Abstract
Influenza A viruses are classified into 16 subtypes according to the serotypes of hemagglutinin (HA). It is generally thought that neutralizing antibodies (Abs) are not broadly cross-reactive among HA subtypes. We examined the repertoire of neutralizing Abs against influenza viruses in humans. B lymphocytes were collected from donors by apheresis, and Ab libraries were constructed by using phage-display technology. Anti-HA clones were isolated by screening with H3N2 viruses. Their binding activity was examined, and four kinds of Abs showing broad strain specificity were identified from one donor. Two of the Abs, F045-092 and F026-427, were extensively analyzed. They neutralized not only H3N2 but also H1N1, H2N2, and H5N1 viruses, although the activities were largely varied. Flow cytometry suggested that they have the ability to bind to HA and HA1 artificially expressed on the cell surface. They show hemagglutination inhibition activity and do not compete with C179, an Ab thought to bind to the stalk region. F045-092 competes with Abs that recognize sites A and B for binding to HA. Furthermore, the serine at residue 136 in site A could be a part of the epitope. Thus, it is likely that F045-092 and F026-427 bind to a conserved epitope in the head region formed by HA1. Interestingly, while the VH1-69 gene can encode MAbs against the HA stem that are group 1 specific, F045-092 and its relatives that recognize the head region also use VH1-69. The possible epitope recognized by these clones is discussed.
INTRODUCTION
Antibodies (Abs) play important roles in protection against and recovery from influenza virus infection, and hemagglutinin (HA) is the main target for virus-neutralizing Abs (5). In the last century, H1, H2, and H3 subtype viruses infected humans and caused three major pandemics (20). In 2009, a novel H1N1 virus originating in swine caused the first pandemic influenza in the 21st century. The highly pathogenic avian influenza (HPAI) H5N1 virus is still the most serious threat because of its potential to cause a future pandemic. Passive immunization with neutralizing monoclonal Abs (MAbs) is considered to be a prophylactic and therapeutic strategy to combat the pandemic possibly caused by HPAI virus (22).
There are at least two mechanisms by which anti-HA Abs neutralize influenza viruses: the prevention of HA binding and the prevention of membrane fusion (23). HA is responsible for the binding of influenza virus to the cell surface receptor. Neutralizing Abs can prevent this binding process. The epitopes recognized by these Abs are located in variable regions surrounding the sialic acid-binding pocket. Viruses with mutations in the critical residues in HA preferentially survive under the pressure of neutralizing Abs and can cause the next epidemic. The mutations accumulate mainly in five sites (A, B, C, D, and E) that include neutralizing epitopes (27, 29). Thus, most MAbs with neutralizing activity recognize one of the five sites. The strain specificity of such neutralizing Abs is generally narrow because the sequence of the epitope rapidly changes.
There have been a few reports that describe neutralizing MAbs with broadly cross-reactive specificity among the HA subtypes (19). Recently, three groups successfully isolated human MAbs that neutralize all viruses belonging to group 1, including the H1, H2, and H5 subtypes (11, 24, 25). After the virus binds to cells through HA/sialic acid interactions, it is internalized by endocytosis. Acidification of endosomal compartments results in major structural rearrangements in HA2, leading to the fusion of the viral envelope with the internal host membrane. Low-pH exposure converts the connecting segment between the A and CD helices in HA2 to an additional α-helical segment (2). The second type of neutralizing Abs, which corresponds to the recently isolated ones, can prevent this structural change in HA, causing the inhibition of membrane fusion. Since the sequence of this region has been well conserved among group 1 viruses, these Abs show a broad specificity. The MAbs with this activity were first isolated as mouse MAb C179 by Okuno et al. (19) in 1993 and recently isolated as human MAbs (4, 24, 25). These MAbs did not neutralize H3 virus, which is classified into group 2. Although a cross-reactive MAb to many viruses, including H1, H2, H3, H5, H9, and H13, was generated by immunizing mice with the Aichi/68 H3N2 virus (31), there has been no report describing human MAbs that can neutralize both group 1 and group 2 viruses.
To reveal the repertoire of neutralizing Abs generated by virus infection in humans, we collected large numbers of B lymphocytes by apheresis from three donors born in 1944, 1960, and 1974. Ab libraries were constructed and screened with H3 influenza viruses. Clones that bound to virus particles were isolated, and their binding and neutralizing activities were examined. We previously reported the results obtained from the donor born in 1960 (16, 17). In the present report, we focus on clones with extremely broad strain specificity that were isolated from the donor born in 1974.
MATERIALS AND METHODS
Viruses.
The following influenza virus strains were used for experiments and analyses: A/H3N2 strains A/Aichi/2/1968 (Aic68 or A68), A/Fukuoka/1/1970 (Fuk70 or F70), A/Tokyo/6/1973 (Tok73 or T73), A/Yamanashi/2/1977 (Yam77 or Y77), A/Niigata/102/1981 (Nii81 or N81), A/Fukuoka/C29/1985 (Fuk85 or F85), A/Guizhou/54/1989 (Gui89 or G89), A/Kitakyushu/159/1993 (Kit93 or K93), A/Sydney/5/1997 (Syd97 or S97), A/Panama/2007/1999 (Pan99 or P99), A/Wyoming/3/2003 (Wyo03 or W03), and A/New York/55/2004 (NY04); A/H1N1 strains A/New Caledonia/20/1999 (NC99) and A/Solomon Islands/3/2006 (SI06); A/H1N1pdm strains A/California/7/2009 pdm (Cal09) and A/Suita/1/2009 pdm (Sui09pdm); swine A/H1N1 strain A/Swine/Hokkaido/2/1981 (swHok81); A/H3N8 strains A/Budgerigar/Aichi/1/1977 (budAic77) and A/wedge-tailed shearwater/Western Australia/405/1977 (aviAus77); A/H2N2 strains A/Okuda/1957 (OK57), A/Duck/Hong Kong/273/1978 (dukHK78), and A/Japan/305/1957 (Jpn57); and A/H5N1 strains A/duck/Mongolia/54/2001-A/duck/Mongolia/47/2001 (9) (Duck/Mongolia/2001 or RdukMon01), A/Vietnam/1194/2004/NIBRG-14 (Viet04), A/Anhui/1/2005/PR8-IBCDC-RG5 (Anh05), and A/Indonesia/5/2005/PR8-IBCDC-RG2 (Ind05). Abbreviations for the strains are shown in parentheses. The A/H3N2 and A/H1N1 strains listed above and A/California/7/2009 pdm have been used for influenza vaccines in Japan.
Construction of Ab library.
Mononuclear cells from a donor born in 1974 were collected by apheresis from the equivalent of 3 liters of blood. A total of 1.3 × 109 B lymphocytes were collected. Large combinatorial Ab libraries were constructed by using the phage-display method as previously described (12). A total of 3.7 × 109 clones were established for heavy (H)-chain genes, and 1.6 × 108 clones were established for light (L)-chain genes. The genes encoding the Fab form of Ab fused with a truncated cp3 (Fab-cp3) were constructed by combining the V regions of the H (VH) and L (VL) chain genes (7). The resulting Ab library contained 2.9 × 1010 clones.
Screening of Ab library.
Phage bound to virus particles was selected by a panning method as described previously (16). In brief, formalin-treated virus particles were used as antigens (Ags) in the screenings. After three time pannings, Escherichia coli (DH12S) cells were infected with the eluted phage and spread onto LB plates containing 100 μg/ml ampicillin and 0.2% glucose. Colonies were picked up, and the E. coli colonies harboring the phagemid were grown in 2× yeast extract–tryptophan (YT) medium containing 100 μg/ml ampicillin, 0.05% glucose, and 1 mM isopropyl-β-d-thiogalactopyranoside at 30°C overnight. The Fab-cp3 form of Ab was secreted into the medium (13). The culture supernatants containing Fab-cp3 molecules were subjected to enzyme-linked immunosorbent assay (ELISA) against various H3 strains of influenza viruses and an H1 strain of influenza virus. Clones that bound to H3 but not to H1 were selected and subjected to sequencing for classification. Clones that reacted equally with H3 and H1 were not subjected to further analysis because results from our previous study (16) revealed that these types of clones are antinucleoprotein (anti-NP) Abs. Clones that bound strongly to H3 but weakly to H1 were subjected to Western blotting to determine if their target is HA or NP.
ELISA.
Inactivated virus particles were coated onto Maxisorp immunoplates (Nunc). The plates were incubated with human IgG Ab, and then peroxidase-conjugated goat anti-human IgG (H + L chain; MBL) was added. When Fab-cp3 Ab in the supernatant of E. coli culture was added to the virus-coated plate, rabbit anti-cp3 Ab (MBL) and peroxidase-conjugated goat anti-rabbit IgG (H+L chain; MBL) were used as the 2nd and 3rd Abs, respectively. Finally, horseradish peroxidase (HRP) substrate (o-phenylenediamine [OPD]; Wako) was added, and the optical density (OD) at 492 nm was measured.
Preparation of MAbs.
Fab-cp3 Abs were purified with an anti-cp3 MAb-conjugated column. The Fab-PP Abs (10) (P denotes a single Fc-binding domain of protein A) were purified with IgG Sepharose (GE Healthcare). IgG was prepared from a high-expression vector (1) and purified with protein A-Sepharose (GE Healthcare).
Western blotting.
Formalin-inactivated virus particles were separated by SDS-PAGE under nonreducing conditions and transferred to an Immobilon-P membrane (Millipore). The membranes were incubated with the Fab-cp3 Abs. For detection, rabbit anti-cp3 Ab (MBL) was used as the primary Ab, and peroxidase-conjugated goat anti-rabbit IgG (H+L chain; MBL) was used as the secondary Ab. Then, the immunoreactive bands were visualized by using ECL Plus Western blotting detection regents (GE Healthcare).
Neutralization test.
Neutralizing activity was measured by the focus reduction assay as described previously (14).
Construction of HA-expressing vectors.
HA-expressing vectors were constructed by the following two-step strategy. DNAs corresponding to entire HA genes were amplified by reverse transcription-PCR from virus RNA and cloned. DNAs encoding the HA ectodomain (residues 1 to 513) or the HA1 domain (residues 1 to 329) were amplified by PCR and inserted into the KpnI-ApaI site of pYD1 (Invitrogen Inc.), resulting in HA or HA1 connected with a V5 epitope tag. DNAs encoding HA or HA1 with a V5 tag were amplified by PCR again and inserted into the SfiI-SalI site of pDisplay (Invitrogen Inc.). The resultant plasmid DNA encodes a V5 tag between the transmembrane region (a transmembrane domain of platelet-derived growth factor receptor) and the extracellular domain of HA or HA1.
Expression of HA or HA1 on the cell surface.
293T cells in a 150-mm dish were transiently transfected with 24 μg of plasmid DNA (HA-expressing vector) or no DNA (mock transfection) with 60 μl of Lipofectamine LTX (Invitrogen Inc.) and recovered after culture at 37°C for 24 h. The cells were incubated with 10 μg/ml of Fab-PP Ab, 1 μg/ml of mouse anti-H3 MAb F49 (26), or 1 μg/ml of rabbit anti-V5 tag Ab (Millipore). Then, these cells were detected with Alexa Fluor 488 anti-human IgG, Alexa Fluor 488 anti-mouse IgG, or Alexa Fluor 488 anti-rabbit IgG (Molecular Probes), respectively, and subjected to flow cytometry (FCM) analyses.
Hemadsorption assay.
Hemadsorption activity of HA-expressing cells was checked by using the previously described method (15, 17). In brief, 0.3 ml of 2% guinea pig red blood cells was mixed with 0.3 ml of HA-expressing 293T cell suspension (5 × 105) in a microtube, and the mixture was rotated slowly at 4°C. After 1 h, the microtube was allowed to stand for 10 to 15 min, so that the 293T cells were precipitated at the bottom of the microtube, while almost all of the free blood cells remained suspended. The suspended blood cells were removed, and the cell pellet was suspended in 1 ml of phosphate-buffered saline (PBS) in 0.05% NaN3. The microtube was allowed to stand for 10 to 15 min, and the suspended blood cells were removed again. The resulting cell pellet was suspended in 40 μl of PBS in 0.05% NaN3, and the complexes of the HA-expressed 293T cells and the blood cells were observed under an optical microscope.
HI assay.
The hemagglutination inhibition (HI) test was performed as described previously (18). Briefly, serial dilutions of 100 μg/ml of purified Fab-PP or 100 μg/ml of purified IgG in PBS were prepared. Serial dilutions of Fab-PP were preincubated with 4 HA units of virus per well. Guinea pig red blood cells were added to a final concentration of 0.5%, and the plate was incubated at room temperature for 30 to 60 min.
Competition ELISA.
Competition ELISA was performed by using Fab-PP to detect binding to virus particles and Fab-cp3 as a competitor. Fab-cp3 molecules in the supernatant of the E. coli culture were concentrated 20-fold. Fab-cp3 of clone F045-092 was purified and used at a concentration of 20 μg/ml. Formalin-inactivated virus particles were coated onto a Maxisorp immunoplate. A total of 50 μl of Fab-PP at an optimized concentration was mixed with 50 μl of 20-fold-concentrated Fab-cp3 or 20 μg/ml of Fab-cp3 of F045-092 and added to a virus-coated immunoplate. Then, peroxidase-conjugated rabbit Ab was added. Finally OPD was added, and the OD at 492 nm was measured.
Nucleotide sequence accession numbers.
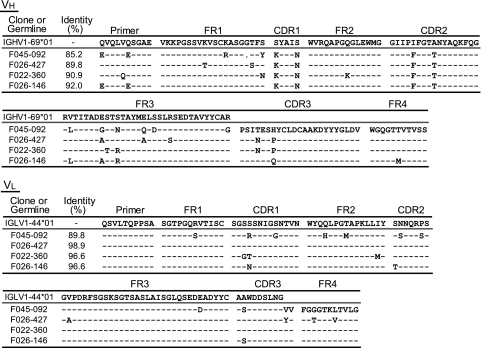
The nucleotide sequences of the VH and VL domains of four Abs, F045-092, F026-427, F022-360, and F026-146 (see Fig. 2) have been deposited in the DDBJ database (accession numbers AB649264 to AB649271).
Fig. 2.
Amino acid sequences of four Abs, F045-092, F026-427, F022-360, and F026-146. Amino acid sequences of the VH and VL domains were compared with those of the relevant germ line genes, VH1-69 and IGLV1-44, respectively. Bars indicate the same amino acids as in the germ line gene. A dot at the 27th residue of F045-092 indicates a one-amino-acid deletion. Differences in the primer portion were not included in the calculation of identity between germ line and somatic sequences.
RESULTS
Isolation of MAbs that bound to HA of H3 strains from the library of the donor born in 1974.
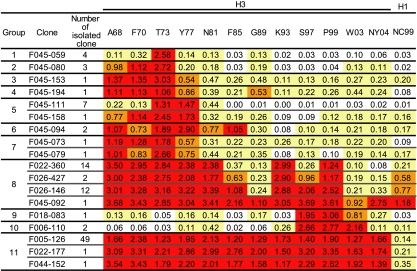
To reveal the repertoire of neutralizing Abs generated by virus infection in humans, we collected large numbers of B lymphocytes by apheresis from three donors born in 1944, 1960, and 1974. Ab libraries were constructed by using phage-display technology and screened against influenza viruses of 12 different H3 vaccine strains isolated between 1968 and 2004. Clones that bound to virus particles were isolated, and their binding activity to the 12 H3 strains and neutralizing activity were examined. Previously, we reported the results obtained from the library of the donor born in 1960 (16, 17). In the present study, we examined the clones isolated from the library of the donor born in 1974. Previous studies indicated that the majority of clones showing neutralizing activity are anti-HA MAbs and that the anti-HA MAbs have neutralizing activity, with the exception of a few clones. Therefore, we examined target Ags of respective clones by Western blotting, and anti-HA MAbs were selected during the screening process. A total of 104 anti-HA clones were isolated and sequenced. These clones were composed of 18 unique MAbs with different VH sequences. On the basis of the sequence similarities of VH fragments, the 18 clones were classified into 11 groups. Their binding activities to 12 H3N2 viruses and 1 H1N1 virus were examined by ELISA (Fig. 1). Nine clones belonging to groups 1 to 7 showed HA-binding activity to various restricted strains, such as strains 1973, 1968 to 1973, 1968 to 1977, and 1968 to 1981, but all of these clones bound to the 1973 strain the most strongly. Two clones belonging to groups 9 and 10 bound to HAs of the 1997 to 2003 strains. Other than these clones, only two types of clones, groups 8 and 11, were found among the anti-HA MAbs. Three clones belonging to group 11 were able to bind to all 12 H3N2 strains but did not bind to the H1N1 strain. Four clones belonging to group 8 (Fig. 2) also showed broad strain specificity and appeared to bind to not only H3N2 but also H1N1. Since bands that correspond to HAs were detected by Western blotting (Fig. 3 A), these clones should bind to HA. In the libraries of the donors born in 1960 and 1944, we could not find any clones that bind to HAs of both H3 and H1 (16). Therefore, we decided to focus on the group 8 clones from the donor born in 1974.
Fig. 1.
Binding activity of 18 unique clones to 12 H3 strains and 1 H1 strain. A total of 104 clones were isolated. They produced 18 unique MAbs, and they were classified into 11 groups. The HA-binding ability of these MAbs was shown by Western blotting with representative clones of the respective groups. The binding activity to HAs of 12 H3 strains and 1 H1 strain was examined by ELISA. The OD values at 492 nm are indicated. Values greater than 1.0 are marked by red. Values between 0.5 and 1.0 are marked by orange. Values between 0.1 and 0.5 are marked by yellow.
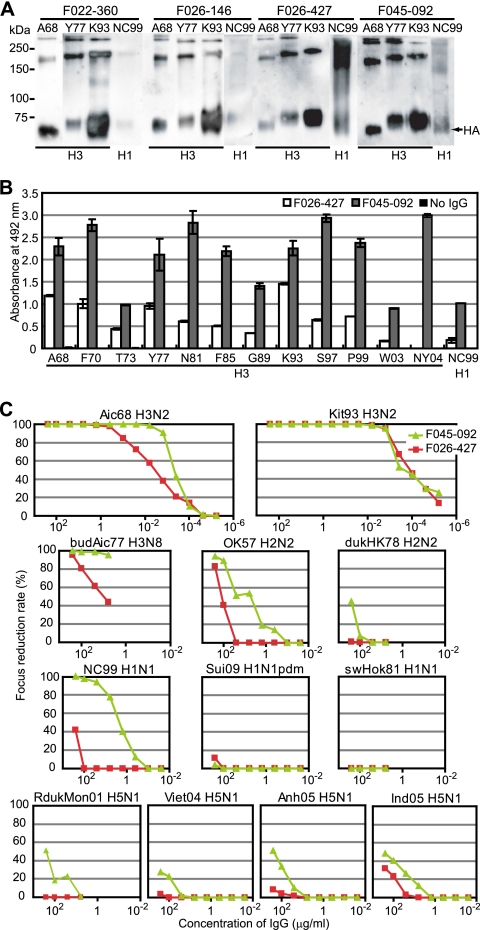
Fig. 3.
F045-092 and F026-427 binding to and neutralizing activities against virus strains. (A) Western blot of three H3 strain viruses and one H1 strain virus with Fab-cp3 of four clones under nonreducing conditions. (B) Binding activities of IgG from F045-092 (gray) and F026-427 (white) to 12 H3 viruses and 1 H1 virus were measured by ELISA. The OD values at 492 nm are indicated. (C) Virus-neutralizing activities of F045-092 (green) and F026-427 (red) against H3N2 (Aichi/68, Kitakyushu/93), H3N8 (Budgerigar/Aichi/77), H2N2 (Okuda/57, Duck/Hong Kong/78), H1N1 (New Caledonia/99, Suita/2009 pdm, Swine/Hokkaido/81), and H5N1 (Duck/Mongolia/2001, Vietnam/2004, Anhui/2005, Indonesia/2005) viruses were measured by focus reduction assay.
F045-092 neutralizes H3N2, H1N1, H2N2, and H5N1 viruses.
A comparison of the amino acid sequences of H chains among the four clones indicated that they were derived from the same B cell and matured in vivo (Fig. 2). Clone F045-092 showed the strongest binding activity and appeared to be the most deviated from the other clones, and the other three clones seemed to be similar to each other; therefore, F045-092 and F026-427 were selected for further study, and IgG1 Abs of both clones were prepared. The binding activity to HAs of 12 H3 strains and 1 H1 strain was examined by ELISA (Fig. 3B). F045-092 showed much stronger HA-binding activity than F026-427. The neutralizing activity against various influenza viruses, including human H3N2, H1N1, H2N2, and H5N1 viruses and avian H5N1 virus, was examined (Fig. 3C). Both clones neutralized the H3N2 Kitakyushu/93 virus with extremely strong activity (50% inhibitory concentration [IC50], 0.1 ng/ml [0.6 pM]). F045-092 neutralized H1N1 New Caledonia/99 (IC50, 26 nM), although it did not neutralize other H1N1 strains, Suita/2009 and sw/Hokkaido/81. Furthermore, F045-092 had the capability to neutralize H2N2 virus (IC50 against Okuda/57, 40 nM) and even H5N1 viruses (IC50 against Indonesia/2005, 1.6 μM). In the case of F026-427, the neutralizing activities against H2N2, H1N1, and H5N1 viruses were extremely weak or nonexistent.
The epitope recognized by F045-092 and F026-427 is located in the head region formed by HA1.
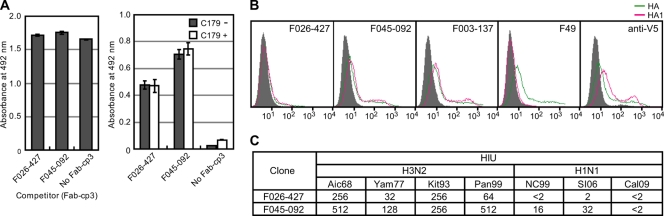
Since our clones that neutralized H3, H1, H2, and H5 viruses utilized the germ line VH1-69 gene (Fig. 2), it appeared that the epitope recognized by them is located in a region similar to that for the epitope recognized by CR6261 and F10 (4, 24). The presence of a common epitope among HAs of group 1 influenza viruses was originally demonstrated by isolation of the mouse MAb C179 in 1993 (19). Sui et al. (24) reported that F10 efficiently competed with C179 for binding to H5, suggesting that the epitopes recognized by both clones overlapped each other. Therefore, we tested whether F045-092 and F026-427 could compete with C179 for binding to H1. As shown in Fig. 4 A, they did not compete with C179.
Fig. 4.
F045-092 and F026-427 recognize HA1 and show HI activities. (A) Competition for binding to HA in ELISA between C179 and F045-092 or F026-427. Formalin-inactivated New Caledonia/99 virus particles were coated onto an immunoplate. (Left) A total of 50 μl of 0.5 μg/ml of C179 was mixed with 50 μl of 2 μg/ml of Fab-cp3 of F026-427, F045-092, or PBS (No Fab-cp3) and applied to the immunoplate. Bound C179 was detected with anti-mouse IgG-HRP. (Right) A total of 50 μl of 0.5 μg/ml of Fab-cp3 of F026-427, F045-092, or PBS (No Fab-cp3) was mixed with 50 μl of 2 μg/ml of C179 (C179+) or with 50 μl PBS (C179−) and applied to the immunoplate. The bound Fab-cp3 was detected with rabbit anti-cp3 polyclonal antibody followed by anti-rabbit IgG-HRP. Values are the averages of duplicate experiments, and the standard deviation is depicted by an error bar. (B) FCM analyses of the cells expressing HA and HA1 of Aichi/68 virus. FCM signals for mock transfection (gray-filled area), HA-expressing cells (green), and HA1-expressing cells (pink) are shown. F49 Ab binds to an epitope on HA2 that is commonly present on all H3 subtype viruses (26). Anti-V5 Ab binds to the V5 tag located at the membrane-proximal end of HA. F003-137 binds to the B2/D site located on the globular head of HA (17). (C) HI activity of IgG from F045-092 and F026-427 against H3N2 (Aichi/68, Yamanashi/77, Kitakyushu/93, Panama/99) and H1N1 (New Caledonia/99, Solomon Islands/2006, California/2009 pdm) viruses.
Next, we examined whether clones F045-092 and F026-427 bind to HA1 or HA2. HA and HA1 of the Aichi/68 strain were artificially expressed on the cell surface, and the ability of both clones to bind to the cell surface HA and HA1 was examined by FCM. Anti-V5 Ab bound to cells transfected with either the HA gene or the HA1 gene, indicating that HA and HA1 molecules were expressed on the cell surface. F003-137, which binds to site B2/D (17) in the head region formed by HA1, bound to HA and HA1 (Fig. 4B). F49, which binds to HA2 (26), bound to HA but not HA1. F045-092 bound to HA and HA1 equally. F026-427 also bound to HA and HA1, although the binding was much weaker than that of F045-092. These results indicate that the epitope recognized by these clones is located on HA1.
One mechanism by which anti-HA Abs neutralize influenza viruses (23) is the prevention of the binding of HA to sialic acid on the cell surface. Since this type of Ab shows HI activity, we examined the HI activity of these clones. As indicated in Fig. 4C, both F045-092 and F026-427 have HI activity, and the relative strength of both clones against four kinds of H3N2 viruses almost perfectly matched the relative strength of their binding activity measured by ELISA and their neutralizing activity measured by a focus reduction assay (18). The HI activity and the neutralizing activity against H1N1 strain NC99 were also consistent between F045-092 and F026-427. These observations strongly suggest that the mechanism of virus neutralization by these clones is the inhibition of the interaction between HA and the receptor, sialic acid; therefore, the epitope should be localized in the head region formed by HA1.
F045-092 competes with Abs that recognize sites A and B for binding to HA.
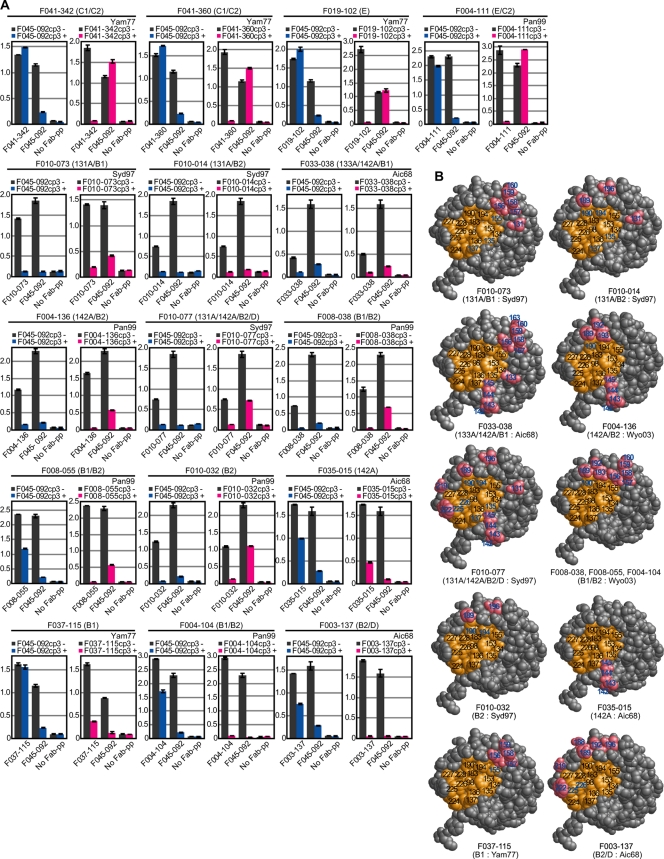
Previously, we isolated many MAbs that neutralize H3N2 viruses and determined the location of epitopes recognized by them. Original HAs of several strains and chimeric variants, in which one of the seven sites (A, B1, B2, C1, C2, D, and E) was replaced by some other strain-derived sequence, were artificially expressed on the cell surface. By examining the binding activity of MAbs to the HAs, the location of epitopes was determined. To identify the region recognized by F045-092, we performed competition experiments with F045-092 and 16 MAbs that bind to various sites on HA1 (17). Table 1 summarizes the sites recognized by the 16 MAbs and the residues on HAs that negatively affect binding when replaced. As indicated in Fig. 5 A, three competition patterns were revealed. Four clones, F041-342, F041-360, F019-102, and F004-111, which bind to site C or E, did not compete with F045-092. Eight clones, F010-073, F010-014, F033-038, F004-136, F010-077, F008-038, F008-055, and F010-032, which bind to site A or B, competed with F045-092. In addition, a third type of competition pattern was observed. Although a large excess of F045-092 did not disturb the binding of a site-specific Ab to HA, a large excess of the site-specific Ab disturbed the binding of F045-092 to HA. Four clones, F035-015, F037-115, F004-104, and F003-137, which bind to site A or B, showed this competition pattern. The localization of epitopes recognized by MAbs showing mutual inhibition and one-sided inhibition is depicted in Fig. 5B. These observations clearly indicate that the epitope recognized by F045-092 is localized to the region close to both sites A and B. The implications of the third type of competition pattern are described in the Discussion.
Table 1.
Summary of binding characteristics of antibodies used in this study
| Clone | H3N2 strains bounda | Mutations impacting bindingb |
Recognized sitec | ||
|---|---|---|---|---|---|
| Strain | Name of chimeric HA | Mutation(s) | |||
| F003-137 | A68, F70, T73 | A68 | 188B2 | N188D, Q189S, E190D, T192I, V196A | B2/D |
| A68 | 219D | S219Y, W222R, G215D, L219I | |||
| F004-104 | K93, S97, P99, W03 | S97 | 155B1 | H155T, Q156K, L157S, K158G, Y159S, K160T | B1/B2 |
| S97 | 189B2 | S189Q, D190E, I194L, A196V | |||
| W03 | 156B1 | Q156K, L157S, K158G, Y159S, K160T | |||
| W03 | 189B2 | S189Q, D190E, I192T, S193K | |||
| F004-111 | S97, P99, W03, NY04 | S97 | 82E | K82E, E83T | E/C2 |
| S97 | 275C2 | G275D, K276T, N278I | |||
| W03 | 82E | K82E, K83T | |||
| W03 | 275C2 | G275D, K276T, N278I | |||
| F004-136 | S97, P99, W03 | W03 | 142A | R142G, S143P, N144G, K145S, S146G | A/B2 |
| W03 | 189B2 | S189Q, D190E, I192T, S193K | |||
| F008-038 | S97, P99, W03 | S97 | 155B1 | H155T, Q156K, L157S, K158G, Y159S, K160T | B1/B2 |
| W03 | 156B1 | Q156K, L157S, K158G, Y159S, K160T | |||
| W03 | 189B2 | S189Q, D190E, I192T, S193K | |||
| F008-055 | S97, P99, W03, NY04 | S97 | 155B1 | H155T, Q156K, L157S, K158G, Y159S, K160T | B1/B2 |
| W03 | 156B1 | Q156K, L157S, K158G, Y159S, K160T | |||
| W03 | 189B2 | S189Q, D190E, I192T, S193K | |||
| F010-014 | S97, P99 | S97 | 131A | A131T, T135G, Y137N | A/B2 |
| S97 | 189B2 | S189Q, D190E, I194L, A196V | |||
| F010-032 | S97, P99 | S97 | 189B2 | S189Q, D190E, I194L, A196V | B2 |
| F010-073 | K93, S97 | S97 | 131A | A131T, T135G, Y137N | A/B1 |
| S97 | 155B1 | H155T, Q156K, L157S, K158G, Y159S, K160T | |||
| F010-077 | S97, P99 | S97 | 131A | A131T, T135G, Y137N | A/B2/D |
| S97 | 142A | S142G, S143P, I144G, K145S, S146G | |||
| S97 | 189B2 | S189Q, D190E, I194L, A196V | |||
| S97 | 219D | S219Y, W222R, G215D | |||
| F019-102 | Y77, N81, F85, G89, K93 | Y77 | 82E | E82K, K83E | E |
| F85 | 82E | E82K, K83E | |||
| F033-038 | A68, F70 | A68 | 133A | N133D | A/B1 |
| A68 | 142A | G142R, P143S, G144N, S145K, G146S | |||
| A68 | 154B1 | V154L, K156H, S157L, G158K, S159Y, T160K, V163A | |||
| F035-015 | A68, F70 | A68 | 142A | G142R, P143S, G144N, S145K, G146S | A |
| F037-115 | Y77, N81, F85, G89 | Y77 | 155B1 | Y155T, E156Q, S157L, E158K, S159Y | B1 |
| F85 | 155B1 | H155T, E156Q, S157L, E158K | |||
| F041-342 | Y77, N81, F85, G89, K93 | Y77 | 50C1 | R50K, D53N, S54N | C1/C2 |
| Y77 | 276C2 | T276K, S278N | |||
| F85 | 50C1 | R50K, D53N, S54N | |||
| F85 | 276C2 | T276K, S278N | |||
| F041-360 | Y77, N81, F85, G89, K93 | Y77 | 50C1 | R50K, D53N, S54N | C1/C2 |
| Y77 | 276C2 | T276K, S278N | |||
| F85 | 50C1 | R50K, D53N, S54N | |||
| F85 | 276C2 | T276K, S278N | |||
The binding activities of the clone to H3N2 virus strains were detected by ELISA.
Abs show no binding activity to the chimeric HA with mutations expressed on the cell surface.
The antigenic sites recognized by the respective clones.
Fig. 5.
Competition for binding to HA between F045-092 and MAbs that bind to various sites on HA1. (A) Competition ELISA was performed by using Fab-PP for detection of binding activity and Fab-cp3 as a competitor. The results indicate the binding activity of Fab-PP to virus particles in the absence (−) or presence (+) of 10 μg/ml of Fab-cp3 of F045-092 or an excess of Fab-cp3 of each Ab. The antigenic sites recognized by the Ab are indicated above the graph. The H3N2 virus strain used in each assay is indicated in the upper right of the graph. (B) Antigenic sites for various MAbs, as determined by epitope mapping through analysis of chimeras (EMAC) methods (17), are indicated in the 3D structure of HA1. The illustrations were constructed by using the structure of H3 protein (Protein Data Bank [PDB] accession code 1HA0) at amino acid residues 91 to 260 according to molecular graphic viewer Rasmol (version 2.7.5). The amino acids that form a receptor-binding site are marked in orange. The amino acids replaced in the chimera, to which each clone could not bind, are marked in pink. A number representing the amino acid position replaced in the chimera is indicated in blue, even if the amino acid was localized to a receptor-binding site. These positions correspond to the epitope recognized by the Ab used in the competition assay. The antigenic sites recognized by Ab and the H3N2 virus strain used in the EMAC method are indicated under the illustration.
F045-092 may bind to serine at residue 136.
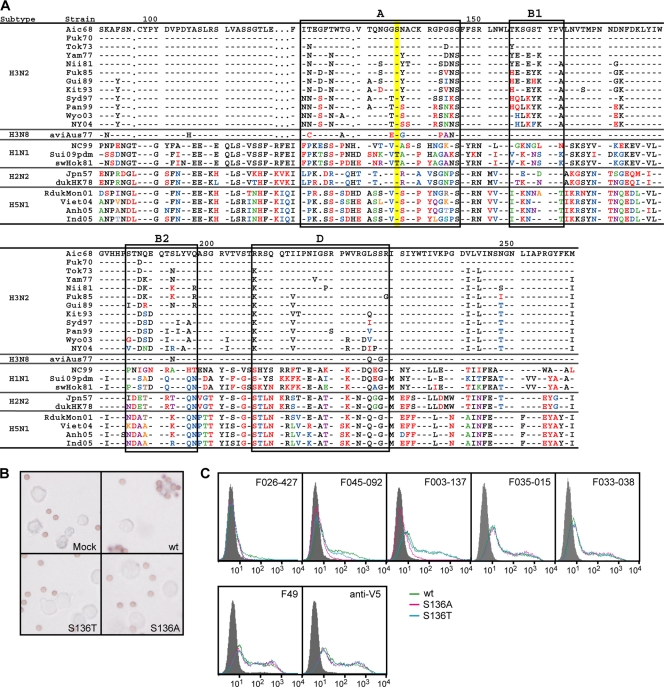
According to the data shown in Fig. 3C, F045-092 could not neutralize the Suita/2009 H1N1 or sw/Hokkaido/81 H1N1 virus, but it did neutralize all other viruses. California/2009, to which F045-092 did not show HI activity, is almost identical to Suita/2009 H1N1. Assuming that this difference might be assigned to a specific amino acid residue located at the receptor-binding site, the amino acid sequences of the HAs of the strains used in the neutralization test were compared (Fig. 6A). Residue 136 of Suita/2009 H1N1 and sw/Hokkaido/81 H1N1 is threonine, but all of the other strains contain serine at this position. Serine at residue 136 is directly involved in forming a receptor-binding site (28).
Fig. 6.
Effect of one amino acid substitution at residue 136 of HA on the binding activity of F045-092 and F026-427. (A) Amino acid sequences of HA1 of various strains. The sequence of the Aichi/68 H3N2 virus strain was used as a standard for comparison. The bars indicate the same amino acid as in the standard. Amino acids that differ from those of the standard are indicated by using a different color. Amino acid sequences in sites A, B1, B2, and D are boxed. The amino acids at residue 136 are shaded in yellow. (B) Attachment of reticulocytes to cells expressing wild-type (wt) HA or two variant HAs of the Aichi/68 H3N2 influenza strain with amino acid substitutions at residue 136. (C) Reactivity of F045-092 and F026-427 with mutant HAs of Aichi/68 on 293T cells. FCM signals for mock transfection (gray-filled area), Aic68 wild type (green), and two kinds of mutants, Aic68/S136A (pink) and Aic68/S136T (blue), are shown. Ser136 of the wild type was changed to alanine and threonine in Aic68/S136A and Aic68/S136T, respectively. While positive-control antibody, anti-V5 antibody, F49, F035-015, and F033-038 reacted with both the wild type and the mutants to the same extent, F045-092 and F026-427 reacted with the mutants more weakly than the wild type.
We examined the effects of a change from serine to threonine at residue 136 on the reactivity of F045-092 and F026-427 to HA of Aichi/68. Two variants of HA were constructed and expressed on the cell surface. As indicated in Fig. 6B, neither of the variants retained hemadsorption activity (15), suggesting that the sialic acid-binding activity was lost by mutation at residue 136. FCM analysis indicated that F035-015 and F033-038, which recognize sites A and A/B1, respectively, were identically bound to wild-type HA and the two variant HAs (Fig. 6C). On the other hand, F003-137, which recognizes site B2/D, was identically bound to wild-type HA and the serine-to-threonine variant but only weakly bound to the serine-to-alanine variant. These observations suggest that the serine-to-threonine variant retained conformational integrity in sites A, A/B1, and B2/D but lost the sialic acid-binding activity, while the serine-to-alanine variant lost both the conformational integrity in site B2/D and the sialic acid-binding activity. The reactivity of F026-427 to HA of Aichi/68 was lost by the change from serine to threonine at residue 136, and the reactivity of F045-092 was weakened by this change, suggesting that serine at residue 136 of HA is directly involved in the formation of the contact surface with these Abs.
DISCUSSION
In a series of studies, we examined the repertoire of neutralizing MAbs against influenza viruses in humans. We analyzed B lymphocytes collected by apheresis from three donors born in 1944, 1960, and 1974. Since phage Ab libraries were constructed by combinatorially mixing H and L chains, there was a suspicion that the clones isolated from libraries may not correctly reflect the in vivo MAbs. As indicated in Fig. 2, however, four clones showing similar anti-HA activities are encoded by a set of VHDJH and VLJL genes that appear to be derived from a single B cell and deviated by mutation. Therefore, we concluded that the Ag specificity detected by our method correctly reflects the specificity of the in vivo clones. According to our experimental strategy, we analyzed the Ab repertoire formed by B cells in the peripheral blood of a donor at the time of blood collection. Therefore, it is likely that the B cells that did not become memory cells or that were relatively short-lived memory cells are not included in our analyses.
Under this limitation, the analyses of B lymphocytes of the donor born in 1960 indicated that the majority of clones showing neutralizing activity against H3N2 viruses could be divided into three major groups with distinct strain specificity: 1968 to 1973, 1977 to 1993, and 1997 to 2003 (16). The clones isolated from the donor born in 1944 showed essentially the same characteristics as those from the donor born in 1960. However, the clones isolated from the donor born in 1974 were very different from the clones of the other two donors. The clones belonging to groups 1 to 7 in Fig. 1 showed HA-binding activity to various restricted strains but bound the most strongly to the 1973 strain. Therefore, it is likely that they were generated by infection with the 1973 strain virus after birth. The Abs in groups 9 and 10, which bound to the 1997 to 2003 strains, probably reflected the time of blood collection, June 2004. The other two types of clones showed extremely broad strain specificity. Thus, it is possible that neutralizing Abs showing broad strain specificity have been major players in protection against influenza viruses during this donor's life.
In the present study, we focused on the Abs that neutralize not only H3 but also group 1 viruses. The sequence of CDR3 clearly indicated that the four clones belonging to group 8 in Fig. 1 should have been derived from a single B cell. Since F045-092 is the most deviated among the four clones, the following scenario is likely. B cells producing this type of Ab were generated by infection with H3N2 viruses. Since the epitope recognized by this Ab is extremely stable among H3N2 viruses, B cells producing such Abs matured and survived as long-lived memory cells. The epitope may even include a structure formed by the HAs of group 1 viruses. Then, F045-092, which can neutralize not only H3 but also group 1 viruses, was generated by mutation.
The crystal structure of Fabs from CR6261 and F10 in complex with HA revealed that there is an epitope commonly present on the HAs of group 1 viruses and that the Abs encoded by the germ line VH1-69 gene are capable of binding this common epitope (4, 24). Although the four clones of group 8 bound to the head region of HA, they also utilize the VH1-69 gene. Abs encoded by VH1-69 have unique characteristics. Two successive hydrophobic residues in CDR2, isoleucine at residue 53 and phenylalanine at residue 54, form a hydrophobic tip and frequently interact with solvent-exposed hydrophobic clusters that may require electroneutral interactions. This unique capability has been utilized in neutralizing Abs against not only influenza viruses but also HIV (6) and hepatitis C virus (3). Thus, HCDR2 is directed into the hydrophobic groove at the junction between the A helix and HA1. If we presume the presence of a hydrophobic patch in the globular head of HA, whose structure is highly conserved among all influenza viruses, the sialic acid-binding pocket could be a candidate (28).
Results obtained in the present study indicate that the group 8 Abs bind to the globular head of HA and show HI activity. F045-092 competes with Abs that recognize sites A and B for binding to HA. The epitope should be commonly present among the HAs of all H3 viruses. Although our results do not contradict the hypothesis that the group 8 Abs bind to the sialic acid-binding pocket, there are other possible binding sites. The two types of competition shown in Fig. 5 may support the hypothesis that F045-092 binds to the receptor-binding site. Generally, when mutual inhibition was observed, the site-specific Ab recognized a relatively large space surrounding the sialic acid-binding site. On the other hand, when one-sided inhibition was observed, the site recognized by the site-specific Ab appeared to be restricted.
A three-dimensional (3D) analysis of HA/Ab complexes by X ray is required to understand how the common epitope can be formed by apparently divergent sequences and be recognized by these Abs. However, it should be true that the epitope recognized by these Abs is immunogenic in humans. If the epitope whose antigenic structure has been highly conserved is generally immunogenic in humans, it might be possible to develop a new type of vaccine that can specifically stimulate the growth of B cells that produce Abs against such a neutralizing epitope. Moreover, the production of broadly cross-reactive Abs against influenza virus infection by humans could occur much more frequently than previously believed (30).
If the sialic acid-binding pocket is the epitope recognized by F045-092, the structural analysis of the HA/Ab complex might provide the design for a chemical drug against type A influenza viruses. The VH segments of the clones analyzed in the present study have the following distinctive characteristics: the VH1-69 gene was utilized in the formation of the VHDJH structure; isoleucine at residue 53 was mutated to phenylalanine; the CDR3 is very long, 23 amino acids in length, while the average CDR3 length in human Abs is 11 or 12 amino acids; and since CLDC at residues 9 to 12 in CDR3 could not be encoded by any of the germ line D genes (8), the peptide should have been produced by terminal deoxynucleotidyltransferase. If these unique characteristics are directly involved in the formation of the antigen-binding site, the formation of Ab with this broad neutralization ability might be a rare event. The neutralizing activity of F045-092 is much stronger than that of F026-427. When the amino acid sequence of both clones was compared with that of the germ line counterpart, we noticed two differences that could cause large conformational changes in F045-092. One amino acid at residue 27 was deleted, and arginine at residue 94, which could be involved in the formation of a salt bridge with aspartic acid (21), was changed to glycine in the VH domain of F045-092 (Fig. 2). Judging from the extremely high neutralizing activity shown by F045-092, these changes should have occurred during maturation, when the donor's immune system was actively combating influenza virus infection. Therefore, the analysis of the 3D structure formed at the interface between HA and the Ab will provide precious information that can be used to develop a therapeutic drug against influenza viruses.
ACKNOWLEDGMENTS
This work was supported in part by a grant-in-aid for the 21st Century Center of Excellence (COE) program of Fujita Health University from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a grant for Research on Pharmaceutical and Medical Safety from the Ministry of Health, Labor, and Welfare of Japan, and a grant from the Research Foundation for Microbial Diseases, Osaka University.
Footnotes
Published ahead of print on 24 August 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Bebbington C. R., et al. 1992. High-level expression of a recombinant antibody from myeloma cells using a glutamine synthetase gene as an amplifiable selectable marker. Biotechnology (NY) 10:169–175 [DOI] [PubMed] [Google Scholar]
- 2. Bullough P. A., Hughson F. M., Skehel J. J., Wiley D. C. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37–43 [DOI] [PubMed] [Google Scholar]
- 3. Chan C. H., Hadlock K. G., Foung S. K. H., Levy S. 2001. VH1-69 gene is preferentially used by hepatitis C virus-associated B cell lymphomas and by normal B cells responding to the E2 viral antigen. Blood 97:1023–1026 [DOI] [PubMed] [Google Scholar]
- 4. Ekiert D. C., et al. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerhard W., Mozdzanowska K., Furchner M., Washko G., Maiese K. 1997. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 159:95–103 [DOI] [PubMed] [Google Scholar]
- 6. Huang C.-C., et al. 2004. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc. Natl. Acad. Sci. U. S. A. 101:2706–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iba Y., Ito K., Kurosawa Y. 1997. Expression vectors for the introduction of highly diverged sequences into the six complementarity-determining regions of an antibody. Gene 194:35–46 [DOI] [PubMed] [Google Scholar]
- 8. Ichihara Y., Matsuoka H., Kurosawa Y. 1988. Organization of human immunoglobulin heavy chain diversity gene loci. EMBO J. 7:4141–4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Isoda N., et al. 2006. Pathogenicity of a highly pathogenic avian influenza virus, A/chicken/Yamaguchi/7/04(H5N1) in different species of birds and mammals. Arch. Virol. 151:1267–1279 [DOI] [PubMed] [Google Scholar]
- 10. Ito W., Kurosawa Y. 1993. Development of an artificial antibody system with multiple valency using an Fv fragment fused to a fragment of protein A. J. Biol. Chem. 268:20668–20675 [PubMed] [Google Scholar]
- 11. Kashyap A. K., et al. 2008. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc. Natl. Acad. Sci. U. S. A. 105:5986–5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marks J. D., et al. 1991. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 222:581–597 [DOI] [PubMed] [Google Scholar]
- 13. Morino K., et al. 2001. Antibody fusions with fluorescent proteins: a versatile regent for profiling protein expression. J. Immunol. Methods 257:175–184 [DOI] [PubMed] [Google Scholar]
- 14. Nakagawa N., Kubota R., Nakagawa T., Okuno Y. 2001. Antigenic variants with amino acid deletions clarify a neutralizing epitope specific for influenza B virus Victoria group strains. J. Gen. Virol. 82:2169–2172 [DOI] [PubMed] [Google Scholar]
- 15. Nakajima K., Nobusawa E., Nagy A., Nakajima S. 2005. Accumulation of amino acid substitutions promotes irreversible structural changes in the hemagglutinin of human influenza AH3 virus during evolution. J. Virol. 79:4672–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okada J., et al. 2010. Monoclonal antibodies in man that neutralized H3N2 influenza viruses were classified into three groups with distinct strain specificity: 1968-1973, 1977-1993 and 1997-2003. Virology 397:322–330 [DOI] [PubMed] [Google Scholar]
- 17. Okada J., et al. 2011. Localization of epitopes recognized by monoclonal antibodies that neutralized the H3N2 influenza viruses in man. J. Gen. Virol. 92:326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okuno Y., et al. 1990. Rapid focus reduction neutralization test of influenza A and B viruses in microtiter system. J. Clin. Microbiol. 28:1308–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okuno Y., Isegawa Y., Sasao F., Ueda S. 1993. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 67:2552–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palese P. 2004. Influenza: old and new threats. Nat. Med. 10:S82–S87 [DOI] [PubMed] [Google Scholar]
- 21. Shirai H., Kidera A., Nakamura H. 1999. H3-rules: identification of CDR-H3 structures in antibodies. FEBS Lett. 455:188–197 [DOI] [PubMed] [Google Scholar]
- 22. Simmons C. P., et al. 2007. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 4:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skehel J. J., Wiley D. C. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531–569 [DOI] [PubMed] [Google Scholar]
- 24. Sui J., et al. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Throsby M., et al. 2008. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3:e3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ueda M., et al. 1998. Application of subtype-specific monoclonal antibodies for rapid detection and identification of influenza A and B viruses. J. Clin. Microbiol. 36:340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Underwood P. A. 1982. Mapping of antigenic changes in the haemagglutinin of Hong Kong influenza (H3N2) strains using a large panel of monoclonal antibodies. J. Gen. Virol. 62:153–169 [DOI] [PubMed] [Google Scholar]
- 28. Weis W., et al. 1988. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333:426–431 [DOI] [PubMed] [Google Scholar]
- 29. Wiley D. C., Wilson I. A., Skehel J. J. 1981. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature 289:373–378 [DOI] [PubMed] [Google Scholar]
- 30. Wrammert J., et al. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoshida R., et al. 2009. Cross-protective potential of a novel monoclonal antibody directed against antigenic site B of the hemagglutinin of influenza A viruses. PLoS Pathog. 5:e1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]