusB and rnhA in E. coli suggest that yeaZ, ygjD, and yjeE are involved in transcriptional regulation and/or DNA replication.
To examine the transcriptional profile of the ygjD(Ts) mutant, cells were incubated at 30°C to avoid inducing a heat shock response. The ygjD(Ts) mutant grew more slowly at 30°C than the isogenic ygjD+ strain, indicating that this temperature was semipermissive for growth. Genes exhibiting altered transcription in the ygjD(Ts) strain compared to the wild-type strain at 30°C are shown in Table 1 (see Table S1 in the supplemental material). Expression of the thr operon was markedly increased in the ygjD(Ts) strain at 30°C (see Fig. S3A in the supplemental material). A similar analysis at 37°C (Table 2; see Table S2 in the supplemental material) showed that the expression of the ilv operon was also increased in the mutant strain (see Fig. S3B in the supplemental material). To confirm these results, the expression of thrA and ilvG, the upstream genes of those operons, was examined using a lacZ reporter gene assay (see Materials and Methods in the supplemental material for details). Expression of thrA was significantly increased in yeaZ(Ts), ygjD(Ts), and yjeE(Ts) mutants grown at 42°C (Fig. 2 A, B, and C). The relative changes at 30°C were similar to those at 42°C, although there was a significant difference between the total expression levels at 30°C and 42°C. In addition, the expression of ilvG was increased in the ygjD(Ts) mutant (Fig. 2D).
Table 1.
Up-regulated genes in the ygjD(Ts) mutant (30°C)
| Gene | ygjD(Ts) signal intensity | Wild-type signal intensity | Ratio |
|---|---|---|---|
| thrC | 2,320.2 | 472.5 | 4.9105 |
| thrB | 2,495.2 | 536.5 | 4.6509 |
| thrA | 3,494.8 | 871.2 | 4.0115 |
| cirA | 499.6 | 152.6 | 3.2739 |
| ilvA | 431.0 | 138.3 | 3.1164 |
| ygdI | 373.5 | 129.3 | 2.8886 |
| osmE | 342.3 | 126.9 | 2.6974 |
| grpE | 1,967.9 | 800.5 | 2.4583 |
| fimA | 1,710.6 | 708.4 | 2.4147 |
| fimC | 401.2 | 175.5 | 2.2860 |
| rpiB | 582.9 | 263.5 | 2.2121 |
| ygjD | 803.3 | 371.1 | 2.1646 |
| rmf | 240.5 | 113.4 | 2.1208 |
| elaB | 314.6 | 148.9 | 2.1128 |
| ilvE | 950.1 | 450.8 | 2.1076 |
| cspD | 280.2 | 134.1 | 2.0895 |
| yiaG | 467.3 | 224.0 | 2.0862 |
| ybfH | 288.6 | 140.4 | 2.0556 |
| ryfA | 713.3 | 348.8 | 2.0450 |
| nlpA | 985.2 | 483.0 | 2.0398 |
Table 2.
Up-regulated genes in the ygjD(Ts) mutant (37°C)
| Gene | ygjD(Ts) signal | Wild-type signal | Ratio |
|---|---|---|---|
| ilvG_2 | 1,023.5 | 137.2 | 7.4599 |
| ilvG_1 | 2,380.2 | 471.7 | 5.0460 |
| ilvE | 632.4 | 203.0 | 3.1153 |
| thrB | 3,726.3 | 1,255.7 | 2.9675 |
| dadA | 487.7 | 171.4 | 2.8454 |
| gcvT | 670.5 | 239.6 | 2.7984 |
| ybiJ | 301.5 | 111.0 | 2.7162 |
| ilvD | 452.2 | 173.5 | 2.6063 |
| rof | 385.6 | 153.4 | 2.5137 |
| lysA | 333.8 | 133.0 | 2.5098 |
| pheA | 1,249.6 | 499.2 | 2.5032 |
| thrC | 3,913.0 | 1,587.1 | 2.4655 |
| yjjJ | 246.9 | 101.0 | 2.4446 |
| aceB | 443.9 | 188.8 | 2.3512 |
| gdhA | 514.9 | 219.8 | 2.3426 |
| dadX | 419.7 | 179.2 | 2.3421 |
| thrA | 5,104.3 | 2,184.8 | 2.3363 |
| yicH | 242.3 | 104.6 | 2.3164 |
| putA | 367.7 | 160.5 | 2.2910 |
| yfdZ | 248.7 | 109.8 | 2.2650 |
Fig. 2.
Enhanced expression of thr and ilv operons in yeaZ(Ts), ygjD(Ts), and yjeE(Ts) mutant strains. β-Galactosidase activity was measured in temperature-sensitive mutants and their isogenic strains in which the thrA and ilvG upstream regions containing the promoter-leader peptide coding regions were inserted into the chromosomal lac operon to generate thrA′-lacZ and ilvG′-lacZ gene fusions, respectively. The fusion junctions are the initiation codons of the genes. See Materials and Methods and Fig. S5 to S7 in the supplemental material for details. (A) thrA ygjD(Ts). (B) thrA yjeE(Ts). (C) thrA yeaZ(Ts). (D) ilvG ygjD(Ts). β-Galactosidase activity in the ygjD(Ts) mutant and the parental strains carrying a thrL′-lacZ gene fusion (E) or a thrA′-lacZ terminator deletion mutant gene fusion (F). The amino acid sequences of the leader peptides of the thr and ilv operons (G). The codons for Thr, Ile, and Val are underlined, and the ANN codons are in bold font. Cells were grown overnight in Antibiotic Medium 3 and subcultured in fresh medium at a dilution of 1:100 at 30°C. Cells were incubated for 4 h, and 5 ml of the culture was used for the β-galactosidase assay. Cells were also incubated at 42°C for 2 h and assayed.
Both the thr operon (containing thrL, thrA, thrB, and thrC) and the ilv operon (containing ilvL, ilvG, ilvM, ilvE, ilvD, and ilvA) are regulated by attenuation (13). The thrL and ilvL genes encode the leader peptides. This mechanism controls the ability of RNA polymerase to read through an attenuator, which is an intrinsic terminator located at the end of the leader region. Attenuation is controlled by the position of the ribosome on the leader transcripts. When the ribosome is prevented from translating the leader (thrL and ilvL), the terminator is inactive and downstream genes are transcribed.
The mechanisms that underlie the increased expression of the thr and ilv operons may be either increased transcription initiation or decreased efficiency of transcription attenuation. To discriminate between these two possibilities, the expression of thrL was examined using a lacZ reporter gene assay. The upstream region of thrA was inserted into the chromosomal lac operon to generate a fused gene in which the lacZ initiation codon was replaced with that of thrL. No difference in expression at 30°C and 42°C was observed, and the expression of thrL did not differ between the ygjD(Ts) and ygjD+ strains, indicating that transcriptional initiation is not affected by the ygjD mutation (Fig. 2E). To investigate the effects on attenuation, a thr mutant was constructed in which the Rho-independent terminator involved in attenuation was deleted (see Materials and Methods in the supplemental material for details). The effect of the expression of this gene fusion in the ygjD(Ts) mutant was similar to that in ygjD+, which suggests that attenuation at the thr operon is inhibited by the ygjD mutation (Fig. 2F).
The leader peptides of the thr and ilv operons are rich in threonine and isoleucine, respectively, which are encoded by the ANN codons (Fig. 2G). A deficiency in t6A modification in the yeaZ, ygjD, and yjeE temperature-sensitive mutants would slow the translation of the leader peptides, resulting in readthrough and the subsequent expression of the downstream genes responsible for the biosynthesis of Thr, Ile, and Val. If the cellular concentration of Thr is sensed via the modification of t6A, then this mechanism for regulating the expression of the thr and ilv operons represents a feedback regulation loop. Since Ile is synthesized, at least in part, from Thr, low concentrations of Thr would result in the increased expression of the ilv operon. High levels of Ile would also lead to increased levels of Thr because the Thr-degradative enzyme threonine deaminase is inhibited by Ile (2).
Expression profiling of the ygjD(Ts) mutant identified the effects of the mutation on transcription but did not explain why growth was inhibited by the overexpression of nusB and rnhA. To probe the mechanisms underlying nusB-mediated growth inhibition, the expression profile of a nusB deletion mutant was compared to that of the ygjD(Ts) mutant (see Tables S3 and S4 in the supplemental material). The results showed very little overall correlation between the two strains. However, the expression of one essential gene, dnaG, was decreased in the ygjD(Ts) mutant and increased in the nusB mutant (see Table S5 and Fig. S3C in the supplemental material). To test whether dnaG expression is involved in the growth inhibition mediated by the overexpression of nusB, individual multicopy plasmids encoding nusB or dnaG were introduced into the ygjD(Ts) mutant strain and cell growth was examined. The growth of the ygjD(Ts) mutant was inhibited by the overexpression of nusB, but this effect was abrogated by the cointroduction of nusB and dnaG (Fig. 3). Interestingly, the introduction of dnaG alone into the ygjD(Ts) strain suppressed the slow-growth phenotype at the semipermissive temperature (30°C), suggesting that the growth defect in the ygjD(Ts) mutant may be due, in part, to decreased levels of DnaG primase (Fig. 3). Growth of the yjeE(Ts) mutant (but not the yeaZ(Ts) mutant) was slightly, but significantly, suppressed by the expression of dnaG alone (see Fig. S4 in the supplemental material).
Fig. 3.
Suppression of the ygjD(Ts) mutant phenotype by introduction of a dnaG multicopy plasmid. The ygjD(Ts) mutant and the isogenic parental strain (ygjD+) were transformed with pACYC184-nusB or an empty vector, together with pBAD-dnaG (or an empty vector as a control). Transformants were plated on Antibiotic Medium 3 containing chloramphenicol and ampicillin and grown at 30°C, 37°C, or 42°C.
A lack of DnaG primase causes not only defects in DNA replication but also defects in chromosomal segregation (16, 20). Loss of nucleoids in the absence of ygjD expression was observed in a conditional ygjD mutant (17). Interestingly, cells with irregular chromosomes were also observed when yeaZ, ygjD, and yjeE temperature-sensitive mutants were incubated at nonpermissive temperatures (data not shown). A yeast mutant lacking the YgjD ortholog, Qri7, which localizes to the mitochondria, exhibited abnormal mitochondrial morphology and no detectable DNA. Some of these phenotypes can be attributed to decreased levels of primase.
Although growth suppression was observed at 30°C, it was not observed at 37°C or 42°C (Fig. 3) and the introduction of dnaG did not suppress the growth defect observed in the yeaZ, ygjD, and yjeE deletion mutants (data not shown). These results suggest that the growth defects seen in these temperature-sensitive mutant strains cannot be explained entirely by the decreased expression of dnaG.
Microarray data accession numbers.
Data for experiments with E. coli ygjD(Ts) at 30°C, E. coli ygjD(Ts) at 37°C, and E. coli nusB have been deposited in Array Express under accession numbers E-MEXP-3333, E-MEXP-3334, and E-MEXP-3335.
Supplementary Material
Acknowledgments
We thank Akira Iwamoto for RNA preparation and helpful discussion. We also thank Y. Oguro, Y. Murakoshi, and M. Kobayashi for technical assistance.
This work was supported by KAKENHI from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 26 August 2011.
REFERENCES
- 1. Abdullah K. M., Lo R. Y., Mellors A. 1991. Cloning, nucleotide sequence, and expression of the Pasteurella haemolytica A1 glycoprotease gene. J. Bacteriol. 173:5597–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Changeux J. P. 1961. The feedback control mechanism of biosynthetic l-threonine deaminase by l-isoleucine. Cold Spring Harbor Symp. Quant. Biol. 26:313–318 [DOI] [PubMed] [Google Scholar]
- 3. Downey M., et al. 2006. A genome-wide screen identifies the evolutionarily conserved KEOPS complex as a telomere regulator. Cell 124:1155–1168 [DOI] [PubMed] [Google Scholar]
- 4. El Yacoubi B., et al. 2011. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J. 30:882–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galperin M. Y., Koonin E. V. 2004. ‘Conserved hypothetical’ proteins: prioritization of targets for experimental study. Nucleic Acids Res. 32:5452–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Handford J. I., et al. 2009. Conserved network of proteins essential for bacterial viability. J. Bacteriol. 191:4732–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hashimoto M., et al. 2005. Cell size and nucleoid organization of engineered Escherichia coli cells with a reduced genome. Mol. Microbiol. 55:137–149 [DOI] [PubMed] [Google Scholar]
- 8. Hecker A. 2007. An archaeal orthologue of the universal protein Kae1 is an iron metalloprotein which exhibits atypical DNA-binding properties and apurinic-endonuclease activity in vitro. Nucleic Acids Res. 35:6042–6051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kato J., Ikeda H. 1996. Construction of mini-F plasmid vectors for plasmid shuffling in Escherichia coli. Gene 170:141–142 [DOI] [PubMed] [Google Scholar]
- 10. Kato J., Hashimoto M. 2007. Construction of consecutive deletions of the Escherichia coli chromosome. Mol. Syst. Biol. 3:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kato J., Hashimoto M. 2008. Construction of long chromosomal deletion mutants of Escherichia coli and minimization of the genome, p. 279–293 In Osterman A. L., Gerdes S. Y. (ed.), Microbial gene essentiality, protocols and bioinformatics: methods in molecular biology. Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 12. Kisseleva-Romanova E., et al. 2006. Yeast homolog of a cancer-testis antigen defines a new transcription complex. EMBO J. 25:3576–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landick R., Turnbough C. L., Jr., Yanofsky C. 1996. Transcription attenuation, p. 1263–1286 In Neidhardt F. C., et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed ASM Press, Washington, DC [Google Scholar]
- 14. Marians K. J. 1996. Replication fork propagation, p. 749–763 In Neidhardt F. C., et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed ASM Press, Washington, DC [Google Scholar]
- 15. Murphy F. V., IV, Ramakrishnan V., Malkiewicz A., Agris P. F. 2004. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat. Struct. Mol. Biol. 11:1186–1191 [DOI] [PubMed] [Google Scholar]
- 16. Norris V., Alliotte T., Jaffé A., D'Ari R. 1986. DNA replication termination in Escherichia coli parB (a dnaG allele), parA, and gyrB mutants affected in DNA distribution. J. Bacteriol. 168:494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oberto J., et al. 2009. Qri7/OSGEPL, the mitochondrial version of the universal Kae1/YgjD protein, is essential for mitochondrial genome maintenance. Nucleic Acids Res. 37:5343–5352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Richardson J. P., Greenblatt J. 1996. Control of RNA chain elongation and termination, p. 822–848 In Neidhardt F. C., et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed ASM Press, Washington, DC [Google Scholar]
- 19. Srinivasan M., et al. 2011. The highly conserved KEOPS/EKC complex is essential for a universal tRNA modification, t6A. EMBO J. 30:873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Versalovic J., Lupski J. R. 1997. Missense mutations in the 3′ end of the Escherichia coli dnaG gene do not abolish primase activity but do confer the chromosome-segregation-defective (par) phenotype. Microbiology 143(Pt. 2):585–594 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.