Abstract
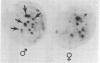
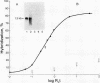
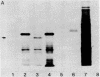
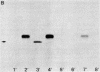
We describe a simple cloning procedure for alpha 2u-globulin that requires neither enrichment of mRNA for cloning nor purification of a specific probe for screening recombinant colonies. Total adult male liver poly(A)+RNA was used as template for cloning, and the subsequent recombinant colonies were screened by comparing hybridization to radioactive cDNA probes prepared from hepatic male and female mRNA, respectively. Almost all of the selected "male-specific" clones were later shown to contain alpha 2u-globulin sequences. This cloned alpha 2u-globulin cDNA has been shown to specifically hybridize to male rat liver RNA, which, when isolated and translated in vitro, codes for a 21,000-dalton protein (pro-alpha 2u-globulin) immunologically identical to alpha 2u-globulin. When translation occurs in the presence of pancreatic microsomes this in vitro synthesized pro-alpha 2u-globulin is processed to the 19,000-dalton mature form of alpha 2u-globulin. The nucleotide sequence of the alpha 2u-globulin cDNA has been determined, thus elucidating the complete amino acid sequence of alpha 2u-globulin and most of the hydrophobic "leader" sequence of pro-alpha 2u-globulin. The amino acid sequence deduced from the cDNA is in agreement with the partial sequence that we previously determined by sequential Edman degradation of the purified protein. alpha 2u-Globulin cDNA clones contain within the 3'-untranslated region one or both of the two putative polyadenylylation/transcription termination sites (A-A-T-A-A-A and A-A-T-T-A-A-A). Either of these can be used, generating alpha 2u-globulin mRNA species of two lengths. A codon usage analysis of the cDNA showed that, although all six leucine codons are used for the 14 leucine residues in mature alpha 2u-globulin, the seven leucines in the partial leader sequence reported are all encoded by the same codon, CTG. The primary amino acid sequence contains a unique Asn-Gly-Ser sequence, likely to be in beta-turn conformation, as the probable site of glycosylation for this glycoprotein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. M., Kurtz D. T., Feigelson P. Transcription of the alpha2u-globulin gene in male rat liver nuclei in vitro. Biochemistry. 1978 Jul 25;17(15):3092–3096. doi: 10.1021/bi00608a023. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Feigelson P. Glucocorticoid induction of alpha2u-globulin protein synthesis and its mRNA in rat hepatocytes in vitro. J Biol Chem. 1978 Nov 10;253(21):7880–7885. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Beta-turns in proteins. J Mol Biol. 1977 Sep 15;115(2):135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. V., Smardo F. L., Jr, Morrissey J. J. Evidence for internal homology in bovine preproparathyroid hormone. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1469–1471. doi: 10.1073/pnas.76.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigelson P., Kurtz D. T. Hormonal modulation of specific messenger RNA species in normal and neoplastic rat liver. Adv Enzymol Relat Areas Mol Biol. 1978;47:275–312. doi: 10.1002/9780470122921.ch4. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haars L. J., Pitot H. C. Hormonal and developmental regulation of glycosylated alpha 2u-globulin synthesis. Arch Biochem Biophys. 1980 May;201(2):556–563. doi: 10.1016/0003-9861(80)90545-7. [DOI] [PubMed] [Google Scholar]
- Hobart P., Crawford R., Shen L., Pictet R., Rutter W. J. Cloning and sequence analysis of cDNAs encoding two distinct somatostatin precursors found in the endocrine pancreas of anglerfish. Nature. 1980 Nov 13;288(5787):137–141. doi: 10.1038/288137a0. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Synthesis of extensive, possibly complete, DNA copies of poliovirus RNA in high yields and at high specific activities. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2191–2195. doi: 10.1073/pnas.73.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. T., Chan K. M., Feigelson P. Glucocorticoid induction of hepatic alpha2u-globulin synthesis and messenger RNA level in castrated male rats in vivo. J Biol Chem. 1978 Nov 10;253(21):7886–7890. [PubMed] [Google Scholar]
- Kurtz D. T., Feigelson P. Multihormonal induction of hepatic alpha2u-globulin mRNA as measured by hybridization to complementary DNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4791–4795. doi: 10.1073/pnas.74.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. T., Sippel A. E., Ansah-Yiadom R., Feigelson P. Effects of sex hormones on the level of the messenger RNA for the rat hepatic protein alpha 2u globulin. J Biol Chem. 1976 Jun 25;251(12):3594–3598. [PubMed] [Google Scholar]
- Kurtz D. T., Sippel A. E., Feigelson P. Effect of thyroid hormones on the level of the hepatic mRNA for alpha2u globulin. Biochemistry. 1976 Mar 9;15(5):1031–1036. doi: 10.1021/bi00650a013. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane S. E., Neuhaus C. W. Further studies on the isolation and characterization of a sex-dependent protein from the urine of male rats. Biochim Biophys Acta. 1972 Feb 29;257(2):461–470. doi: 10.1016/0005-2795(72)90299-1. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Crerar M. M., Swain W. F., Pictet R. L., Thomas G., Rutter W. J. Structure of a family of rat amylase genes. Nature. 1980 Sep 11;287(5778):117–122. doi: 10.1038/287117a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhasi H. L., Lynch K. R., Dolan K. P., Unterman R. D., Feigelson P. Covalent modification and repressed transcription of a gene in hepatoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):834–837. doi: 10.1073/pnas.78.2.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhasi H. L., Quasba P. K. Quantitation of milk proteins and their mRNAs in rat mammary gland at various stages of gestation and lactation. J Biol Chem. 1979 Jul 10;254(13):6016–6025. [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Pennica D., Lynch K. R., Cohen P. S., Ennis H. L. Decay of vesicular stomatitis virus mRNAs in vivo. Virology. 1979 Apr 30;94(2):484–487. doi: 10.1016/0042-6822(79)90480-x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. K. Androgen-dependent synthesis of aplha-2u globulin in the rat: role of the pituitary gland. J Endocrinol. 1973 Feb;56(2):295–301. doi: 10.1677/joe.0.0560295. [DOI] [PubMed] [Google Scholar]
- Roy A. K., Neuhaus O. W., Harmison C. R. Preparation and characterization of a sex-dependent rat urinary protein. Biochim Biophys Acta. 1966 Sep 26;127(1):72–81. doi: 10.1016/0304-4165(66)90477-6. [DOI] [PubMed] [Google Scholar]
- Roy A. K., Neuhaus O. W. Identification of rat urinary proteins by zone and immunoelectrophoresis. Proc Soc Exp Biol Med. 1966 Mar;121(3):894–899. doi: 10.3181/00379727-121-30917. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Shine J., Martial J. A., Baxter J. D., Goodman H. M. Nucleotide sequence and amplification in bacteria of structural gene for rat growth hormone. Nature. 1977 Dec 8;270(5637):486–494. doi: 10.1038/270486a0. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Feigelson P., Roy A. K. Hormonal regulation of the hepatic messenger RNA levels for alpha2u globulin. Biochemistry. 1975 Feb 25;14(4):825–829. doi: 10.1021/bi00675a028. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Kurtz D. T., Morris H. P., Feigelson P. Comparison of in vivo translation rates and messenger RNA levels of alpha2U-globulin in rat liver and Morris hepatoma 5123D. Cancer Res. 1976 Oct;36(10):3588–3593. [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Rosbash M. The use of R-looping for structural gene identification and mRNA purification. Nucleic Acids Res. 1979 Jun 11;6(7):2483–2497. doi: 10.1093/nar/6.7.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]