Abstract
Antibiotic-resistant microbes, such as methicillin-resistant Staphylococcus aureus, seriously threaten human health. The outbreak of “superbugs” in recent years emphasizes once again the need for the development of new antimicrobial agents or resources. Antimicrobial peptides have an evident bactericidal effect against multidrug-resistant pathogens. In the present study, a new antimicrobial peptide, ctriporin, was cloned and characterized from the venom of the scorpion Chaerilus tricostatus, an animal which has not yet been explored for toxic peptide resources. The MICs of ctriporin against Staphylococcus aureus, Bacillus thuringiensis, Bacillus subtilis, Micrococcus luteus, and Candida albicans are 5 to 20 μg/ml. Meanwhile, it MIC against clinical antibiotic-resistant bacterial strains is 10 μg/ml. Furthermore, the potential for ctriporin to be used as a topical antibiotic for treating staphylococcal skin infections was investigated. External use of the peptide ctriporin dramatically decreased the bacterial counts and cured skin infections in mice. In addition, ctriporin demonstrates antimicrobial efficacy via the bactericidal mechanism of rapid cell lysis. Together, these results suggest the potential of developing ctriporin as a new topical antibiotic.
INTRODUCTION
“Superbug” infections are being reported frequently worldwide and have gained more and more attention. These superbugs emerged when antibiotics were overused in recent decades. Unfortunately, methicillin-resistant Staphylococcus aureus (MRSA), one of the most lethal superbugs, has been transmitted rapidly all over the world. The first case of MRSA infection was reported in the United Kingdom in 1961 (21, 48). At present, MRSA infections have become pandemic in many medical institutions (hospital-acquired MRSA) and the community (community acquired MRSA) worldwide (17, 29). Moreover, MRSA has been found to colonize or infect livestock and humans exposed to infected animals in several countries, and such MRSA strains has been termed livestock-associated MRSA (36). MRSA infections cause many health problems. It has become a primary cause of skin and soft tissue infections among persons without extensive exposure to professional healthcare (46). Such settings and skin-to-skin contact have become a risk factor, which will accelerate the transmission of MRSA in humans (4, 9, 16, 25). MRSA-induced sepsis, endocarditis, pneumonia, and soft tissue infections are difficult to treat because of the limited range of effective antibiotics. Vancomycin is one of the most effective antibiotics against MRSA, but it is associated with many adverse effects (30). Moreover, the overuse of vancomycin in treating MRSA infections and the existence of vancomycin-resistant enterococci has led to the emergence of vancomycin-intermediate S. aureus (2, 3). In order to prevent additional MRSA infections, more effective antimicrobial drugs are needed.
Antimicrobial peptides (AMPs), as a part of the ancient and nonspecific innate immune system, play an important role in defending against invading pathogenic organisms (18, 22, 28). AMPs are typically relatively short (12 to 100 amino acids), positively charged (net charge of +2 to +9), and amphiphilic and have been isolated from single-celled microorganisms, insects and other invertebrates, plants, amphibians, birds, fish, and mammals (including humans) (34, 49). AMPs demonstrate a broad spectrum of activity against Gram-negative and Gram-positive bacteria, fungi as well as against viruses and parasites (26, 39, 45). Moreover, some antimicrobial peptides have been shown to have inhibitory activity against antibiotic-resistant bacteria (40).
Scorpions are one of the most ancient animals on earth, having survived more than 400 million years (52). They have been used as a traditional Chinese medicine for more than 2,000 years for their outstanding pharmaceutical effects, which include antimicrobial and anti-inflammatory activities (41, 42). It has long been widely believed that scorpion venom is a mixture of natural bioactive substances (35). At present, some antimicrobial peptides have been found in scorpion venom and hemolymph (8, 20), such as hadrurin (47), scorpine (11), opistoporins, parabutoporin (33), IsCTs (14), pandinins (12), mucroporin (13), imcroporin (53), and StCT1 (51), indicating that scorpion venom is also a rich resource of crude antimicrobial peptides.
In the present study, we cloned and identified a new antimicrobial peptide, ctriporin, the first bioactive peptide characterized from the venom of the scorpion Chaerilus tricostatus. The mature peptide of ctriporin was composed of 19 amino acid residues with an amidated C terminus. The synthetic peptide ctriporin showed potent growth-inhibitory activity against standard Gram-positive bacteria and Candida albicans at low concentrations. Moreover, the in vitro treatment of clinically isolated pathogens showed that ctriporin is able to inhibit antibiotic-resistant pathogens, including MRSA, methicillin-resistant coagulase-negative Staphylococcus (MRCNS), and penicillin-resistant Staphylococcus epidermidis (PRSE) strains. We also explored its in vivo antibacterial activity in topical treatment of mouse skin infection model. Our findings showed that ctriporin displays reasonable antibacterial activity in vivo using an ointment application. Finally, we chose a standard Gram-positive bacterium, S. aureus, as a model bacterial strain to further explore the antimicrobial mechanism of ctriporin. Our findings show that ctriporin is an effective antimicrobial agent, acting via the bactericidal mechanism of the rapid lysis of cells. All of these results suggest that the antimicrobial activity of ctriporin against Gram-positive bacteria and antibiotic-resistant pathogens makes it a promising candidate for the treatment of skin infection by topical application.
MATERIALS AND METHODS
cDNA library construction.
The Chaerilus tricostatus scorpions were gathered in Medog Xizang Autonomous Region, China. We cut their glands 2 days after electrical extraction of the venom to provoke their toxin-producing cells into a secretory phase. Their total RNA was extracted using TRIzol reagent (Invitrogen). Poly(A) mRNA was purified by using a Poly(A) Tract mRNA isolation system (Promega). The cDNA library construction was made using a Superscript plasmid system library construction kit (Gibco-BRL). The cDNAs were cloned into the pSPORT1 plasmid (Gibco-BRL) and introduced into Escherichia coli DH5α cells by transformation. cDNA clones were chosen randomly and sequenced to acquire a reliable representation of toxic peptides and proteins in the glands.
cDNA sequencing and computer analysis.
Positive clones were identified by using an ABI Prism 377XL DNA sequencer with a universal T7 promoter primer. Analysis of the sequence was carried out by using GENERUNR and BLASTX. All homologue sequences of ctriporin were retrieved from GenBank (http://www.ncbi.nlm.nih.gov/) by the BlastP method.
Chemical synthesis and identification.
The ctriporin mature peptide was synthesized by solid-phase synthesis method and was amidated at the C terminus (More Biotechnology, Ltd., China). Identification of the synthesized peptide and determination of its purity was carried out by reversed-phase high-performance liquid chromatography (RP-HPLC) analysis. Agilent 1100 series HPLC equipment and a C18 column (Agilent Eclipse XDB-C18; 5 μm; 4.6 by 150 mm) were used. The gradient chromatography solvents were eluent A (0.1% [vol/vol] trifluoroacetic acid) and eluent B (90% [vol/vol] acetonitrile with 0.1% [vol/vol] trifluoroacetic acid). The average molecular mass of the synthetic ctriporin peptide was measured by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF-MS) analysis. A Voyager DE STR MALDI-TOF (Applied Biosystems) was used, and the matrix was cinnamic acid.
Microorganism strains.
The standard bacterial strains Bacillus thuringiensis (AB92037), Pseudomonas aeruginosa (AB93066), S. aureus (AB94004), B. subtilis (AB91021), Micrococcus luteus (AB93113), and E. coli (AB94012) and the standard fungi strain C. albicans (AY93025) were purchased from the China Center of Type Culture Collection.
Antibiotic-resistant bacterial strains were obtained from the 302nd Military Hospital of Beijing, China, including MRSA strains P1386 and P1374, MRCNS strain P1369, and PRSE strain P1389.
Antibacterial assay in vitro. (i) MIC determination.
The MICs of the peptide against bacterial strains were tested by a liquid growth inhibition assay in 96-well microtiter plates. Bacteria in the logarithmic phase were diluted with Luria-Bertani (LB) medium to about 104 to 106 CFU/ml, and fungi in the logarithmic phase were diluted with yeast extract-peptone-dextrose medium. The peptide was diluted to a series of concentrations of 400, 200, 100, 50, 25, 12.5, 6.25, and 3 μg/ml, respectively. The bacterial suspension and the serially diluted peptides were added to 96-well plates at a ratio of 4:1 in a final volume of 100 μl; thus, the final concentrations of these peptides were 80, 40, 20, 10, 5, 2.5, 1.25, and 0.6 μg/ml. The microplates were incubated at 37°C with continuous shaking for 12 to 16 h. Inhibitory growth was examined by monitoring the absorbance at 630 nm with a microplate reader. The concentrations of peptides and commercial antibiotics were all determined by weight. The MIC was determined based on the optical density (OD). Each concentration reading was repeated three times. All of the above experiments were repeated at least twice.
(ii) Growth-inhibitory effect.
Time-growth curves of S. aureus, C. albicans, and MRSA strains treated by ctriporin peptide were measured at 630 nm at each time point. The tested strain added with different concentrations of peptide was cultured in 96-well plates, as described for the method used above. Ampicillin, cefotaxime, penicillin, vancomycin, and amphotericin B were used as control treatments.
Antibacterial assay in vivo.
(i) Experimental animals. Adult female BALB/c mice, 6 to 8 weeks old and weighing 17 to 21 g, were obtained from the Hubei Center for Disease Control and Prevention, Wuhan, China. The animals were housed one per cage and maintained on a 12-h light-dark cycle with access to food and water. All animal studies were approved by the Institutional Animal Care and Use Committee of Wuhan University.
(ii) Preparation of inoculum.
Standard S. aureus (AB94004) was cultured in LB medium at 37°C with continuous shaking. A 1-ml portion of AB94004 logarithmic-phase broth was quantified and concentrated to 20 μl using phosphate-buffered saline (PBS).
(iii) Compound preparation.
The synthetic peptide ctriporin was prepared as an ointment in 0.5% hydroxypropylcellulose to a final concentration of 5 mg/ml.
(iv) Mouse skin abrasion and infection model.
In order to foster a more vulnerable environment in the mice for infection, mice were injected with two doses of cyclophosphamide at days 4 and 1 before the infection. For the first dose, mice were injected with 150 mg of cyclophosphamide per kilogram of the mouse's body weight (150 mg/kg). The second dose was 100 mg/kg. This treatment reduced peripheral blood neutrophils to <100/ml of blood (15).
Before creation of the wounds, the mice were anesthetized with injections of 20% ethyl carbamate (7 ml/kg [mouse body weight]) and then shaved on the dorsal surfaces. Skin abrasion wounds were made on the dorsal surfaces of mice using a blade by creating 1-by-1-cm2 scratch area. The abrasion wounds only damaged the stratum corneum and the upper layer of the epidermis but not the dermis. At 5 min after the wounding, a pipette tip was used to inoculate 20 μl of concentrated suspension containing 107 to 108 CFU of standard S. aureus into each defined wounded area. In each experiment, a group of mice were killed 4 h after infection to control the infectious dose.
At 4 h after infection, the first treatment was introduced. Ointment treatments (20 μl of a 5-mg/ml ctriporin ointment or a placebo ointment) were applied to the wound or spread over the area, respectively. At 16 h after the first treatment, additional antibiotic applications were made twice daily (in the morning and the evening, at an 8-h interval) for 4 days. After the 4 days of treatment, the mice were killed, and the wound area skin (∼1 cm2) was immediately excised from each animal. The excised skin was homogenized, together with 1 ml of PBS, in a homogenizer. The homogenates were washed once in PBS to decrease the remaining ointment. Suitable dilutions of the homogenates were plated on LB plates to determine the number of living bacteria (expressed as CFU). In order to investigate the reproducibility of the infection with S. aureus, three independent experiments that included untreated and placebo-treated groups were performed.
Statistical analysis.
In each experiment, the mean CFU was calculated by using log10-transformed data. Based on the averages of these three experiments, the mean, range, and coefficient of variation were calculated.
Histological examination.
In order to characterize the histopathology of the model, biopsy specimens were taken after the following treatments: immediately after blade scratching, 4 days after blade scratching, and 4 days after inoculation with S. aureus without treatment, with placebo treatment, or with ctriporin treatment. Immediately after the animals were killed, 5-mm punch biopsy specimens of the excised skin were taken and immediately fixed in 10% phosphate-buffered formalin (pH 7.4). The formalin-fixed biopsy specimens were embedded in paraffin and stained with hematoxylin and eosin.
Mechanism investigation. (i) Bactericidal assay.
Previous studies have suggested that the modes of action of many antibacterial peptides were rapid killing by disrupting the cell membrane of bacteria. Therefore, time-kill curves for ctriporin and ampicillin against S. aureus (AB94004) were measured. The bactericidal effects of ctriporin and ampicillin were tested at 1× and 5× the MIC. Overnight-cultured S. aureus was 1:100 transferred to LB medium and cultured to exponential phase (OD600 = 0.6). The peptide or ampicillin solution and the bacterial suspension were added to a sterilized tube at the volume ratio of 4:1 in a final volume of 2 ml, and the mixture was incubated at 37°C with continuous shaking. At each time point, 200 μl of treated bacterial suspension was transferred to a sterilized 1.5-ml tube. After the tube was centrifuged at 1,000 × g for 5 min, the supernatant was removed, and the pellet was resuspended in 200 μl of LB medium. Suitable dilutions of the bacterial suspension were placed on LB agar plates and incubated at 37°C until the viable colonies could be counted.
(ii) Secondary structure analysis.
The secondary structure of the mature peptide ctriporin was analyzed by using Heliquest (http://heliquest.ipmc.cnrs.fr/cgi-bin/ComputParams.py) and a circular dichroism (CD) assay. CD analysis was performed at room temperature on a 2-mm-path-length, capped, round optical cell using a Jasco J-810 spectropolarimeter. Spectra were obtained at wavelengths of 190 to 260 nm. Each spectrum was obtained from averaging three scans taken at a scan rate of 50 nm/min with a 2-nm spectral bandwidth. In order to simulate the environment of bacterial cell surface and membrane, trifluoroethanol (TFE) was used as a solvent. The synthetic peptide ctriporin was measured at the concentration of 0.1 mg/ml in H2O only, 30% TFE–H2O, or 70% TFE–H2O. The peptide configuration was obtained by analyzing the data with CD deconvolution software.
Transmission electron microscopy.
Overnight-cultured S. aureus was transferred to LB medium and cultured to the exponential phase. A portion (200 μl) of the peptide solution was added to an 800-μl bacterial suspension, and the mixture was incubated at 37°C with continuous shaking (∼150 rpm/min). After 2 min of incubation, the bacterial suspension was immediately centrifuged at 1,000 × g for 10 min, and the pellet was washed with 0.1 M PBS several times and then fixed for 1 h with 2% glutaraldehyde in 0.1 M PBS, followed 1 h later with 1% osmium tetroxide at 4°C. After fixation, the bacteria were stained with 1% uranyl acetate and then dehydrated by using a series of graded ethyl alcohols (60% for 15 min, 70% for 15 min, 80% for 15 min, and 90% for 15 min and then two changes of 100% for 10 min each). After this, the samples were embedded in epoxy resins and stained with 1% uranyl acetate and lead citrate. The samples were then semi-thin sectioned and ready to view on the Hitachi X650 transmission electron microscope.
RESULTS
Sequence analysis.
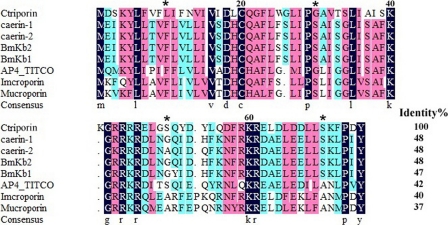
After systemic screening of 884 clones from the venom gland cDNA library of the scorpion Chaerilus tricostatus, we obtained 10 positive clones. Sequencing of all of these positive clones revealed a new precursor, termed ctriporin (Fig. 1). It has high similarity to caerin-1, caerin-2, BmKb2, and a number of antimicrobial peptides, as shown in Fig. 2.
Fig. 1.
Precursor nucleotide sequences and deduced amino acid sequences of ctriporin (accession number JN172934). The deduced amino acid residues are shown below the corresponding nucleotide sequences. The signal peptide residues are underlined. The mature peptide residues are shaded in gray. The C-propeptide residues were not marked. The potential polyadenylation signal aataaa is double underlined.
Fig. 2.
Sequence alignments of ctriporin and its related peptides. The sequence alignments of ctriporin with other antimicrobial peptides were carried out using DNAMAN. The residues shaded in dark blue are highly conserved sites (100%), the residues in pink and light blue are less conserved sites (≥75 and ≥50%), and the residues without any background color are highly varied sites. Identity%, the percentage of sequence identity relative to ctriporin.
The ctriporin precursor consists of three parts: a 5′ untranslated region (5′UTR), an open reading frame (ORF), and a 3′UTR. The lengths of the 5′UTR and 3′UTR are 86 and 71 nucleotides (nt), respectively. At the 3′UTR end of the cDNA, a single aataaa (double underlined in Fig. 1) polyadenylation signal is found 16 nt upstream of the poly(A) tail. An ORF of 225 nt encodes a precursor of 75 amino acid residues.
Ctriporin has a putative 22-residue signal peptide, identified with the SignalP 3.0 program (http://www.cbs.dtu.dk/services/SignalP/), followed by a presumed 19-residue mature peptide and an uncommon acidic propeptide at the C terminus. The 34-residue propeptide starts with a conserved posttranslational processing signal, Gly-Arg-Arg, at positions 42 to 44. Conventionally, removal of a propeptide with such a processing signal would result in a mature peptide with C-terminal amidation, as described previously (19). The sequence of ctriporin has been deposited in the GenBank NCBI database (accession number JN172934).
Chemical synthesis and characterization of ctriporin.
Based on the amino acid sequence analysis of ctriporin, the presumed 19-residue mature peptide was synthesized by solid-phase synthesis method and amidated at the C terminus. As described previously, Gly followed by either a single residue (Arg) or pairs of residues (Lys-Arg or Arg-Arg) is a potential cleavage site for prohormone convertases (27, 43). The presence of multiple copies of peptide sequences flanked by pairs of basic residues is a very common feature of the prohormones of insect peptides. Generally, the processing of a prohormone to biologically active peptide with an amidated C terminus requires the removal of the basic residues to give a peptide intermediate with a C-terminal Gly, which then undergoes oxidation to generate a peptide terminating with an amidated residue (5, 6). C-terminal amidated will increase solubility and potency of the peptide.
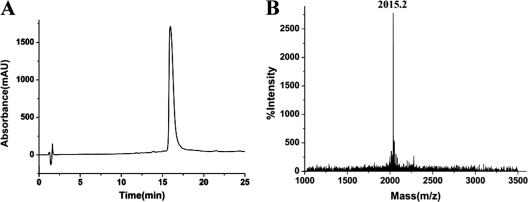
HPLC analysis showed that the synthetic peptide ctriporin had a purity of >95% (Fig. 3A). Moreover, MALDI-TOF-MS analysis showed that the average molecular mass of the synthetic peptide ctriporin was 2,014.2 Da, corresponding to an m/z signal 2,015.2 (Fig. 3B). The average mass tested by MALDI-TOF-MS confirmed the theoretical molecular mass of 2,014.5 Da calculated by ProtParam (http://us.expasy.org/tools/protparam.html).
Fig. 3.
HPLC profile and mass spectrum of the synthetic ctriporin peptide. (A) HPLC profile of the synthetic ctriporin peptide. The fraction containing the synthetic ctriporin peptide was indicated. (B) Mass spectrum of the synthetic ctriporin peptide measured by MALDI-TOF-MS. The measured molecular mass corresponding to the m/z signal (2,015.2) was 2,014.2 Da, and the theoretical calculated molecular mass was 2,014.5 Da.
MIC.
We tested the MICs of the synthetic peptide ctriporin against standard microorganism strains and antibiotic-resistant pathogens with the microdilution method. As shown in Table 1, ctriporin peptide can specifically inhibit Gram-positive organisms and fungi but not Gram-negative bacteria. The MICs of ctriporin against S. aureus (AB94004), B. thuringiensis (AB93066), B. subtilis (AB91021), M. luteus (AB93113), and C. albicans (AY93025) were 5, 10, 10, 5, and 20 μg/ml, respectively. In contrast, E. coli (AB94012) and P. aeruginosa (AB92037) were insensitive to >100 μg of ctriporin peptide/ml.
Table 1.
Antibacterial effects of ctriporin peptide against standard strains
| Bacterial or fungal strain | MIC (μg/ml) |
|||
|---|---|---|---|---|
| Ctriporin | Penicillin | Cefotaxime | Amphotericin B | |
| Bacillus thuringiensis AB92037 | 10 | 12.5 | 12.5 | ND |
| Bacillus subtilis AB91021 | 10 | 50 | 50 | ND |
| Staphylococcus aureus AB94004 | 5 | 6.25 | 3 | ND |
| Micrococcus luteus AB93113 | 5 | 25 | 3 | ND |
| Escherichia coli AB94012 | >100 | NDa | 3 | ND |
| Pseudomonas aeruginosa AB93066 | >100 | ND | 12.5 | ND |
| Candida albicans AY93025 | 20 | ND | ND | 1.5 |
ND, not determined.
The MICs of ctriporin peptide against MRSA, MRCNS, and PRSE were tested after determination of their antibiotic resistance. The MICs of ctriporin peptide against MRSA strains P1374 and P1386 and MRCNS strain P1369 were all 10 μg/ml. The MIC of the peptide ctriporin against the penicillin-resistant strain P1389 strain (PRSE) was 10 μg/ml. In order to make a direct contrast, the MICs of penicillin G salt, cefotaxime, and vancomycin against penicillin-resistant strains and methicillin-resistant strains were all determined. The MIC of penicillin G against the penicillin-resistant strain P1389 was 10,000 μg/ml, which was nearly 3 orders of magnitude higher than that of the peptide ctriporin. The MICs of cefotaxime against the methicillin-resistant strains were 100 to 400 μg/ml, which was about 10 to 40 times higher than the MICs of ctriporin peptide (Table 2). Thus, the ctriporin peptide not only can inhibit the growth of standard Gram-positive bacteria stains and fungi but also can inhibit the growth of clinical isolated MRSA and PRSE strains. Moreover, ctriporin peptide has a better antibacterial potency than traditional antibiotics in inhibiting antibiotic-resistant pathogens. These results indicated that ctriporin peptide may be an effective peptide molecule or compound to use against antibiotic-resistant bacteria, especially MRSA.
Table 2.
Antibacterial effects of ctriporin peptide against clinical isolates
| Straina | MIC (μg/ml) |
|||
|---|---|---|---|---|
| Ctriporin | Vancomycin | Cefotaxime | Penicillin | |
| Methicillin-resistant S. aureus | ||||
| P1386 | 10 | 4 | 100 | 10,000 |
| P1374 | 10 | 8 | 400 | 4,000 |
| P1369 | 10 | 8 | 400 | 20,000 |
| Penicillin-resistant S. epidermidis | ||||
| P1389 | 10 | 8 | 2 | 10,000 |
| Methicillin-sensitive S. aureus | ||||
| AB94004 | 5 | 3 | 3 | 6.25 |
All strains were clinical isolates obtained from the 302nd military hospital, Beijing, China.
Growth-inhibitory effect.
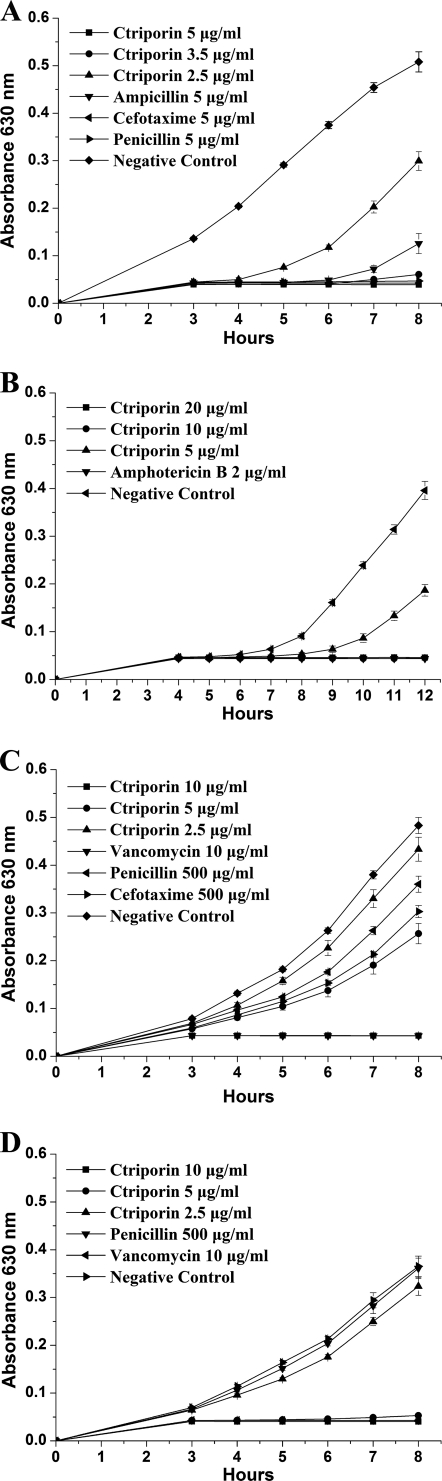
The growth-inhibitory effect of ctriporin against standard microorganism strains and antibiotic- resistant strains was tested by measuring the OD600 of five to nine time points with a microplate reader. As shown in Fig. 4A, a dose of 5 μg of ctriporin peptide/ml could completely inhibit the growth of S. aureus AB94004 as effectively as 5 μg of penicillin G/ml and 5 μg of cefotaxime/ml, whereas ampicillin could not effectively inhibit its growth 7 h after treatment at the same concentration. When the concentration of ctriporin peptide was decreased to 3.5 μg/ml, S. aureus growth could be inhibited for the first 7 h, but when the concentration decreased to 2.5 μg/ml, S. aureus growth was inhibited only in the first 4 h. This result suggested that the inhibitory effect of ctriporin against S. aureus was concentration dependent. For C. albicans, its effect was similar (Fig. 4B).
Fig. 4.
Time-growth curves of S. aureus and C. albicans treated with ctriporin peptide. Growth was measured at 630 nm. (A) Comparison of ctriporin and antibiotics activities against S. aureus AB94004. (B) Comparison of ctriporin and antibiotics activities against C. albicans AY93025. (C) Comparison of ctriporin and antibiotics activities against the clinical isolate MRSA P1374. (D) Comparison of ctriporin and antibiotics activities against the clinical isolated PRSE P1389.
In addition, 500 μg of penicillin/ml or 200 μg of cefotaxime/ml failed to inhibit the growth of MRSA P1374 strain (Fig. 4C). In contrast, 10 μg of ctriporin peptide/ml could completely inhibit the growth of MRSA P1374 strain, although when the concentration of the peptide ctriporin was decreased to 5 μg/ml, it only slightly inhibited bacterial growth. As shown in Fig. 4D, 500 μg of penicillin G/ml failed to inhibit the growth of PRSE strain P1389, whereas both 10 and 5 μg of ctriporin peptide/ml could effectively inhibit the growth of PRSE P1389 within 8 h. However, when the concentration of the peptide ctriporin was decreased to 2.5 μg/ml, ctriporin nearly lost its antibacterial activity against PRSE strain P1389. Therefore, the inhibitory effect of ctriporin peptide against antibiotic-resistant bacteria is also concentration dependent.
CFU statistics.
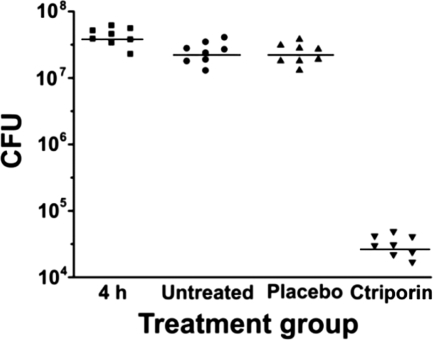
The number of CFU recoverable from a wound 4 h after the application of 107 to 108 CFU of S. aureus was 7.62 ± 0.14 log10. There was no significant difference (P = 1.0) in the numbers of CFU per wound after 4 h, after 4 days of no treatment (7.38 ± 0.16 log10), and after 4 days of placebo treatment (7.36 ± 0.15 log10). However, comparison of placebo treatment (7.36 ± 0.15 log10) and ctriporin treatment (4.48 ± 0.16 log10) revealed a great reduction in the number of CFU per wound (Fig. 5), a difference that was statistically significant (P < 0.001) (Table 3). This result indicates that ctriporin peptide as a topical antibiotic treatment is effective in treating the S. aureus mouse skin infections.
Fig. 5.
Blade-scratched mice infected with S. aureus AB94004. The number of bacteria (CFU) extracted from each mouse is represented by the symbol for the corresponding experimental group. The median value of the data for each group is shown as a horizontal bar. The numbers of mice in each experimental group were as follows: 4 h postinfection, n = 8; untreated, n = 8; placebo treated, n = 8; and ctriporin treated, n = 8.
Table 3.
Bacterial counts obtained in the various treatment groupsa
| Treatment group | Mean S. aureus bacterial count (log10 CFU/wound) ± SD (no. of wounds) |
|---|---|
| All subjects (initial inoculum size) | 7.66 ± 0.09 (32) |
| Subjects at 4 h postinfection | 7.62 ± 0.14 (8) |
| Untreated subjects (day 4) | 7.38 ± 0.16 (8) |
| Placebo-treated subjects (day 4) | 7.36 ± 0.15 (8) |
| Ctriporin-treated subjects (day 4) | 4.48 ± 0.16 (8) |
The S. aureus strain AB94004 was used. Ctriporin ⧣ placebo (P < 0.001); ctriporin ⧣ untreated (P < 0.001); untreated = placebo (P= 1.00).
Wound morphological observation.
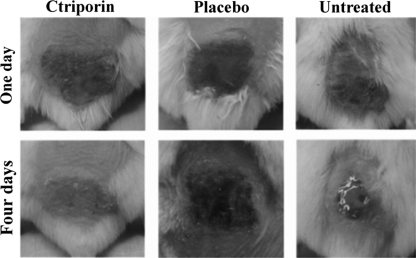
Wound morphologies were observed daily. As shown in Fig. 6, at 1 day after infection, the skin treated with ctriporin peptide was scabbed over, dry, and crusty, whereas skin treated with placebo or no treatment was moist, soft, and red. At 4 days after infection, the scabs fell off, and no visible abrasion was observed in the skin of ctriporin-treated animals, whereas the skin of placebo-treated mice was still red swollen and wet, and untreated skin showed effusion and ulceration, with cave formation.
Fig. 6.
Antibacterial effects of the peptide ctriporin in vivo. Mouse wound morphologies were observed after treatment with ctriporin or placebo or not treated at days 1 and 4.
Histological examination.
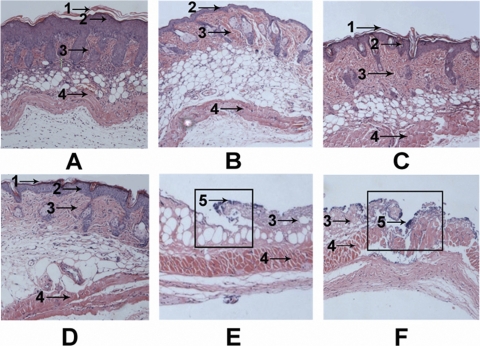
After 4 days of treatment for infection, biopsy specimens were dissected immediately and then fixed in formalin and embedded in paraffin. The biopsy specimens were stained with hematoxylin and eosin, sectioned, and then observed under an optical microscope. As shown in Fig. 7 B, blade-scratched skin had most of the epidermis removed compared to the normal skin (Fig. 7A), although a single layer of epidermal cells or scattered epidermal cells remained. Except for the missing epidermis, the skin appeared normal. At 4 days after blade scratching, the layers of epidermal cells were restored and the skin had recovered (Fig. 7C). At 4 days after infection, the epidermis had nearly disappeared, and the dermis was badly damaged in the placebo-treated group (Fig. 7E) and in the untreated group (Fig. 7F). Moreover, the presence of bacteria was evident on the surface of the ulcer in both the untreated and the placebo-treated groups. However, it was very interesting that, in the ctriporin-treated group, the skin had essentially recovered, and a relatively thin epidermis and normal dermis had formed (Fig. 7D). A thin layer of corneum had also formed on the surface of the skin in the ctriporin-treated group.
Fig. 7.
Histological appearance of normal dorsal skin of mice (A), immediately after blade scratching of the skin (B), 4 days after blade scratching of the skin (C), 4 days after ctriporin treatment of S. aureus-infected skin (D), 4 days after placebo treatment of S. aureus-infected skin (E), and 4 days after no treatment of S. aureus-infected skin (F). Biopsy specimens were obtained immediately after the termination of the experiment, fixed in formalin, and embedded in paraffin. The biopsy specimens were stained with hematoxylin and eosin. Infected lesions are outlined in boxes. Numbered arrows indicate the following: 1, corneum; 2, epidermis; 3, dermis; 4, muscular layer; and 5, bacteria.
Bactericidal assay.
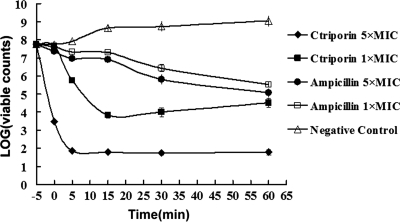
The bactericidal assay results showed that the bactericidal process started from the moment that the bacteria were mixed with ctriporin peptide. As shown in Fig. 8, the visible colonies of bacteria treated with ctriporin at 1× the MIC observably decreased to a plateau, and the colony number of bacteria treated with ctriporin at 5× the MIC decreased steeply to a much lower plateau in 5 min. Thus, we can conclude that the killing rate positively corresponded to the concentration of ctriporin peptide. In contrast, treatment with 5× the MIC of ampicillin sodium (40 μg/ml) yielded a gradual decrease in survival rate, a result which was consistent with its molecular mechanism of inhibiting the synthesis of the bacterial wall. This result suggests that ctriporin peptide exerted a rapid killing effect on microorganisms, and this was probably the reason why it can effectively inhibit the growth of microorganisms.
Fig. 8.
Killing assay. A killing assay was conducted with determination of the count of the surviving bacteria without the supernatant. The set point in the picture refers to the initial bacterial count: 0 min is defined as the time point of the first sample collection, which is immediately after mixing of the bacteria and ctriporin, and the other samples were collected at 5, 15, 30, and 60 min. All of the counts were the average of three dishes. The experiment was repeated with similar results.
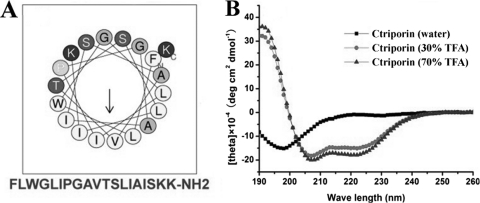
Secondary structure analysis of ctriporin.
The secondary structure of ctriporin peptide predicted online was a typical α-helix (Fig. 9A). The helical wheel of ctriporin peptide is divided into two parts: one part is the hydrophobic face, and the other is the hydrophilic face where there are two positively charged lysines. This suggests that the peptide ctriporin is an amphipathic peptide molecule. Furthermore, the result of CD spectral analysis also confirmed that ctriporin peptide is a typical α-helix molecule. As shown in Fig. 9B, ctriporin showed high percentages of α-helix (>90%) in the structure promoting solution, i.e., 70 and 30% aqueous TFE. However, ctriporin peptide displayed the secondary structure of random coil in water. All of these results indicate that ctriporin peptide is a positively charged molecule that can form an amphipathic helical structure under appropriate environmental conditions. However, it is well known that proline and glycine residues may break up α-helixes. Whether the peptide ctriporin with proline and glycine residues can form a helical structure under appropriate environmental conditions needs to be clarified by determining the 3D structure of the peptide ctriporin. Probably the helix of ctriporin peptide may start after proline or glycine residues.
Fig. 9.
Secondary structure analysis of ctriporin peptide. (A) Helical wheel diagram of ctriporin peptide determined by the Heliquest method. The representation of ctriporin mature peptide as a helical wheel shows the hydrophilic face and hydrophobic face. (B) CD spectra of 0.1 mg of ctriporin/ml in water alone or with 30 or 70% aqueous TFE.
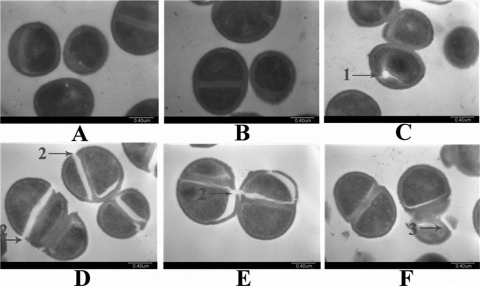
Transmission electron microscopy.
S. aureus cells treated with ctriporin peptide were observed with a transmission electron microscope to show individual bacterial changes. As shown in Fig. 10C and D, some pores and cracks were formed on the surfaces of S. aureus cell walls that had been divorced from the membranes. Moreover, the cracking tended to occur in dividing cells that produced a relatively larger distortion on the cell walls (Fig. 10E). More importantly, some bacterial cells were completely broken into fragments, leading to the leakage of cell contents (Fig. 10F). All of these results were direct evidence supporting the idea that ctriporin peptide treatment caused a fragile cell wall that bursts, thereby killing the bacterium.
Fig. 10.
Transmission electron microscopy images of S. aureus treated with ctriporin peptide. (A and B) Negative control; (C, D, E, and F) 10 min after ctriporin treatment. Numbered arrows indicate in the following: 1, pores on cells; 2, cracks on cell walls; and 3, cell lysis.
The results obtained using bactericidal assay, secondary structure analysis, and transmission electron microscopy provided clear evidence that the bacterial cell walls were broken immediately after ctriporin treatment and that the inhibitory effect of ctriporin on bacteria occurred through the action mode of rapid killing.
DISCUSSION
AMP activation is one of the primary mechanisms used by human skin in the early stages of immune defense (50). Many cell types that permanently reside in skin produce AMPs, including keratinocytes, sebocytes, eccrine glands, and mast cells (7, 31). AMPs are important for skin health; decreased expression of AMPs may result in some skin diseases such as atopic dermatitis (37), as well as skin injury and infection (10, 38). AMPs appear to form a chemical shield on the surface of the skin and protect human skin from the infection of invading pathogens. Damaged skin tends to be more vulnerable to pathogens, especially MRSA.
Just like the AMPs found in mammal skin, the AMPs found in scorpion venom are also important and diverse. Since the first two antimicrobial peptides hadrurin (47) and scorpine (11) were isolated from the scorpion Hadrurus aztecus and the scorpion Pandinus imperator, respectively, some AMPs were isolated and identified from the venoms of different scorpion species. The venom gland cDNA library of the scorpion Chaerilus tricostatus was constructed first. Ctriporin was the first antimicrobial peptide found in this scorpion. It consists of 19 amino acids with a net positive charge of 2, and the amino acid sequence has about 36.8 to 47% similarity to the sequences of BmKb2 (32), imcroporin (53), and mucroporin (13), while the MIC of ctriporin peptide against S. aureus is 5 μg/ml, which was about 3- to 5-fold lower than those of BmKb2, imcroporin, and mucroporin. Moreover, the MIC against MRSA was even 5- to 10-fold lower. So, it provides us a new template for anti-infective drug design and gives us a clue that ctriporin has the potential to be designed as an effective antimicrobial agent for use against some infections.
Since there are no effective treatment strategies for some serious skin infections, especially MRSA infection, our study focused on the effect of the external use of ctriporin peptide. The results suggest that ctriporin may become a potential candidate for treating skin infections. In vitro, ctriporin can effectively inhibit Gram-positive bacteria, including clinically isolated antibiotic-resistant pathogens (MRSA, MRCNS, etc.). Moreover, ctriporin also shows antifungal activity against C. albicans. A test for the efficiency of external use of peptide ctriporin was carried out in vivo. The results show that ctriporin topical treatment can effectively protect skin from infection in a mouse skin S. aureus infection model. Ctriporin treatment with 0.1 mg per wound resulted in a greater decrease in the bacterial counts of S. aureus from untreated controls, yielding a 3.38 log10 decrease on average. Similarly, both morphological observation and histological examination show that the peptide ctriporin-treated skin was back to normal. Moreover, ctriporin has advantages in some aspects compared to pexiganan (MSI-78) and omiganan pentahydrochloride (MBI 226), which have been used as topical agents in gel formulations. Ctriporin is more effective in inhibiting C. albicans. The MICs of pexiganan and omiganan against C. albicans were 64 to >512 μg/ml (24) and 32 to 128 μg/ml (44), respectively, which were about 3- to 5-fold higher than that of ctriporin on average. Besides, the antibacterial activity of ctriporin against MRSA is also better than that of pexiganan (MIC, 16 to 64 μg/ml) (24) and omiganan (MIC, 16 to 32 μg/ml) (23). Thus, ctriporin may be complementary to these developed topical antibiotics in some functions.
Unlike conventional antibiotics, which generally target a metabolic enzyme and may selectively induce resistance in microorganisms, AMPs kill microbes mainly by membrane-targeting pore-forming mechanisms; such mechanisms are inherently more difficult for microbes to circumvent by developing resistance (26). The relatively short length of AMPs makes them reasonably easy to synthesize, which has led to a vast number of studies and clinical trials (1, 21, 39). All of these suggest that the broad-spectrum antibacterial activity and low risk of drug resistance may enable antimicrobial peptides to be one of the alternatives to traditional antibiotics.
In conclusion, we showed here that ctriporin, a new member of antimicrobial peptide family, demonstrates a strong antimicrobial activity, including Gram-positive bacteria, fungi, and antibiotic-resistant pathogens (MRSA, MRCNS, and PRSE). It is a new template for anti-infective drug design. It rapidly killed bacteria and effectively cured skin infections in mice when applied externally. Therefore, we believe ctriporin, because of its high antimicrobial activity and therapeutic potential in treating skin infection, shows promise in the area of clinical application.
ACKNOWLEDGMENTS
We thank Ruiming Zhao from University of Chicago for help with the language correction.
This study was supported by the NSFC (grant 31071942), the China Specific Project for Developing New Drugs (grants 2009ZX09103-612 and 2008ZX10001-015-9), the National Basic Research Program of China (grants 2010CB529800 and 2010CB530100), and Fundamental Research Funds for the Central Universities (grant 1102001).
Footnotes
Published ahead of print on 29 August 2011.
REFERENCES
- 1. Andres E., Dimarcq J. L. 2004. Cationic antimicrobial peptides: update of clinical development. J. Intern. Med. 255:519–520 [DOI] [PubMed] [Google Scholar]
- 2. Appelbaum P. C. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 12(Suppl. 1):16–23 [DOI] [PubMed] [Google Scholar]
- 3. Appelbaum P. C. 2007. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 30:398–408 [DOI] [PubMed] [Google Scholar]
- 4. Baggett H. C., et al. 2003. An outbreak of community-onset methicillin-resistant Staphylococcus aureus skin infections in southwestern Alaska. Infect. Control Hosp. Epidemiol. 24:397–402 [DOI] [PubMed] [Google Scholar]
- 5. Birnbaum R. S., O'Neil J. A., Muszynski M., Aron D. C., Roos B. A. 1982. A non-calcitonin secretory peptide derived from preprocalcitonin. J. Biol. Chem. 257:241–244 [PubMed] [Google Scholar]
- 6. Bradbury A. F., Smyth D. G. 1991. Peptide amidation. Trends Biochem. Sci. 16:112–115 [DOI] [PubMed] [Google Scholar]
- 7. Braff M. H., Zaiou M., Fierer J., Nizet V., Gallo R. L. 2005. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect. Immun. 73:6771–6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cociancich S., et al. 1993. Purification and characterization of a scorpion defensin, a 4-kDa antibacterial peptide presenting structural similarities with insect defensins and scorpion toxins. Biochem. Biophys. Res. Commun. 194:17–22 [DOI] [PubMed] [Google Scholar]
- 9. Cohen P. R. 2005. Cutaneous community-acquired methicillin-resistant Staphylococcus aureus infection in participants of athletic activities. South Med. J. 98:596–602 [DOI] [PubMed] [Google Scholar]
- 10. Cole A. M., et al. 2001. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood 97:297–304 [DOI] [PubMed] [Google Scholar]
- 11. Conde R., Zamudio F. Z., Rodriguez M. H., Possani L. D. 2000. Scorpine, an anti-malaria and antibacterial agent purified from scorpion venom. FEBS Lett. 471:165–168 [DOI] [PubMed] [Google Scholar]
- 12. Corzo G., et al. 2001. Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion Pandinus imperator. Biochem. J. 359:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai C., et al. 2008. Mucroporin, the first cationic host defense peptide from the venom of Lychas mucronatus. Antimicrob. Agents Chemother. 52:3967–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai L., et al. 2001. IsCT, a novel cytotoxic linear peptide from scorpion Opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 286:820–825 [DOI] [PubMed] [Google Scholar]
- 15. Dai T., Tegos G. P., Zhiyentayev T., Mylonakis E., Hamblin M. R. 2010. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg. Med. 42:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. David M. Z., Rudolph K. M., Hennessy T. W., Boyle-Vavra S., Daum R. S. 2008. Molecular epidemiology of methicillin-resistant Staphylococcus aureus, rural southwestern Alaska. Emerg. Infect. Dis. 14:1693–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deurenberg R. H., et al. 2007. The molecular evolution of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 13:222–235 [DOI] [PubMed] [Google Scholar]
- 18. Diamond G., Beckloff N., Weinberg A., Kisich K. O. 2009. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 15:2377–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Douglass J., Civelli O., Herbert E. 1984. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu. Rev. Biochem. 53:665–715 [DOI] [PubMed] [Google Scholar]
- 20. Ehret-Sabatier L., et al. 1996. Characterization of novel cysteine-rich antimicrobial peptides from scorpion blood. J. Biol. Chem. 271:29537–29544 [DOI] [PubMed] [Google Scholar]
- 21. Enright M. C., et al. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. U. S. A. 99:7687–7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Finlay B. B., Hancock R. E. 2004. Can. innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2:497–504 [DOI] [PubMed] [Google Scholar]
- 23. Fritsche T. R., Rhomberg P. R., Sader H. S., Jones R. N. 2008. Antimicrobial activity of omiganan pentahydrochloride tested against contemporary bacterial pathogens commonly responsible for catheter-associated infections. J. Antimicrob. Chemother. 61:1092–1098 [DOI] [PubMed] [Google Scholar]
- 24. Fuchs P. C., Barry A. L., Brown S. D. 1998. In vitro antimicrobial activity of MSI-78, a magainin analog. Antimicrob. Agents Chemother. 42:1213–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Groom A. V., et al. 2001. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 286:1201–1205 [DOI] [PubMed] [Google Scholar]
- 26. Hancock R. E., Sahl H. G. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 27. Isaac R., et al. 1998. A novel peptide-processing activity of insect peptidyl-dipeptidase A (angiotensin I-converting enzyme): the hydrolysis of lysyl-arginine and arginyl-arginine from the C terminus of an insect prohormone peptide. Biochem. J. 330(Pt. 1):61–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jenssen H., Hamill P., Hancock R. E. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19:491–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kock R., et al. 2010. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Eur. Surveill. 15:19688. [DOI] [PubMed] [Google Scholar]
- 30. Kupstaite R., et al. 2010. Severe vancomycin-induced anaphylactic reaction. Medicina (Kaunas) 46:30–33 [PubMed] [Google Scholar]
- 31. Lee D. Y., et al. 2008. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill Propionibacterium acnes. J. Invest. Dermatol. 128:1863–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo F., et al. 2005. Genomic organization of four novel non-disulfide-bridged peptides from scorpion Mesobuthus martensii Karsch: gaining insight into evolutionary mechanism. Peptides 26:2427–2433 [DOI] [PubMed] [Google Scholar]
- 33. Marothi Y. A., Agnihotri H., Dubey D. 2005. Enterococcal resistance: an overview. Indian J. Med. Microbiol. 23:214–219 [PubMed] [Google Scholar]
- 34. Martin E., Ganz T., Lehrer R. I. 1995. Defensins and other endogenous peptide antibiotics of vertebrates. J. Leukoc. Biol. 58:128–136 [DOI] [PubMed] [Google Scholar]
- 35. Nair R. B. 1980. Chemical nature of toxic protein of venom of the South Indian scorpion Heterometrus scaber. Indian J. Exp. Biol. 18:1142–1144 [PubMed] [Google Scholar]
- 36. Nemati M., et al. 2008. Antimicrobial resistance of old and recent Staphylococcus aureus isolates from poultry: first detection of livestock-associated methicillin-resistant strain ST398. Antimicrob. Agents Chemother. 52:3817–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ong P. Y., et al. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151–1160 [DOI] [PubMed] [Google Scholar]
- 38. Ortega M. R., Ganz T., Milner S. M. 2000. Human beta defensin is absent in burn blister fluid. Burns 26:724–726 [DOI] [PubMed] [Google Scholar]
- 39. Oyston P. C., Fox M. A., Richards S. J., Clark G. C. 2009. Novel peptide therapeutics for treatment of infections. J. Med. Microbiol. 58:977–987 [DOI] [PubMed] [Google Scholar]
- 40. Parisien A., Allain B., Zhang J., Mandeville R., Lan C. Q. 2008. Novel alternatives to antibiotics: bacteriophages, bacterial cell wall hydrolases, and antimicrobial peptides. J. Appl. Microbiol. 104:1–13 [DOI] [PubMed] [Google Scholar]
- 41. Petricevich V. L. 2006. Balance between pro- and anti-inflammatory cytokines in mice treated with Centruroides noxius scorpion venom. Mediators Inflamm. 2006:54273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petricevich V. L., Hernandez Cruz A., Coronas F. I., Possani L. D. 2007. Toxin gamma from Tityus serrulatus scorpion venom plays an essential role in immunomodulation of macrophages. Toxicon 50:666–675 [DOI] [PubMed] [Google Scholar]
- 43. Rouille Y., et al. 1995. Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front. Neuroendocrinol. 16:322–361 [DOI] [PubMed] [Google Scholar]
- 44. Sader H. S., Fedler K. A., Rennie R. P., Stevens S., Jones R. N. 2004. Omiganan pentahydrochloride (MBI 226), a topical 12-amino-acid cationic peptide: spectrum of antimicrobial activity and measurements of bactericidal activity. Antimicrob. Agents Chemother. 48:3112–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sang Y., Blecha F. 2008. Antimicrobial peptides and bacteriocins: alternatives to traditional antibiotics. Anim. Health Res. Rev. 9:227–235 [DOI] [PubMed] [Google Scholar]
- 46. Stevens A. M., et al. 2010. Methicillin-resistant Staphylococcus aureus carriage and risk factors for skin infections, Southwestern Alaska, U.S.A. Emerg. Infect. Dis. 16:797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Torres-Larios A., Gurrola G. B., Zamudio F. Z., Possani L. D. 2000. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. Eur. J. Biochem. 267:5023–5031 [DOI] [PubMed] [Google Scholar]
- 48. Waness A. 2010. Revisiting methicillin-resistant Staphylococcus aureus infections. J. Glob. Infect. Dis. 2:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Z., Wang G. 2004. APD: the antimicrobial peptide database. Nucleic Acids Res. 32:D590–D592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamasaki K., Gallo R. L. 2008. Antimicrobial peptides in human skin disease. Eur. J. Dermatol. 18:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yuan W., et al. 2010. Cloning and functional characterization of a new antimicrobial peptide gene StCT1 from the venom of the scorpion Scorpiops tibetanus. Peptides 31:22–26 [DOI] [PubMed] [Google Scholar]
- 52. Zeh D. W. 1990. The biology of scorpions. Science 249:1176–1177 (Review)17831996 [Google Scholar]
- 53. Zhao Z., et al. 2009. Imcroporin, a new cationic antimicrobial peptide from the venom of the scorpion Isometrus maculates. Antimicrob. Agents Chemother. 53:3472–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]