Abstract
Over the past decade, molecular details of lymphatic vessels (lymphatics) have been rapidly acquired due to the identification of lymphatic endothelial-specific markers. Separate from the cardiovascular system, the lymphatic system is also an elaborate network of vessels that are important in normal physiology. Lymphatic vessels have the unique task to regulate fluid homeostasis, assist in immune surveillance, and transport dietary lipids. However, dysfunctional lymphatic vessels can cause pathology, while normal lymphatics can exacerbate pathology. This review summarizes the development and growth of lymphatic vessels in addition to highlighting their critical roles in physiology and pathology. Also, we discuss recent work that suggests a connection between lymphatic dysfunction and cardiovascular disease.
Keywords: Cardiovascular disease, inflammation, lymphedema, lymphatic vessels, vegf receptor-3
INTRODUCTION
Lymphatic vessels were described dating back to the 17th century.[1] As the second component of the human vasculature, they are less well characterized relative to blood vessels. At the turn of the 21st century, the identification of lymphatic endothelial markers such as Prox-1,[2] podoplanin,[3] and lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1)[4] has advanced study of lymphatic endothelial cells (LECs) during the past decade. For some time, it has been known that lymphatic vessels complement blood vessels by absorbing fluid, proteins, and cells (collectively known as lymph) from the interstitial space. Therefore, lymphatic vessels are found in most, but not all, vascularized tissues. However, it is appreciated that the lymphatic vasculature serves critical and nonredundant roles apart from the blood vascular system. It assists in immune surveillance, transport of nutrients from the intestine, and regulation of tissue pressure. Furthermore, the blood and lymphatic vasculatures are fundamentally different in their mode of operation. The blood system is a closed circuit system of high pressure that transports its content throughout the body, with the heart providing the force necessary for circulation. On the other hand, the lymphatic system is a unidirectional, low-pressure system that is relatively “passive” in its mode of action. Hence, lymph is propelled forward by respiration, skeletal muscle contraction, and intrinsic contraction of smooth muscle cells that surround the larger collecting lymphatic vessels. Unlike blood capillaries, lymphatic capillaries are more firmly attached to the extracellular matrix by anchoring filaments. Despite their functional and morphological differences, they do have some characteristics in common. Similar to blood vessels, larger lymphatic vessels contain a basement membrane in addition to valves that aid in unidirectional flow. In addition, certain signaling molecules common to both vasculatures are necessary during development and tissue remodeling, as discussed later.
Lymphatic vessel development
For the most part, the molecular mechanisms that dictate the development and growth of lymphatic vessels contrast to those of blood vessels. In the early 20th century, two models were put forward regarding how the lymphatic system develops. In 1902, Florence Sabin proposed that the blood vasculature gave rise to the lymphatic vasculature. Through meticulous ink-injection experiments, she concluded that the lymphatics originated in the cardinal vein of fetal pigs, form lymph sacs that migrate toward the periphery, and form lymph vessels that spread throughout the body.[5] A few years later, Huntington and McClure proposed that lymphangioblasts form the original lymph sacs and later establish venous connections.[6] While there is evidence in non-mammalian species that suggest embryonic veins and mesenchymal lymphangioblasts contribute to lymphatic vessels, much molecular support for Sabin's model has been generated thus far, in addition to lineage tracing experiments that further support the existing data that lymphatics are derived from the venous system.[7]
In mouse development, a subset of venous endothelial cells in the anterior cardinal vein express Sox-18, a member of the Sox family of transcription factors, which have been shown to have a pivotal role in cardiovascular and blood vascular development.[8,9] Sox-18 is expressed in the cardinal vein at E9.0 in a subpopulation of endothelial cells. Sox-18 can then bind to the promoter of Prox-1, also a homeobox transcription factor, to initiate the lymphatic specification program [Figure 1a]. In addition, Coup-TFII, an orphan nuclear receptor, assists in turning on and maintaining the expression of Prox-1 [Figure 1a].[10] Prox-1 expression in this subset of cells in the anterior cardinal vein around embryonic (E) 9.75 of mouse development is necessary for these blood endothelial cells to subsequently commit to the LEC lineage.[2] After commitment to the lymphatic cell lineage, vascular endothelial growth factor receptor-3 (VEGFR-3) is critical for sprouting and migration in response to its ligand, vascular endothelial growth factor-C [VEGF-C, Figure 1b].[11] Sprouting is necessary for the formation of the primary lymph sacs [Figure 1c]. Peripheral lymphatic vessels are thought to subsequently form by centrifugal sprouting from the primary lymph sacs, followed by maturation of large collecting lymphatic vessels [Figure 1e].
Figure 1.
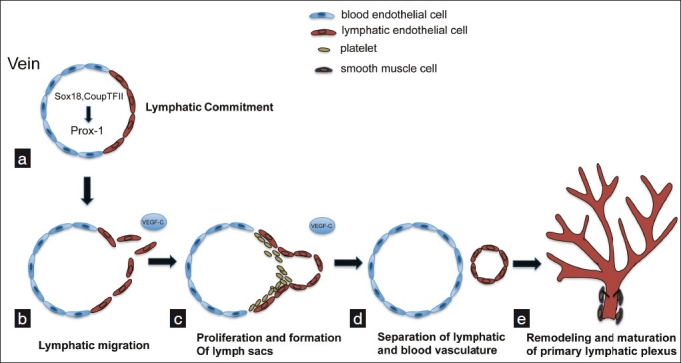
Development of the murine lymphatic vasculature. (a) Lymphatic competent “precursors” begin to express Sox-18 and CoupTFII, which subsequently induce expression of Prox-1, which is responsible for induction of and maintenance of the LEC phenotype. (b) A mesenchymal source of VEGF-C initiates sprouting of LECs and formation of primitive lymph sacs, through VEGFR-3. With the assistance of platelets and a continued source of VEGF-C, lymphatic cells separate from veins and proliferate to form a primitive lymphatic network (c, d), which is later remodeled into the mature lymphatic vascular system that features organization into capillaries and larger collecting vessels (e)
A cadre of genes have been found to be important for each stage of lymphatic development, which include lymphatic commitment, migration and proliferation, separation, and remodeling/maturation. These molecular mediators are thoroughly summarized elsewhere.[12] Furthermore, recent work has suggested that micro-RNAs are involved in the regulation of lymphatic vascular lineage-specific differentiation from blood ECs in vitro and lymphatic vascular development in vivo.[13,14] Also, attention has refocused on the role of the hematopoietic system and its role in allowing lymphatic vessels to separate from blood vessels. Mice lacking certain signaling mediators have been shown to develop blood-lymphatic mixing during embryonic development.[15–18] This is the product of misconnections between lymphatics and blood vessels. Podoplanin, a transmembrane glycoprotein and surface marker for lymphatic endothelium, has recently gained interest. Podoplanin allows aggregation of platelets through interaction with the C-type lectin-like receptor 2 (CLEC-2) on platelets.[19,20] Recently, it was found that podoplanin-deficient mice phenocopy the blood-lymphatic mixing found in knockout mice other genes that are critical for separation of lymphatic vessels from blood vessels.[21–23] Therefore, platelets are also important for lymphatic separation from the blood endothelium [Figures 1c and 1d]. This has raised interesting questions concerning the nature of the interactions between platelets and the endothelium during lymphatic separation. Further work is needed to characterize mechanisms of lymphatic development and determine if similar mechanisms are recapitulated in pathological lymphangiogenesis.
THE PHYSIOLOGICAL ROLE OF LYMPHATICS
Lymphatics and fluid/fat uptake
Our cardiovascular system forces blood through the microcirculation. The dynamics of blood pressure and osmotic pressure is responsible for leakage of a relatively small amount of fluid from the blood into the interstitial space. However, collectively, in humans, tissue fluid and lymph make up a volume of approximately 12 liters.[24] In normal physiology, the blind-ended lymphatic vascular system drains this extravasated interstitial fluid from peripheral tissue and returns it to the blood. Through specialized junctions, the lymphatic capillaries are responsible for uptake of this fluid along with immune cells, antigens, lipids, macromolecules, and particulate matter, collectively referred to as lymph,[24] once inside lymphatic vessels. Having little or no basement membrane, lymphatic capillaries are composed of a single layer of thin-walled LECs[25] and are attached to the extracelluar matrix by anchoring filaments.[26,27] From the capillaries, lymph travels toward larger collecting lymphatic vessels, which are significantly different from lymphatic capillaries in that they have a basement membrane, contain intraluminal valves to ensure unidirectional flow, and are surrounded by smooth muscle cells, which serve as an intrinsic pump for lymphatic flow.[28] Indeed, removal of this fluid is critical in order to conserve tissue homeostasis, as will be discussed later.
The discovery of lymphatic vessels dates back hundreds of years. They were reported to be found in the mesentery of well fed dogs in 17th century by Aselli.[1] Consumption of foods rich in lipids have been shown to increase lymph flow.[29] This observation underscores the role of the lymphatics in lipid transport. In the Western diet, the majority of dietary lipids are long-chain triglycerides that are digested and absorbed in distinct steps. Luminal hydrolysis in the intestine produces fatty acids and monoglycerides, which form mixed micelles with bile salts and enter mucosal enterocytes, where endoplasmic reticulum enzymes convert them back into triglycerides. Through mostly unknown mechanisms, these dietary lipids, known as chylomicrons, enter the lymphatic system in intestinal villi through lacteals, which are specialized lymphatic vessels. From the lacteals, these large lipoproteins then travel through the submucosal lymphatics and larger mesenteric lymphatics. Next, chylomicrons enter the blood to deliver triglycerides to adipose and muscle before going to the liver to deliver cholesterol. Due to the important role in fat absorption, lymphatic dysfunction often leads to accumulation of fat in mice and humans.
Lymphatics and immunity
Lymphatic vessels are abundant in many organs of the body, such as the skin. Perhaps, this is an evolutionary advantage to protect the host from foreign microbes, as lymphatic vessels are a critical component of the immune response. It is known that dendritic cells (DCs) in peripheral tissues upregulate the chemokine receptor, CCR7, after encountering pathogen-associated molecular patterns (PAMPS). This upregulation increases responsiveness to its ligand chemokine (C-C motif) ligand 21 (CCL21), which is expressed on lymphatic vessels.[30,31] DCs and other antigen-presenting cells (APCs) travel through afferent lymphatic vessels en route to lymph nodes where they present antigen to prime T cells and mount an adaptive immune response. Efferent lymphatic vessels are critical for allowing activated lymphocytes to exit from lymph nodes and return to the blood where they are transported to tissues throughout the body to serve their effector functions. In addition to priming T cells, lymph node-resident and incoming DCs are important for T-cell education and maintenance of peripheral tolerance. After entry into the lymph nodes, DCs via CCR7 migrate to the paracortex region of the lymph node, which produces CCL19 and CCL21.[32] Here, they interact with naive T cells that have also exited peripheral tissue via CCR7.[33] It is appreciated that the continuous sampling of antigen by DCs is necessary for the maintenance of peripheral tolerance.[34] However, DCs and other APCs do not have to gather all peripheral antigens. The flow of lymph, via lymphatic vessels, can assist in peripheral tolerance by bringing soluble antigen to lymph node resident APCs. Furthermore, this afferent lymphatic flow is critical for maintaining lymph node architecture,[35] an additional positive regulator of peripheral tolerance. Recent work has shown that lymphatic vessels in the lymph node can directly maintain peripheral tolerance through expression of certain peripheral tissue antigens that cause CD8 T-cell deletion upon presentation by MHC class I.[36,37] Furthermore, the relationship between lymphatic vessels and lymphocytes in the lymph node appears to be even more dynamic and complex. T cells, through the production of interferon gamma (IFNγ), inhibit lymph node lymphangiogenesis.[38] On the other hand, B cells can positively regulate lymphangiogenesis,[39] through production of lymphangiogenic factors. These results indicate that the lymphatic vasculature is more than a passive conduit or a waste disposal for but is very active in immunological surveillance and lymph node homeostasis, thereby enhancing immunity.
LYMPHATIC SIGNALING
VEGF family
One of the most potent inducers of angiogenesis, vascular endothelial growth factor (or VEGF-A), was discovered more than two decades ago.[40] Since then, the family has grown and consists of additional members including placenta growth factor (PlGF), VEGF-B, VEGF-C, VEGF-D, and VEGF-E. Collectively, the VEGFs and their receptors are critical regulators of angiogenesis and lymphangiogenesis. Of interest to the lymphatic system, VEGF-A, VEGF-C, and VEGF-D have been shown to stimulate lymphangiogenesis, through binding specific receptors. VEGF-A binds to VEGFR-1/fms-like tyrosine kinase 1 (FLT-1) and VEGFR-2/human kinase insert domain receptor (KDR), while VEGF-C and VEGF-D bind to VEGFR-3/FLT4 and upon proteolytic processing can bind to VEGFR-2.[41] VEGFR-1 and VEGFR-2 are abundantly expressed on blood endothelial cells and function mainly in angiogenesis, while VEGFR-2 and VEGFR-3 are highly expressed in LECs and primarily function in lymphangiogenesis.[42] VEGFRs contain an extracellular domain consisting of seven immunoglobulin (Ig)-like domains, a transmembrane domain, and an intracellular tyrosine kinase domain that undergoes dimerization and autophosphorylation at several tyrosine kinase residues after binding of ligand.[43,44] This recruitment of downstream signaling molecules leads to biological responses such as survival, proliferation, and migration [Figure 2].
Figure 2.
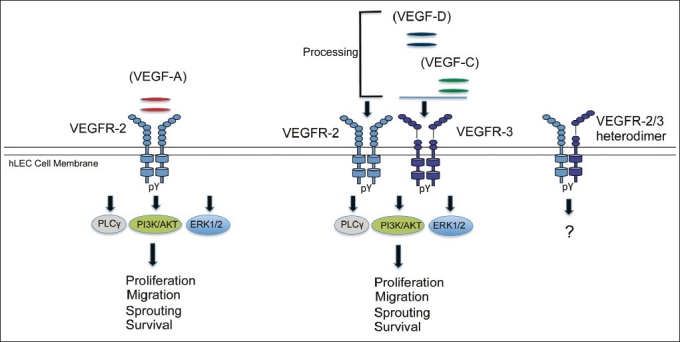
VEGF-VEGFR signaling in lymphangiogenesis. Of the VEGFR family proteins, VEGFR-2 and VEGFR-3 are strongly expressed in hLECs in vivo and exist as homodimers and heterodimers. VEGF-A activates VEGFR-2, whereas VEGF-C and VEGF-D activate both VEGFR-2 (after proteolytic cleavage) and VEGFR-3. In human lymphatic EC, VEGF-A preferentially induces phosphorylation of PLC-γ. In contrast, VEGF-C and D preferentially activate the Akt pathway. Both growth factors strongly activate the MAPK pathway. These signaling pathways have been shown to lead to effector functions of LECs, namely migration, sprouting, proliferation, enlargement (growth), survival and tube formation in vivo and in vivo. pY, tyrosine phosphorylation
VEGFR-3 is critical for lymphangiogenesis. The first ligand identified for VEGFR-3 was VEGF-C.[45] The spatiotemporal expression of VEGF-C with lymphatics suggested a role for VEGF-C in lymphatic development.[46] Indeed, a critical role for VEGF-C in lymphangiogenesis was seen in VEGF-C null mice, which die before birth due to an inability of committed lymphatic vessels to migrate and proliferate.[11] In addition to VEGF-C, VEGF-D has also been identified as a ligand for VEGFR-3.[47] Additional proof of the ability of VEGF-C and VEGF-D to stimulate lymphatic growth can be seen in individual overexpression of these ligands using transgenic mice. Under the control of a skin-specific promoter, VEGF-C and VEGF-D induce lymphatic hyperplasia.[48,49] Similarly, a mutant form of VEGF-C (VEGF-C156S, which only binds VEGFR-3) was sufficient for lymphatic growth. In vitro, VEGF-C/VEGFR-3 signaling is important for proliferation, migration, and survival of LECs through the activation of Akt and MAPK pathways.[50] It is worth noting that VEGFR-3 is expressed on certain tumor-associated blood vessels and blood vessels undergoing active angiogenic sprouting.[51] Furthermore, VEGFR-3-deficient mice die from failure of the primary vascular network to remodel, suggesting an important role for VEGFR-3 in blood vessels.
While VEGF-A is an undisputed inducer of angiogenesis, several lines of recent evidence support its role in lymphangiogenesis. Transgenic overexpression of VEGF-A promoted lymphangiogenesis.[52] Moreover, local injection of VEGF-A adenovirus into mouse ears induced significant lymphatic vessel growth, locally and systemically.[53,54] In addition, chronically inflamed tissue produced VEGF-A, which resulted in lymphangiogenesis.[55] It is unclear whether all these effects are accounted for through the VEGFR-2 receptor, or indirectly through recruitment of another cell type or upregulation of VEGF-C/D. However, it has been documented that systemic blockade of VEGFR-2 or VEGF-A prevented VEGF-A-induced lymphatic vessel formation in vivo.[55,56] In vitro, LECs signal through VEGFR-2, similarly to blood endothelial cells, but VEGF-C appears to be more potent for tube formation.[54] Our results also show that VEGF-A strongly activates the PLCγ pathway, while VEGF-C strongly activates the Akt pathway. Differential signaling “downstream” of VEGF receptors may lead to differences in functional outcomes. One molecule that we have identified downstream of VEGFR signaling is Bmx, a nonrecptor tyrosine kinase. Upon silencing Bmx, VEGFR -2/3 signaling was partially reduced, suggesting additional mediators of VEGF signaling. However, mechanistically, Bmx can interact with VEGFR-2 and VEGFR-3 to mediate downstream signaling of VEGF-A and VEGF-C.
It is known that VEGFR-2 and VEGFR-3 can form heterodimers, potentially in response to VEGF-A and VEGF-C.[57] It will be worthwhile to further dissect the differential roles of VEGFR-2/3 heterodimers on LECs in vitro and in vivo, as this interaction has shown to contribute to lymphangiogenic sprouting.[57] In addition, there may be unique signaling pathways activated in response to heterodimer formation, compared with either receptor alone. As mentioned earlier, VEGF/VEGFR-2 signaling can promote lymphangiogenesis in lymph nodes. However, VEGF/VEGFR-2 signals seem to mainly promote lymphatic vessel enlargement but not vessel sprouting in the skin. It will also be interesting to investigate if organ-specific differences account for the contrasting phenotypes. If so, what is the difference in the signaling repertoire between skin and lymph node lymphatics? These answers may be relevant for therapeutic organ-specific targeting of lymphatic vessels.
OTHER REGULATORS OF LYMPHANGIOGENESIS
While the VEGF family of receptors is well characterized, a plethora of other molecules are known to stimulate lymphangiogenesis. Other growth factors such as insulin-like growth factor (IGF) 1 and 2, fibroblast growth factor (FGF)-2, platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), and angiopoietin-1 have all been shown to be lymphangiogenic.[58–63] In addition, cytokines such as tumor necrosis factor (TNF-α) and lymphotoxin-α have been shown to drive lymphangiogenesis.[64,65] Unexpected inducers of lymphangiogenesis have been found such as Netrin-4, a secreted protein involved in axon guidance.[66] Adrenomedullin and endothelin-1, peptide vasodilator and vasoconstrictor, respectively, contribute to lymphangiogenesis.[67,68] Hormones such as luteinizing hormone, follicle-stimulating hormone, and growth hormone can also stimulate lymphangiogenesis.[69] Some aforementioned effects are direct, while others have been indirect, through upregulation of VEGF-C, for example. Conversely, transforming growth factor -beta (TGF-β), IFN γ, and thrombospondin-1 have been shown to inhibit lymphangiogenesis.[38,70–73] While the molecular mechanism of TGF-β-mediated inhibition is unknown, IFNγ downregulates Prox-1 and expression of LEC-specific genes, thus leading to decreased lymphangiogenesis in vitro. Thrombospondin-1 inhibits expression of VEGF-C/D via a CD36-dependent mechanism in monocytes. With knowledge of many ligands involved in lymphangiogenesis, further insight will be gained by exploring intracellular signaling pathways in response to these molecules.
LYMPHATICS IN PATHOLOGY
Lymphedema
When lymphatic vessels are dysfunctional, the importance of the lymphatics in tissue drainage is manifested by lymphedema, in which the main symptom is persistent (chronic) swelling, usually of extremities. Lymphedema is classified as two forms: primary and secondary lymphedema. Primary lymphedema has a genetic etiology that leads to inadequate functioning of the lymphatic vasculature. It can present at birth or arise later in life. The oldest report of congenital lymphedema is known as Milroy's disease. Multiple reports have linked Milroy disease with a mutation in the tyrosine kinase domain of the VEGFR-3 gene.[74–76] Interestingly, the Chy mouse mutant, a model for congenital lymphedema that contains a heterozygous mutation to deactivate VEGFR-3, has abnormal cutaneous lymphatic vessels and symptoms of lymphedema.[77] A list of genes related to primary lymphedema is summarized elsewhere.[12]
Secondary lymphedema is the most common cause of edema. It is caused by obstruction or damage to normal lymphatic vessels. In industrialized countries, a common cause of edema is from surgery.[78] In tropical and subtropical countries, pathogenic filarial parasites are the major cause of lymphedema.[79] These mosquito-borne parasites reside in and cause damage to the lymphatic vessels, leading to an inhibition of lymphatic function.[79]
Currently, there is no cure for lymphedema. Therapeutic approaches include massage therapy, exercise, dietary restrictions, compression garments, skin care, manual drainage, and liposuction. VEGF-C/D-based therapeutics appear to be a promising alternative, as VEGF-C/D regenerated collecting lymphatic vessels improved the outcome of lymph node transplantation.[80]
Lymphatic vessels in chronic inflammation and cancer
In adulthood, lymphangiogenesis occurs primarily during tissue regeneration, tumor growth, and acute and chronic inflammation. Much attention has been focused on lymphangiogenesis in the context of inflammation. Inflammation is a well-known phenomenon that occurs in response to infection or injury. During inflammation, a variety of cell types are recruited to the inflamed sites. Among these, macrophages have been extensively implicated in the production of VEGF-C and VEGF-D, leading to lymphangiogenesis.[81] In addition to secretion of growth factors, macrophages have been reported to transdifferentiate and incorporate into lymphatic vessels in the context of inflammation in the cornea.[84] Although lymphangiogenesis is well documented during inflammation, the biological role of lymphangiogenesis during inflammation is not well understood. Presumably, one would expect lymphangiogenesis to be beneficial by allowing immune trafficking and clearance of pathogen infection and inflammation. This would resolve inflammation and enhance antigen presentation. Indeed, activation of lymphatic vessels by overexpression of VEGFR-3-specific ligands inhibits acute and chronic inflammation.[82,83] However, several reports suggest that lymphatic vessels generated during inflammation are not beneficial to the host. For example, a murine ovarian cancer model resulted in significant lymphangiogenesis. However, lymphatic vessels in this case were nonfunctional, as determined by functional assays.[85] Crohn's disease, an autoimmune inflammatory bowel disease, is often associated with lymphatic vessel dysfunction. Similarly, lymphatic contractile activity was compromised in a model of intestinal inflammation.[86] In addition, results suggest that even in acute inflammation, the function of the endothelial barriers in the initial lymphatics may be compromised.[87] Interestingly, during inflammation, cytokines are produced which have been shown to have negative effects on lymphatic vessels directly.[88] The presence of these cytokines may have a detrimental effect on lymphatic vessels during inflammation. Further work is needed to determine the temporal and spatial function of these cytokines in specific pathologies.
In addition to angiogenesis, tumors stimulate lymphangiogenesis in experimental murine models and in human cancers.[89] Lymphatic vessels have also been found to contribute to the metastasis of primary tumor cells to draining lymph nodes and distant organs. Tumor cells can disseminate through preexisting lymphatic vessels. In addition, it has been found that tumors can secrete prolymphangiogenic growth factors such as VEGF-A, VEGF-C, and VEGF-D which can directly induce lymphangiogenesis in order to advance their spread to the draining lymph nodes and distant organs.[90] In particular, VEGF-C has been found to upregulate CCL21 production in lymphatic endothelium, which in turn can promote lymphatic entry, by CCR7 -expressing tumor cells.[91] VEGF-C is also a chemoattractant for macrophages via VEGFR-3, which is expressed by a population of peripheral blood monocytes and activated tissue macrophages.[92] This recruitment may lead to further lymphangiogenesis. Therefore, the presence of VEGF-C (and VEGF-D) is associated with increased metastasis and poor prognosis in human patients.[89]
Lymphatic vessels and cardiovascular disease
The metabolic syndrome (MetS) is characterized by a cluster of metabolic risk factors and includes abdominal obesity, dyslipidemia, hypertension, insulin resistance, and proinflammation and prothrombiotic conditions. Those with MetS are at increased risk for cardiovascular disease.[93] Obesity is considered a key contributing factor leading to the increased prevalence of the metabolic syndrome. Interestingly, several mouse models and cases of human pathology show the association between dysfunctional lymphatic vessels and obesity [Figure 3].
Figure 3.
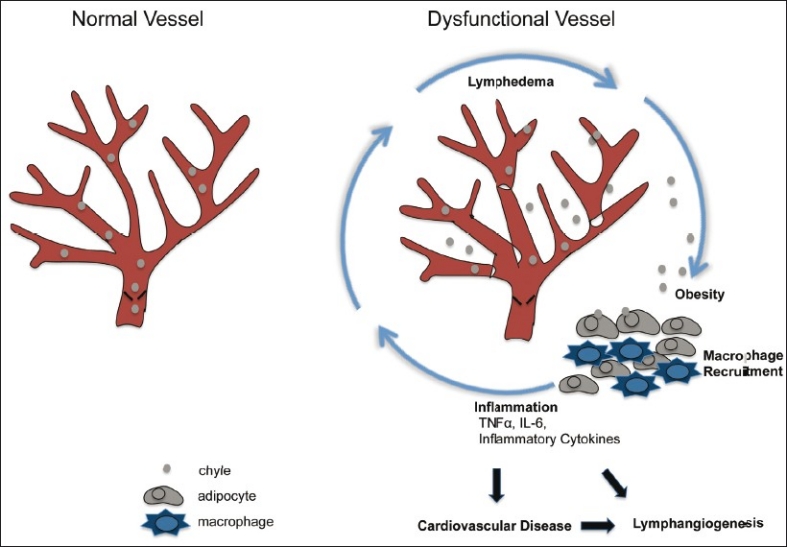
Proposed working model for dysfunctional lymphatic vessel contribution to cardiovascular disease (evidence from literature in text). Persons diagnosed with metabolic syndrome are at greater risk to develop cardiovascular disease. The metabolic syndrome is a condition diagnosed with the appearance of several risk factors in an individual, and obesity is considered a major risk factor. Dysfunctional lymphatic vessels are unable to properly drain lymph and chyle (shown here). This can cause edema and increased fluid volume leading to chylous ascites and chylothorax. Furthermore, chyle (including chylomicrons) can leak from compromised lymphatics and influence surrounding reservoirs of fat. Lymph can cause maturation of preadipocytes and growth of adipose tissue. Secretion of inflammatory cytokines from adipose tissue and macrophages is exacerbated in obese individuals. Recruitment of macrophages to adipose tissue is increased and excess inflammation can increase lymphangiogenesis and add further detriment to lymphatic vessels, amplifying the cycle, and may eventually contribute to cardiovascular disease
The 17th century observation of “milky veins” in fed dogs is now known to be chyle, a milky fluid rich in chylomicrons that consists of cholesterol, phospholipids, triglycerides and apolipoproteins. Chyle is transported by lacteals, which are specialized lymphatic capillaries that absorb dietary fats in the small intestinal villi. One can envision that a failure of lymphatics to absorb or transport lipids can start a cascade of metabolic disorders. Individuals that have genetic mutations associated with genes critical for lymphatic signaling often present with subcutaneous edema and chylothorax as summarized by Schulte-Merker et al.[12] Moreover, several mutant mice created to study lymphatic vessels have abnormal fat accumulation. The Chy mouse, mentioned earlier, is characterized by the accumulation of chylous ascites in the abdomen.[77] In addition, the K14-VEGFR-3-Ig transgenic mouse, which blocks VEGF-C-mediated signaling, develop a lymphedematous-like phenotype that includes increased deposition of subcutaneous fat.[94] An extensive list of murine genes that lead to lymphatic-associated fat accumulation is reviewed in Cueni et al.[95] Perhaps the most striking example is seen with the loss of one allele of Prox-1. Prox-1 heterozygosity resulted in defects of the lymphatic vasculature that lead to chylous ascites and adult onset obesity.[96] Interestingly, the integrity of the lymphatic vasculature was compromised in these mice, notably in the mesenteric lymphatic vessels, suggesting obesity may be a consequence of malfunctioning lymphatic vessels. It is also tempting to speculate that these mice may also have increased inflammation, leading to altered integrity of lymphatic vessels. Nonetheless, lymph was shown to promote differentiation of 3T3-L1 preadipocytes into adipocytes by unknown factors, indicating extravasated lymph, due to compromised lymphatic vessels, may directly influence fat deposition. Indeed, these mice have subcutaneous and intra-abdominal fat accumulation, which has been implicated in metabolic disorders to a greater extent than total body fat.[97] It is known that adipose tissue can function as an endocrine organ, not to mention its function as the major storage site for triglycerides.[98] Adipokines, which are adipocyte-derived factors such as adiponectin and leptin, affect body energy homeostasis through autocrine and endocrine functions. Interestingly, Prox-1 heterozygous mice have increased leptin, an adipose-derived satiety hormone that acts on the hypothalamus to inhibit feeding. Moreover, visceral fat adipokine secretion is associated with systemic inflammation, insulin resistance, and diabetes in obese humans.[99–101] However, it is unclear if inflammation is a cause or consequence of obesity. Data have suggested that an increase in adiposity is accompanied by an increase in the inflammatory response.[99] Interestingly, there was an increased accumulation of macrophages in adipose tissue in the mesentery of the Prox-1 heterozygous mice. In addition to inflammatory cytokines such as TNFα and IL-6 secreted by adipose cells, macrophages have also been shown to be a source of proinflammatory factors in adipose tissue.[102] Collectively, these adipokines are able to recruit and activate more macrophages, which have been associated with insulin resistance.[103] This cyclical dynamic may be in part responsible for the low-grade inflammation linked to MetS.
In addition to inflammatory cytokines, adipose- associated macrophages can secrete angiogenic factors such as VEGF.[104] It is also possible that these adipose- macrophages can stimulate lymphangiogenesis, as macrophages also release growth factors including VEGF-C and VEGF-D, which elicit the formation of lymphatic vessels. Stimulation of lymphangiogenesis would likely lead to enhanced transport of lipids. However, several lines of evidence suggest the contrary. As previously mentioned, lymphatic function was shown to be impaired in several models of inflammation, suggesting that lymphatic function might be compromised in some inflammatory diseases, leading to further exacerbation of edema, inflammation, and obesity.[85,86]
Environmental factors including diet are thought to represent the etiology of the MetS.[105] Diets rich in long-chain triglycerides depend on the lymphatics for absorption after being packaged into chylomicrons. It is plausible that individuals with lymphatic abnormalities are more prone to developing symptoms of the MetS. Evidence has shown that circulating chylomicrons trigger an inflammatory response with the recruitment of neutrophils and activation of monocytes.[106] Furthermore, postprandial lipoproteins can upregulate expression of leukocyte adhesion molecules on the blood endothelium, thereby orchestrating adhesion and migration of inflammatory cells into various tissues.[107] It is possible that a higher level of circulating chylomicrons, due to impaired lymphatics, may exacerbate an otherwise normal physiological response leading to chronic inflammation that negatively affects lymphatic vessels, causing increased deposition of fat. Circulating lipids can then accumulate in both adipocytes and macrophages (foam cells), as found in atherosclerosis. Lipid storage in macrophages is an important step in the development of atherosclerosis, where plaque lesion progression is correlated with accumulation of foam cells.[108] Apolipoprotein E null mice are commonly used to investigate the biological mechanisms of plaque development in arteries. Interestingly, lymphatic vessel functions are also compromised as dyslipidemia advances in apolipoprotein null mice.[109] Elevated levels of chylomicrons may be a significant and independent risk factor for the development of cardiovascular disease by enhancing inflammation, leading to dysfunctional lymphatic vessels, which in turn exacerbates inflammation-associated pathology [Figure 3].
Hypertension is another major risk factor for cardiovascular disease. An excess of dietary salt has been commonly linked to hypertension. Recent work in rats on a high salt diet has shown that excess sodium accumulates in the interstitium of the skin, leading to a hypertonic state.[110] Mononuclear phagocyte system cells, importantly macrophages, sense hypertonicity and produce VEGF-C, which promotes lymphatic vessel growth, providing an additional buffer in response to high salt intake. In cultured macrophages, VEGF-C was regulated through tonicity enhanced binding protein (TonEBP), an osmotic stress responsive transcription factor. This phenomenon was due to VEGFR-3, as blocking VEGFR-3 resulted in increased blood pressure in mice on a high salt diet. In agreement, human subjects with refractory hypertension had higher concentrations of plasma VEGF-C compared with normotensive control subjects, providing further evidence of lymphatic vessels and macrophage contribution to interstitial fluid and blood pressure homeostasis.
In addition to indirect damage to the heart from resulting pathological sequelae, the lymphatic vessels of the heart proper have received little attention, although their existence has been known for some time.[111] The lymphatic system is also involved in fluid homeostasis of the cardiac interstitium, thereby preventing myocardial edema,[112] which can result in cardiac dysfunction. As heart function is significantly compromised with only a small increase in the interstitial fluid volume, it has been proposed that impaired lymph drainage may lead to a variety of human myocardial diseases.[113] Large animal models, such as canines, are commonly used to investigate the outcome of impaired cardiac lymph flow on myocardial function. In an acute myocardial lymph flow impairment model, these animals developed cardiac edema and hemorrhage.[114] In a chronic cardiac lymph flow impairment model, these animals also developed edema, hemorrhages, deposition of fibrous and elastic tissue in addition to reduced cardiac function.[113] While the clinical data to link congenital lymphedema and cardiac dysfunction are sparse, cardiac transplantation serves as a high risk factor for damage of cardiac lymphatic vessels. It has been hypothesized that lymphatic disruption after cardiac transplantation may be a major cause for allograft failure and postoperative mortality.[115] Patients with at least one transplant rejection had a significantly lower density of VEGFR-3-positive vessels after transplantation.[116] Conversely, an investigation by Dashkevich et al. found a significantly higher density of Prox-1-positive lymphatic vessels in rejection grade A1 or A2 biopsy lung transplant recipients.[117] Similar findings were seen in kidney and cornea transplant recipients.[118,119] While increased lymphatic vessels can enhance antigen presentation and the subsequent adaptive immune response that may lead to organ rejection, decreased lymphatic vessels can lead to edema, which accompanies acute organ rejection in many cases. Further studies are warranted to prove the exact cause of organ rejection in specific cases. Such evidence will reveal if it is beneficial to stimulate lymphangiogenesis after cardiac transplantation. Also, to what extent is there a balance between too many or too few lymphatic vessels? Interestingly, several lines of evidence show that lymphatic vessels can grow or remodel in response to pathological changes of the heart. Infective endocarditis was shown to increase the number of lymphatic vessels,[120] but not blood vessels. In addition, lymphangiogenesis accompanies other major cardiac pathological changes, such as acute and chronic ischemia, progressive atherosclerosis, myocarditis, and hypertrophy.[120] Kawasaki disease (KD), characterized by systemic vasculitis, especially of the coronary arteries, results in tissue edema. Increased production of VEGF-D was associated with lymphangiogenesis in patients with acute KD. Consistent with findings in other tissues, coronary/cardiac inflammatory cell infiltration was accompanied by lymphangiogenesis.[121] It is possible that the lymphangiogenesis in certain pathologies may function as a compensatory mechanism to maintain physiologic conditions and reduce tissue edema during resolution of a particular insult, but lymphatic vessels may become compromised due to other inflammatory cytokines present.
CONCLUDING REMARKS
With the emergence of lymphatic-specific markers, further characterization of the underlying molecular mechanisms for lymphangiogenesis may provide a therapeutic avenue for selective inhibition of lymphatic vessels in diseases such as cancer. On the other hand, stimulation of lymphangiogenesis may be beneficial in diseases of lymphatic insufficiency. Additional study of lymphatic vessel regulation will yield further insight into recent implications of their contribution to transplant rejection, obesity, hypertension, and other metabolic and inflammatory disorders.
ACKNOWLEDGMENTS
We thank Myriam Hill for assistance with figure preparation. We also thank Elenoe Smith for critical revision and comments. This work was supported by a Ruth Kirschstein National Research Service Award from the National Institutes of Health, F31CA136316, to D. J.
Footnotes
Source of Support: Ruth Kirschstein National Research Service Award from the National Institutes of Health, F31CA136316, to D.J.
Conflict of Interest: None declared.
REFERENCES
- 1.Aselli G, Bassano C, Falcini D, Settala S, Tadino A. Mediolani: Apud Jo. Baptistam Bidellium; 1627. De lactibus, sive lacteis venis, quarto vasorum mesaraicorum genere, novo invento.Dissertatio, qua sententiae anatomicae multae, vel perperam receptae convelluntur vel parum perceptae illustrantur. [Google Scholar]
- 2.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 3.Breiteneder-Geleff S, Soleiman A, Horvat R, Amann G, Kowalski H, Kerjaschki D. Podoplanin--a specific marker for lymphatic endothelium expressed in angiosarcoma. Verh Dtsch Ges Pathol. 1999;83:270–5. [PubMed] [Google Scholar]
- 4.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. Lyve-1, a new homologue of the cd44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabin F. On the origin of the lymphatic system from the veins, and the development of the lymph hearts and thoracic duct in the pig. Am J Anat. 1902;1:367–89. [Google Scholar]
- 6.Huntington G, Mc Clure C. The anatomy and development of the jugular lymph sacs in the domestic cat (felis domestica) Am J Anat. 1910;10:177–311. [Google Scholar]
- 7.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–32. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–7. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 9.Francois M, Koopman P, Beltrame M. Soxf genes: Key players in the development of the cardio-vascular system. Int J Biochem Cell Biol. 2010;42:445–8. doi: 10.1016/j.biocel.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, et al. The nuclear hormone receptor coup-tfii is required for the initiation and early maintenance of prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24:696–707. doi: 10.1101/gad.1859310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor c is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 12.Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193:607–18. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedrioli DM, Karpanen T, Dabouras V, Jurisic G, van de Hoek G, Shin JW, et al. Mir-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol Cell Biol. 2010;30:3620–34. doi: 10.1128/MCB.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazenwadel J, Michael MZ, Harvey NL. Prox1 expression is negatively regulated by mir-181 in endothelial cells. Blood. 2010;116:2395–401. doi: 10.1182/blood-2009-12-256297. [DOI] [PubMed] [Google Scholar]
- 15.Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, et al. Regulation of blood and lymphatic vascular separation by signaling proteins slp-76 and syk. Science. 2003;299:247–51. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taniguchi K, Kohno R, Ayada T, Kato R, Ichiyama K, Morisada T, et al. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Mol Cell Biol. 2007;27:4541–50. doi: 10.1128/MCB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichise H, Ichise T, Ohtani O, Yoshida N. Phospholipase cgamma2 is necessary for separation of blood and lymphatic vasculature in mice. Development. 2009;136:191–5. doi: 10.1242/dev.025353. [DOI] [PubMed] [Google Scholar]
- 18.Carramolino L, Fuentes J, Garcia-Andres C, Azcoitia V, Riethmacher D, Torres M. Platelets play an essential role in separating the blood and lymphatic vasculatures during embryonic angiogenesis. Circ Res. 2010;106:1197–201. doi: 10.1161/CIRCRESAHA.110.218073. [DOI] [PubMed] [Google Scholar]
- 19.Kato Y, Fujita N, Kunita A, Sato S, Kaneko M, Osawa M, et al. Molecular identification of aggrus/t1 alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J Biol Chem. 2003;278:51599–605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, et al. Involvement of the snake toxin receptor clec-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–6001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 21.Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, et al. Platelets regulate lymphatic vascular development through clec-2-slp-76 signaling. Blood. 2010;116:661–70. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu J, Gerhardt H, McDaniel JM, Xia B, Liu X, Ivanciu L, et al. Endothelial cell o-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118:3725–37. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- 24.Olszewski WL. The lymphatic system in body homeostasis: Physiological conditions. Lymphat Res Biol. 2003;1:11–21. doi: 10.1089/15396850360495655. [DOI] [PubMed] [Google Scholar]
- 25.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–53. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 26.Leak LV, Burke JF. Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am J Anat. 1966;118:785–809. doi: 10.1002/aja.1001180308. [DOI] [PubMed] [Google Scholar]
- 27.Leak LV, Burke JF. Ultrastructural studies on the lymphatic anchoring filaments. J Cell Biol. 1968;36:129–49. [PMC free article] [PubMed] [Google Scholar]
- 28.von der Weid PY, Zawieja DC. Lymphatic smooth muscle: The motor unit of lymph drainage. Int J Biochem Cell Biol. 2004;36:1147–53. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Turner SG, Barrowman JA. Intestinal lymph flow and lymphatic transport of protein during fat absorption. Q J Exp Physiol Cogn Med Sci. 1977;62:175–80. doi: 10.1113/expphysiol.1977.sp002387. [DOI] [PubMed] [Google Scholar]
- 30.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Forster R, Davalos-Misslitz AC, Rot A. Ccr7 and its ligands: Balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–71. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 33.Bromley SK, Thomas SY, Luster AD. Chemokine receptor ccr7 guidest cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 34.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to t cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–44. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mebius RE, Streeter PR, Breve J, Duijvestijn AM, Kraal G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J Cell Biol. 1991;115:85–95. doi: 10.1083/jcb.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via aire-independent direct antigen presentation. J Exp Med. 2010;207:681–8. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689–97. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2010;34:96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–15. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Connolly DT, Olander JV, Heuvelman D, Nelson R, Monsell R, Siegel N, et al. Human vascular permeability factor. Isolation from u937 cells. J Biol Chem. 1989;264:20017–24. [PubMed] [Google Scholar]
- 41.Stacker SA, Stenvers K, Caesar C, Vitali A, Domagala T, Nice E, et al. Biosynthesis of vascular endothelial growth factor-d involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274:32127–36. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- 42.Murakami M, Zheng Y, Hirashima M, Suda T, Morita Y, Ooehara J, et al. Vegfr1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler Thromb Vasc Biol. 2008;28:658–64. doi: 10.1161/ATVBAHA.107.150433. [DOI] [PubMed] [Google Scholar]
- 43.Shibuya M, Claesson-Welsh L. Signal transduction by vegf receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–60. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–83. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 45.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, et al. A novel vascular endothelial growth factor, vegf-c, is a ligand for the flt4 (vegfr-3) and kdr (vegfr-2) receptor tyrosine kinases. EMBO J. 1996;15:290–8. [PMC free article] [PubMed] [Google Scholar]
- 46.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, et al. Vegf-c receptor binding and pattern of expression with vegfr-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–37. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 47.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, et al. Vascular endothelial growth factor d (vegf-d) is a ligand for the tyrosine kinases vegf receptor 2 (flk1) and vegf receptor 3 (flt4) Proc Natl Acad Sci USA. 1998;95:548–53. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, et al. Hyperplasia of lymphatic vessels in vegf-c transgenic mice. Science. 1997;276:1423–5. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 49.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–31. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the vegf-c/d receptor vegfr-3. EMBO J. 2001;20:4762–73. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, et l. Blocking vegfr-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–60. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 52.Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, et al. Induction of cutaneous delayed-type hypersensitivity reactions in vegf-a transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–57. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 53.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Manseau EJ, et al. Vegf-a induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb Symp Quant Biol. 2002;67:227–37. doi: 10.1101/sqb.2002.67.227. [DOI] [PubMed] [Google Scholar]
- 54.Jones D, Xu Z, Zhang H, He Y, Kluger MS, Chen H, et al. Functional analyses of the bone marrow kinase in the x chromosome in vascular endothelial growth factor-induced lymphangiogenesis. Arterioscler Thromb Vasc Biol. 2010;30:2553–61. doi: 10.1161/ATVBAHA.110.214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. Vegf-a produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110:3158–67. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, et al. Vegf-a promotes tissue repair-associated lymphatic vessel formation via vegfr-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–3. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson I, Bahram F, Li X, Gualandi L, Koch S, Jarvius M, et al. Vegf receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J. 2010;29:1377–88. doi: 10.1038/emboj.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:15593–8. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, et al. Dose-dependent response of fgf-2 for lymphangiogenesis. Proc Natl Acad Sci USA. 2004;101:11658–63. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, et al. Pdgf-bb induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–45. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 61.Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, et al. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–6. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- 62.Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, et al. Angiopoietin-1 promotes lyve-1-positive lymphatic vessel formation. Blood. 2005;105:4649–56. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 63.Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–8. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 64.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, et al. Tnf-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–64. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mounzer RH, Svendsen OS, Baluk P, Bergman CM, Padera TP, Wiig H, et al. Lymphotoxin-alpha contributes to lymphangiogenesis. Blood. 2010;116:2173–82. doi: 10.1182/blood-2009-12-256065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larrieu-Lahargue F, Welm AL, Thomas KR, Li DY. Netrin-4 induces lymphangiogenesis in vivo. Blood. 2010;115:5418–26. doi: 10.1182/blood-2009-11-252338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118:40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spinella F, Garrafa E, Di Castro V, Rosano L, Nicotra MR, Caruso A, et al. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res. 2009;69:2669–76. doi: 10.1158/0008-5472.CAN-08-1879. [DOI] [PubMed] [Google Scholar]
- 69.Sapoznik S, Cohen B, Tzuman Y, Meir G, Ben-Dor S, Harmelin A, et al. Gonadotropin-regulated lymphangiogenesis in ovarian cancer is mediated by ledgf-induced expression of vegf-c. Cancer Res. 2009;69:9306–14. doi: 10.1158/0008-5472.CAN-09-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, et al. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol. 2010;177:3202–14. doi: 10.2353/ajpath.2010.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A, et al. Tgf-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol. 2008;295:H2113–27. doi: 10.1152/ajpheart.00879.2008. [DOI] [PubMed] [Google Scholar]
- 72.Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, et al. Inhibition of endogenous tgf-beta signaling enhances lymphangiogenesis. Blood. 2008;111:4571–9. doi: 10.1182/blood-2007-10-120337. [DOI] [PubMed] [Google Scholar]
- 73.Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by cd36 ligation on monocytes. J Exp Med. 2011;208:1083–92. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferrell RE, Levinson KL, Esman JH, Kimak MA, Lawrence EC, Barmada MM, et al. Hereditary lymphedema: Evidence for linkage and genetic heterogeneity. Hum Mol Genet. 1998;7:2073–8. doi: 10.1093/hmg/7.13.2073. [DOI] [PubMed] [Google Scholar]
- 75.Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, et al. Missense mutations interfere with vegfr-3 signalling in primary lymphoedema. Nat Genet. 2000;25:153–9. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- 76.Evans AL, Bell R, Brice G, Comeglio P, Lipede C, Jeffery S, et al. Identification of eight novel vegfr-3 mutations in families with primary congenital lymphoedema. J Med Genet. 2003;40:697–703. doi: 10.1136/jmg.40.9.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001;98:12677–82. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rockson SG. Lymphedema. Am J Med. 2001;110:288–95. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- 79.Pfarr KM, Debrah AY, Specht S, Hoerauf A. Filariasis and lymphoedema. Parasite Immunol. 2009;31:664–72. doi: 10.1111/j.1365-3024.2009.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–66. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 81.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, et al. Vegf-a stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, et al. An important role of lymphatic vessel activation in limiting acute inflammation. Blood. 2011;117:4667–78. doi: 10.1182/blood-2010-10-316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huggenberger R, Ullmann S, Proulx ST, Pytowski B, Alitalo K, Detmar M. Stimulation of lymphangiogenesis via vegfr-3 inhibits chronic skin inflammation. J Exp Med. 2010;207:2255–69. doi: 10.1084/jem.20100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, et al. Inflammation-induced lymphangiogenesis in the cornea arises from cd11b-positive macrophages. J Clin Invest. 2005;115:2363–72. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeon BH, Jang C, Han J, Kataru RP, Piao L, Jung K, et al. Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from cd11b+ macrophages in advanced ovarian cancer. Cancer Res. 2008;68:1100–9. doi: 10.1158/0008-5472.CAN-07-2572. [DOI] [PubMed] [Google Scholar]
- 86.Wu TF, Carati CJ, Macnaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the tnbs model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G566–74. doi: 10.1152/ajpgi.00058.2006. [DOI] [PubMed] [Google Scholar]
- 87.Lynch PM, Delano FA, Schmid-Schonbein GW. The primary valves in the initial lymphatics during inflammation. Lymphat Res Biol. 2007;5:3–10. doi: 10.1089/lrb.2007.5102. [DOI] [PubMed] [Google Scholar]
- 88.Chaitanya GV, Franks SE, Cromer W, Wells SR, Bienkowska M, Jennings MH, et al. Differential cytokine responses in human and mouse lymphatic endothelial cells to cytokines in vitro. Lymphat Res Biol. 2010;8:155–64. doi: 10.1089/lrb.2010.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tobler NE, Detmar M. Tumor and lymph node lymphangiogenesis--impact on cancer metastasis. J Leukoc Biol. 2006;80:691–6. doi: 10.1189/jlb.1105653. [DOI] [PubMed] [Google Scholar]
- 90.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–56. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor-c and c-c chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009;69:349–57. doi: 10.1158/0008-5472.CAN-08-1875. [DOI] [PubMed] [Google Scholar]
- 92.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-c in melanoma. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–8. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 94.Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble vegf receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 95.Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6:109–22. doi: 10.1089/lrb.2008.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, et al. Lymphatic vascular defects promoted by prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–81. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 97.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the framingham heart study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 98.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hagita S, Osaka M, Shimokado K, Yoshida M. Adipose inflammation initiates recruitment of leukocytes to mouse femoral artery: Role of adipo-vascular axis in chronic inflammation. PLoS One. 2011;6:e19871. doi: 10.1371/journal.pone.0019871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Montague CT, O’Rahilly S. The perils of portliness: Causes and consequences of visceral adiposity. Diabetes. 2000;49:883–8. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 101.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 102.Dalmas E, Clement K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32:307–14. doi: 10.1016/j.it.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. 2002;5:551–9. doi: 10.1097/00075197-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 104.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, et al. Angiogenic role of lyve-1-positive macrophages in adipose tissue. Circ Res. 2007;100:e47–57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 105.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA. 2004;292:1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 106.van Oostrom AJ, Rabelink TJ, Verseyden C, Sijmonsma TP, Plokker HW, De Jaegere PP, et al. Activation of leukocytes by postprandial lipemia in healthy volunteers. Atherosclerosis. 2004;177:175–82. doi: 10.1016/j.atherosclerosis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 107.van Oostrom AJ, Sijmonsma TP, Verseyden C, Jansen EH, de Koning EJ, Rabelink TJ, et al. Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. J Lipid Res. 2003;44:576–83. doi: 10.1194/jlr.M200419-JLR200. [DOI] [PubMed] [Google Scholar]
- 108.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: Subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ, et al. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol. 2009;175:1328–37. doi: 10.2353/ajpath.2009.080963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, et al. Osmotically inactive skin na+ storage in rats. Am J Physiol Renal Physiol. 2003;285:F1108–17. doi: 10.1152/ajprenal.00200.2003. [DOI] [PubMed] [Google Scholar]
- 111.Shore LR. The lymphatic drainage of the heart: A preliminary communication. J Anat. 1928;62:125–34. [PMC free article] [PubMed] [Google Scholar]
- 112.Mehlhorn U, Davis KL, Burke EJ, Adams D, Laine GA, Allen SJ. Impact of cardiopulmonary bypass and cardioplegic arrest on myocardial lymphatic function. Am J Physiol. 1995;268:H178–83. doi: 10.1152/ajpheart.1995.268.1.H178. [DOI] [PubMed] [Google Scholar]
- 113.Miller AJ. The grossly invisible and generally ignored lymphatics of the mammalian heart. Med Hypotheses. 2011;76:604–6. doi: 10.1016/j.mehy.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 114.Sun SC, Lie JT. Cardiac lymphatic obstruction: Ultrastructure of acute-phase myocardial injury in dogs. Mayo Clin Proc. 1977;52:785–92. [PubMed] [Google Scholar]
- 115.Kong XQ, Wang LX, Kong DG. Cardiac lymphatic interruption is a major cause for allograft failure after cardiac transplantation. Lymphat Res Biol. 2007;5:45–7. doi: 10.1089/lrb.2007.5108. [DOI] [PubMed] [Google Scholar]
- 116.Geissler HJ, Dashkevich A, Fischer UM, Fries JW, Kuhn-Regnier F, Addicks K, et al. First year changes of myocardial lymphatic endothelial markers in heart transplant recipients. Eur J Cardiothorac Surg. 2006;29:767–71. doi: 10.1016/j.ejcts.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 117.Dashkevich A, Heilmann C, Kayser G, Germann M, Beyersdorf F, Passlick B, et al. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann Thorac Surg. 2010;90:406–11. doi: 10.1016/j.athoracsur.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 118.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–12. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 119.Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing vegf promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–73. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 120.Kholova I, Dragneva G, Cermakova P, Laidinen S, Kaskenpaa N, Hazes T, et al. Lymphatic vasculature is increased in heart valves, ischaemic and inflamed hearts and in cholesterol-rich and calcified atherosclerotic lesions. Eur J Clin Invest. 2011;41:487–97. doi: 10.1111/j.1365-2362.2010.02431.x. [DOI] [PubMed] [Google Scholar]
- 121.Ebata R, Abe J, Yasukawa K, Hamada H, Higashi K, Suwazono Y, et al. Increased production of vascular endothelial growth factor-d and lymphangiogenesis in acute kawasaki disease. Circ J. 2011;75:1455–62. doi: 10.1253/circj.cj-10-0897. [DOI] [PubMed] [Google Scholar]


